Botanical Mixture Stabilizes Cognitive Function in Patients with Mild and Moderate Alzheimer’s Disease
Matsagas K1*, Villeponteau B2, Shankle WR3,4, Cruise RJ1, Morales S1, Goertzel B1, Simmon VF1, Benford G5 and Rizza C1
1Genescient Corporation, Fountain Valley, California, United States of America
2Centagen Inc., Boulder, Colorado, United States of America
3Shankle Clinic, Hoag Hospital, Newport Beach, California, United States of America
4Department of Cognitive Sciences, University of California at Irvine, Irvine, California, United States of America
5Department of Physics and Astronomy, University of California at Irvine, Irvine, California, United States of America
- *Corresponding Author:
- Kennedy Matsagas
Genescient Corporation
Fountain Valley, California, United States of America
Tel: 55622098912
E-mail: kmatsagas@genescient.com
Received Date: July 20, 2018; Accepted Date: August 08, 2018; Published Date: August 17, 2018
Citation: Matsagas K, Villeponteau B, Shankle WR, Cruise RJ, Morales S, et al. (2018) Botanical Mixture Stabilizes Cognitive Function in Patients with Mild and Moderate Alzheimer’s Disease. J Clin Med Ther. 3:14.
Abstract
Context: Currently, there is no treatment that can stop the progression of Alzheimer’s disease. We have used transgenic Drosophila melanogaster models and machine learning to develop an eight component botanical mixture (Geneaire™ ReBuilder™) that targets multiple genetic pathways involved in brain aging and dementia that are homologous between Drosophila and humans.
Objective: To test the effects of ReBuilder on the cognitive function of subjects diagnosed with mild or moderate Alzheimer’s disease.
Methods: We recruited 50 subjects with mild to moderate AD to participate in a double-blind, placebo-controlled clinical study. During the 12-month pilot study, the subjects were evaluated quarterly on the Mini Mental State Exam (MMSE), Alzheimer’s Disease Cooperative Study’s Activities of Daily Living (ADCS-ADL), and the Clinical Dementia Rating Sum of Boxes (CDR-SB).
Results: The addition of ReBuilder to subjects’ existing Namenda and Exelon regimens stabilized cognitive decline in patients with mild AD and slowed cognitive decline in patients with moderate AD.
Conclusions: These results were observed in both sexes and in all ages tested. Importantly, no adverse side effects attributable to ReBuilder were reported. The results of this clinical pilot warrant further study of ReBuilder in AD.
Keywords
Alzheimer's disease; Dementia; Aging; Neurology; APOE; Genetics; Cognition
Introduction
Alzheimer’s Disease (AD) is the 6th leading cause of death in the United States [1]. Today, more than 5 million Americans are living with Alzheimer’s disease (AD), and 1 in 3 seniors’ dies with AD or another form of dementia [2]. By 2050, the number of people expected to be living with AD will rise to 14 million and cost an estimated $1.1 trillion dollars [2]. Deaths of those with AD were about 600,000 in 2010 and are projected to rise to 1.6 million by 2050, which is expected to be some 43% of all older adult deaths [3]. Beta-amyloid (Abeta) plaques and hyperphosphylated Tau (pTau) neurofibrillary tangles have been the dominant focus of research on AD pathophysiology since the disease was first recognized by Alois Alzheimer in 1906 [4,5]. While plaques and tangles are diagnostic for AD [5-7], the cause(s) of their soluble precursors that kill neurons has not been determined. The majority of AD patients are diagnosed after 60 years of age. Many studies point to aging related processes like inflammation [8-14], neural vascular damage [15-21], neural stress [9,22-32], altered cell metabolism [33-37], cellular autophagy [38-45], microglial dysfunction [46-48], mitochondrial dysfunction [49-52], astrocyte function [53-59] and dietary factors [60-65] as potential causal factors in the decline of brain function over the decades that precede an actual AD diagnosis. AD is a multifaceted pathology involving many biochemical pathways and thus a multifaceted therapeutic approach may prove beneficial.
Drosophila melanogaster, the common fruit fly, has been extensively studied and is a highly tractable genetic model organism for understanding molecular mechanisms of human diseases. Many basic biological, physiological, and neurological properties are conserved between mammals and D. melanogaster, and nearly 75% of human disease-causing genes are believed to have a functional homolog in the fly [66,67]. Therefore we used genetic and machine learning approaches on age-related data bases for humans and flies to identify many homologous CNS-specific genetic and biochemical pathways involved in longevity. We targeted genes known to be important in AD and screened botanical products known to interact with the pathways we identified using transgenic Drosophila models [68]. The result is ReBuilder, a 7-component botanical supplement that is effective in reducing neural dysfunction in our Drosophila model of AD. For this human pilot study, we added an eighth component, bioperine, to improve absorption of ReBuilder.
Materials and Methods
Fifty human subjects of mixed gender between the ages of 60 and 90 were recruited and enrolled in this double-blind, placebo-controlled, IRB-approved pilot clinical study. The subjects had been on stable maximum tolerated doses of Memantine and/or Rivastigmine for at least 2 months, and nothing was changed in their standard therapy for the duration of our pilot study. The subjects were randomly assigned to either the active or placebo arm of the study. The subjects were evaluated approximately every three months (quarterly) from October 2013 to December 2015. The subjects were referred to Genescient Corporation by William R. Shankle, MD, and evaluated in the office of Cristina Rizza, MD in Fountain Valley, CA.
At the initial enrollment office visit, we administered the Mini Mental State Examination (MMSE) and the Clinical Dementia Rating Sum of Boxes (CDR-SB) to each subject. Each subject’s caregiver was interviewed using the Alzheimer’s disease Cooperative Study’s Activities of Daily Living (ADCS-ADL) Inventory to assess the subject’s current self-care capabilities. After this initial visit, the subjects were instructed to begin taking one capsule of ReBuilder in the morning and one capsule in the evening daily. Each subject had a caregiver who ensured compliance in taking all their medications. The subjects and their caregivers returned to our office approximately every three months to have the aforementioned tests repeated. ReBuilder is currently distributed by Geneaire.
The components of the ReBuilder treatment used in the clinical study and their known actives and targets are given in Table 1. The formulation is protected by US Patent 9744204.
| Component | Known Active (s) | Targets | References |
|---|---|---|---|
| Astragalus membranaceus (extract) | Astrogalosides I-VII Flavenoids, HDTICs | Telomerase, Mitochondria, ptau, mTOR, TNF-α, ERK, AMPK | [69-75] |
| Berberine HCL | Berberine (98%) | Acetylcholinesterase, AMPK, α-adrenergic receptors, β-Amyloid | [76-81] |
| Vaccinium uliginosum or Pterocarpus marsupium (extracts) | Resveratrol Analogs | PPARα, PGE2, AMPK, phosphodiesterase, Mitochondria | [82-86] |
| L-Theanine | L-Theanine (98%) | NMDA receptors, EAATs, GABA receptors, eNOS, mitochondria | [87-93] |
| Genistein | Genistein (98%) | ERα, AMPK, p450c21, PRPF8 | [94-96] |
| Lithium Orotate | Lithium | NCS-1/Frequenin, Abeta, NMDA, GSK3B, ptau | [97-105] |
| Selenium Glycinate | Selenium | PRPF8, ERCC1, Selenoproteins | [106-108] |
Table 1: Composition, known actives, and targets of treatment (US Patent 9744204).
Ethics
The subjects signed informed consent forms upon enrollment. This study protocol was approved by the Institute of Regenerative and Cellular Medicine Institutional Review Board (approval number: ICSS-2013-007). This study is registered on the Clinical Trails website of the United States government (NCT03611439).
Statistics
Throughout the 12-month period, the subjects’ scores on the MMSE, ADCS-ADL, and CDR-SB were recorded and compared to their initial baseline scores. The subjects’ mean test scores and changes in their mean test score were plotted in relation to their average baseline scores and presented with 95% confidence intervals. Only the subjects for whom we had data for the initial visit and all 4 subsequent quarters were included in the analysis presented here.
To maximize the number of subjects that would be assigned the active botanical compound, 43 subjects were given ReBuilder and only 7 patients were assigned to the placebo arm of the study. To make up for the small number of subjects in the placebo arm (only 5 completed the trial), we compared the data from our active subjects to previously published data from 471 AD subjects of matched demographics and AD symptoms from the study by Bernick et al. The subjects in this previously published study were treated and tested in the same manner as our placebo arm. Our 5 placebo subjects experienced similar loss of cognitive function as measured by MMSE, ADCS-ADL, and CDR-SB as the 471 placebo subjects in the Bernick study. Moreover, with the large clinical study from Bernick et al. we were able to match all the active subjects in our pilot with large numbers of age and condition matched surrogate placebo controls.
Results
Thirty out of the 43 subjects receiving ReBuilder successfully completed our 12-month pilot study. Of those 30, 17 were categorized as “mild” in disease severity based on an initial score at enrollment of 21 to 30 points out of a possible 30 points on the Mini Mental Status Exam (MMSE), a widely used test for dementia. The remaining 13 subjects were categorized as “moderate” in disease severity based on an initial MMSE score of 11 to 20 points.
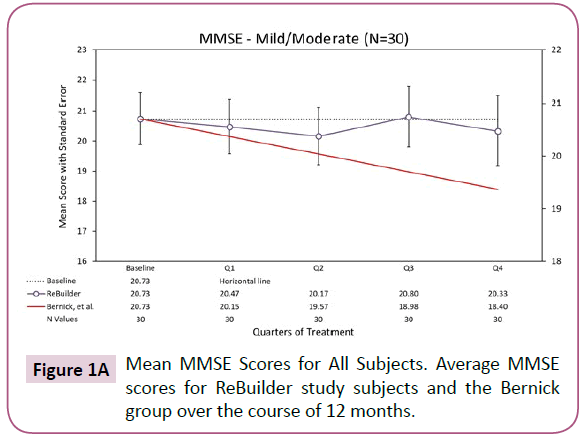
During the course of the 12-month pilot study, the mild and moderate subjects taking ReBuilder maintained MMSE scores close to their initial baseline scores. The ReBuilder subjects did not experience the rate of dec line seen in the subjects from the Bernick study [109], which we used as our benchmark control for the expected rate of decline d urin g the same study (Figure 1A).
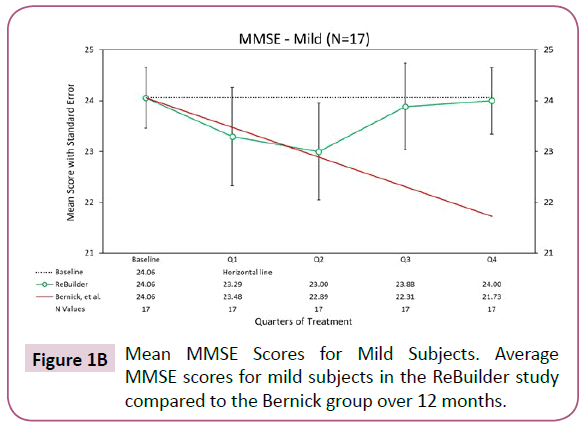
Looking at the 17 mild subjects who completed the pilot, there appears to be a pronounced preservation of the baseline MMSE scores after 12 months of treatment (Figure 1B).
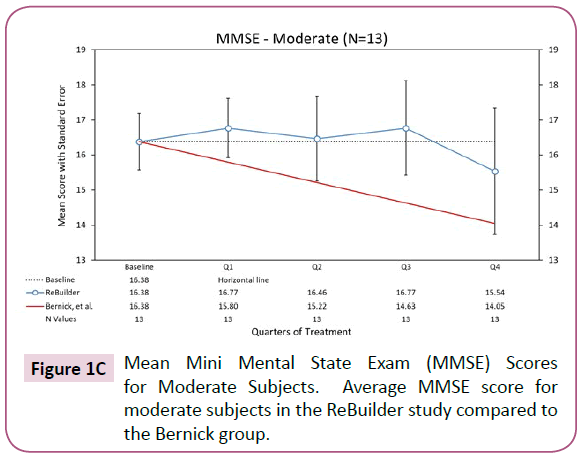
Looking at the 13 subjects with moderate AD who received ReBuilder and completed the pilot study, there is also a pronounced preservation of baseline MMSE score. The moderate AD subjects taking ReBuilder, as with the mild AD subjects, continued to score much closer to their baseline than the subjects in the Bernick study (Figure 1C).
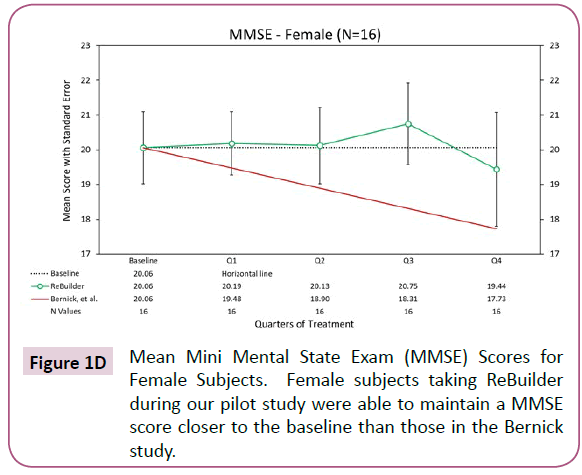
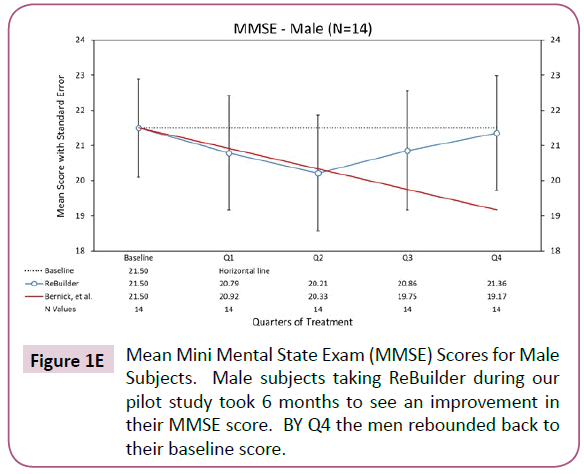
The trend of baseline score maintenance in subjects taking ReBuilder continues if we separate the subjects by gender. Generally, Alzheimer’s disease affects more women than men due to women having longer average lifespans, and possibly because of hormonal differences between the genders. In our pilot study, we had nearly equal numbers of each gender, with 16 women and 14 men. Both genders saw positive effects when ReBuilder was added to their standard therapy (Figures 1D and 1E).
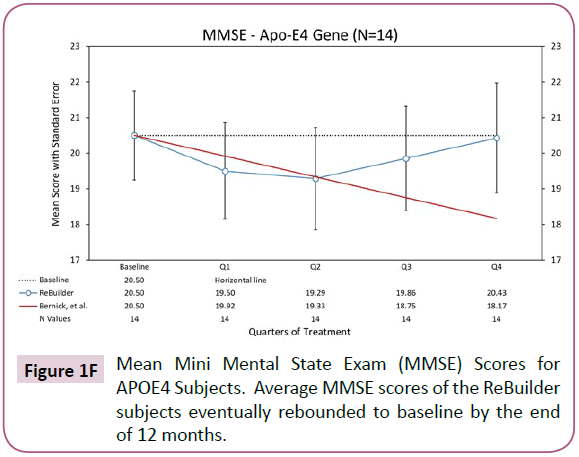
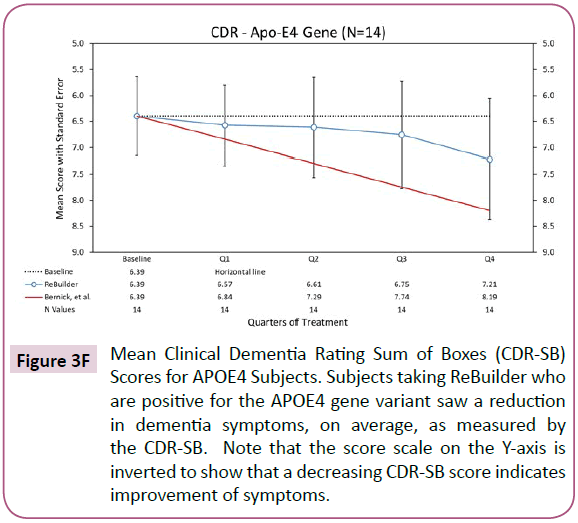
The E4 variant of the Apolipoprotein E (APOE) gene puts an individual at an increased risk of developing Alzheimer’s disease. The risk increase is present in an individual with one copy of the E4 allele, and greater still if the individual has 2 copies. The APOE4 gene variant is associated with an increased number of amyloid protein plaques in the brain tissue of affected individuals and earlier onset of AD symptoms [110]. In our pilot study, we had 14 subjects taking ReBuilder who carried at least one E4 allele. While it took 6 months for these subjects to see an effect from taking ReBuilder, the overall changes in their MMSE scores were positive by Q4 (Figure 1F).
The Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) is a questionnaire widely used to assess the competence of patients with Alzheimer’s Disease (AD) in basic and instrumental activities of daily living (ADL). The ADCS-ADL scores competence in daily activities on a 78-point scale. Slight score changes in this assessment can indicate that a subject is, or is not, able to choose appropriate clothing or prepare a simple meal.
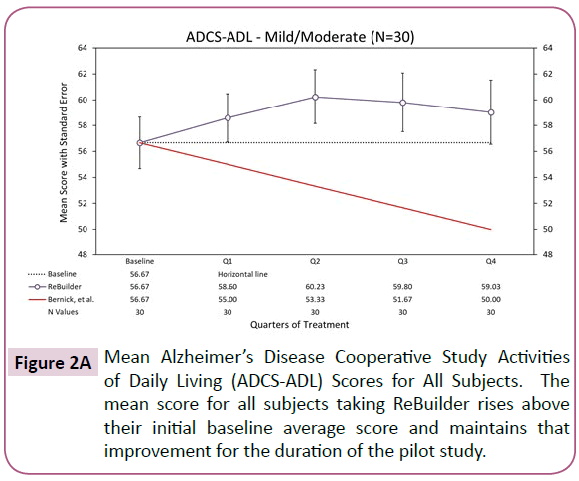
On average, our subjects taking ReBuilder gained 2.34 points on this measure after 12 months of treatment. The control group is expected to have lost 6.67 points during the same amount of time (Figure 2A).
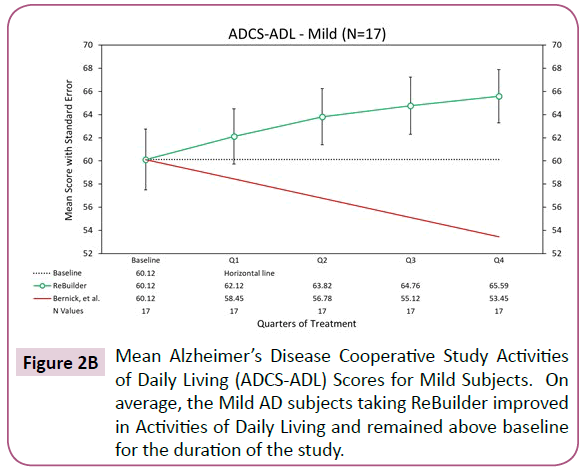
The 17 mild subjects who completed the pilot appear to display an improvement, on average, of ADCS-ADL scores after 12 months of treatment (Figure 2B).
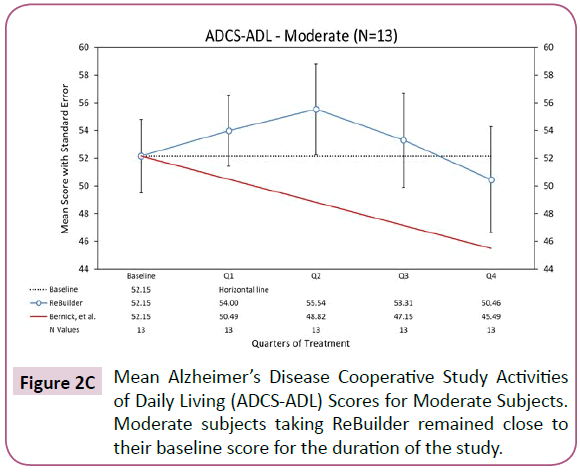
The ADCS-ADL scores of the 13 subjects with moderate AD who completed the pilot study did not see an overall improvement compared to their mean baseline scores after 12 months of treatment. However, the mean point loss of 1.69 still falls quite short of the projected point loss of 6.67 based on the Bernick study (Figure 2C).
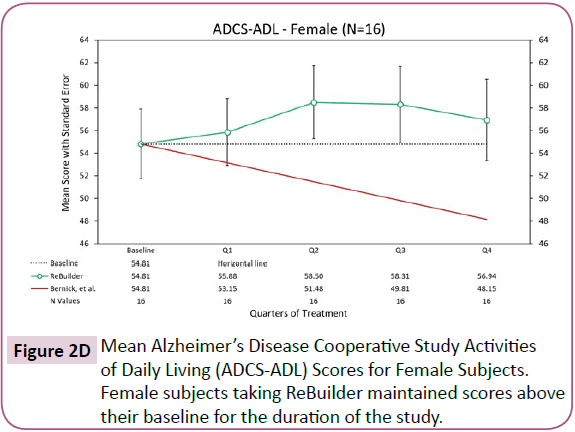
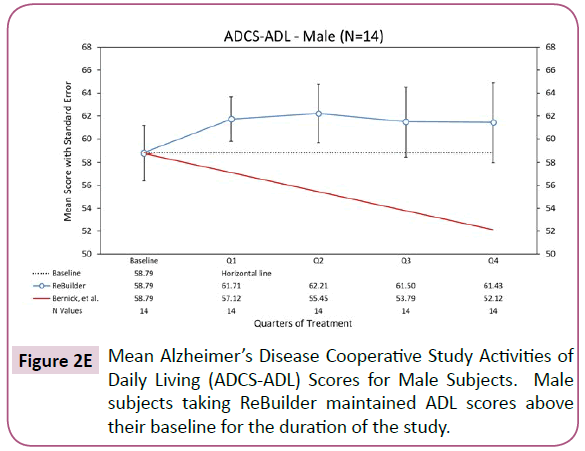
When the subjects in our pilot study are separated by gender, the overall improvement in activities of daily living remains. The subjects taking ReBuilder achieve and maintain a clinically meaningful improvement in the ADCS-ADL assessment that is above their average baseline scores, and far above the score projected based on the Bernick study at the end of 12 months, regardless of gender (Figures 2D and 2E).
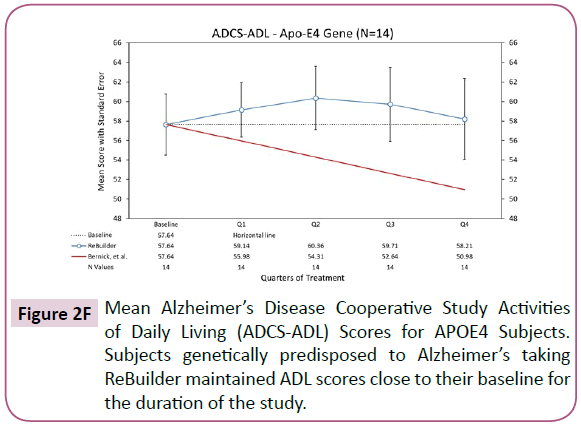
Separating out the subjects with the APOE4 genetic variant, a clinically meaningful improvement in the ADCS-ADL assessment is still observed at the end of 12 months (Figure 2F).
The Clinical Dementia Rating (CDR-SB) is used to quantify the severity of symptoms of dementia. This measure assesses a subject’s cognitive and functional performance in six areas: memory, orientation, judgment & problem solving, community affairs, home and hobbies, and personal care. Scores in each of these are combined to obtain a composite score, with a higher score indicating greater severity of dementia symptoms.
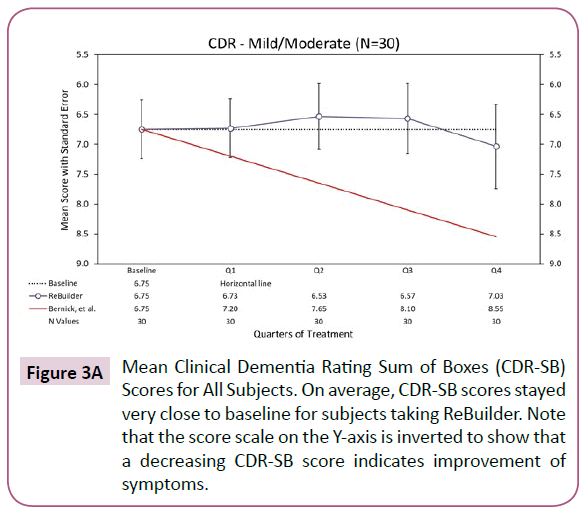
Overall, the subjects taking ReBuilder in our pilot study maintained a CDR-SB score close to their baseline. This suggests that the subjects’ severity of dementia symptoms did not increase as expected over the 12-month pilot study and that they experienced better stability in their symptoms (Figure 3A).
Figure 3A: Mean Clinical Dementia Rating Sum of Boxes (CDR-SB) Scores for All Subjects. On average, CDR-SB scores stayed very close to baseline for subjects taking ReBuilder. Note that the score scale on the Y-axis is inverted to show that a decreasing CDR-SB score indicates improvement of symptoms.
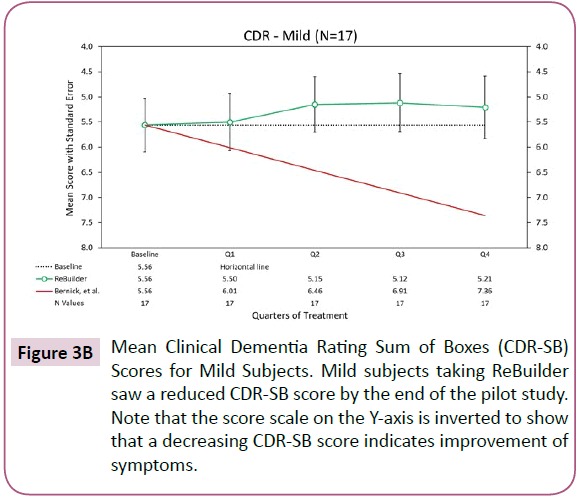
For our mild subjects, the mean CDR-SB scores stayed above baseline for the latter portion of the pilot, possibly indicating a reduction in dementia symptoms (Figure 3B).
Figure 3B: Mean Clinical Dementia Rating Sum of Boxes (CDR-SB) Scores for Mild Subjects. Mild subjects taking ReBuilder saw a reduced CDR-SB score by the end of the pilot study. Note that the score scale on the Y-axis is inverted to show that a decreasing CDR-SB score indicates improvement of symptoms.
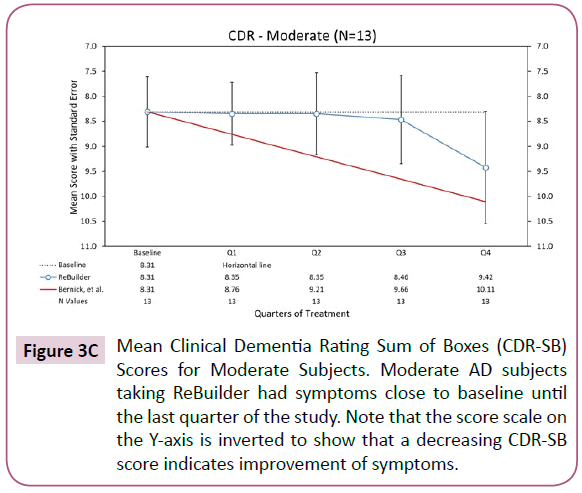
For moderate subjects, the severity of their dementia symptoms does increase by the end of the pilot study, but not to the same degree that is seen in the Bernick group over the same time period (Figure 3C).
Figure 3C: Mean Clinical Dementia Rating Sum of Boxes (CDR-SB) Scores for Moderate Subjects. Moderate AD subjects taking ReBuilder had symptoms close to baseline until the last quarter of the study. Note that the score scale on the Y-axis is inverted to show that a decreasing CDR-SB score indicates improvement of symptoms.
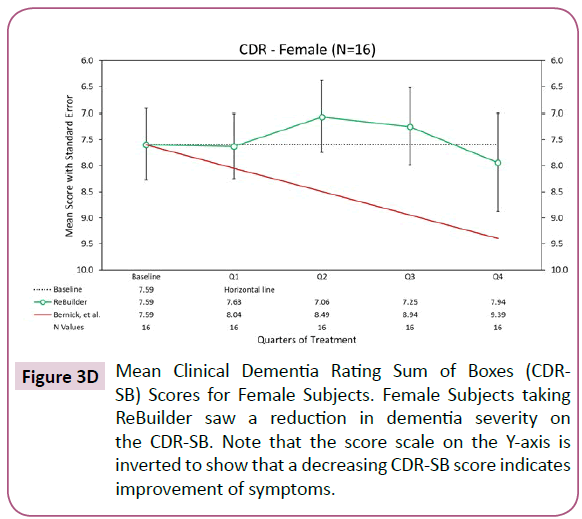
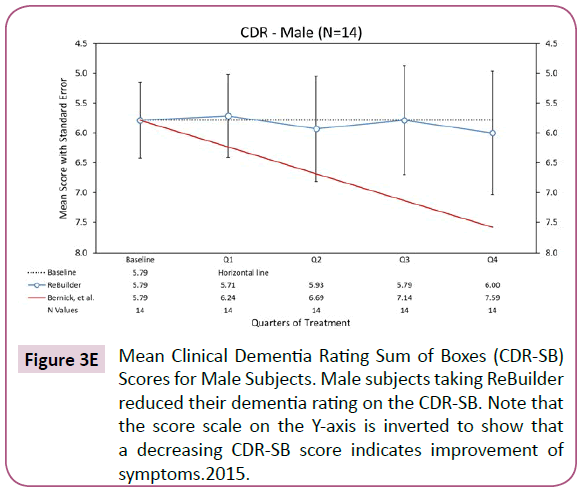
When the subjects taking ReBuilder are separated by gender, we still see a reduction in dementia severity as indicated by the average CDR-SB scores (Figures 3D and 3E).
Figure 3D: Mean Clinical Dementia Rating Sum of Boxes (CDR-SB) Scores for Female Subjects. Female Subjects taking ReBuilder saw a reduction in dementia severity on the CDR-SB. Note that the score scale on the Y-axis is inverted to show that a decreasing CDR-SB score indicates improvement of symptoms.
Looking at the subjects with the APOE4 genetic variant [110], we also see a reduction in dementia symptoms. This suggests that ReBuilder can improve the symptoms of those genetically predisposed to Alzheimer’s disease (Figure 3F).
Figure 3F: Mean Clinical Dementia Rating Sum of Boxes (CDR-SB) Scores for APOE4 Subjects. Subjects taking ReBuilder who are positive for the APOE4 gene variant saw a reduction in dementia symptoms, on average, as measured by the CDR-SB. Note that the score scale on the Y-axis is inverted to show that a decreasing CDR-SB score indicates improvement of symptoms.
On the whole, the above results show that subjects taking ReBuilder during our 12-month pilot at least slowed in their AD progression as indicated by the MMSE, ADCS-ADL, and CDR-SB. In some measures, the subjects appear to stabilize and even reduce their AD symptoms. No adverse side effects such as changes in mood, sleeping habits, balance issues, or digestive disturbances were observed in the subjects treated with ReBuilder.
Discussion
The subjects with mild and moderate AD in our pilot study experienced clinically meaningful stabilization in their disease progression over a 12 month period compared with the decline expected based on the Bernick study [109]. The results for mild and moderate AD subjects occurred regardless of the subjects’ age, gender, or genetic status with respect to the APOE4 genetic variant [111-114]. The benefit of reduced mental decline by all measures was greater in patients diagnosed with mild AD and less pronounced in moderate AD subjects. This suggests that treatment with ReBuilder is beneficial regardless of age, gender, APOE4 status, or disease severity when added to standard AD treatment.
Though no quantitative biomarker studies were done during this pilot study, the positive effects seen in cognitive function suggest that there are biological changes taking place that should be investigated further. ReBuilder is composed entirely of botanical substances that are generally regarded as safe (GRAS) by the FDA, and no deleterious side effects were reported or observed that were attributed to ReBuilder in this pilot study. Therefore, there is the potential for ReBuilder to not only used as a supplemental AD treatment, but also prophylactically as a part of a normal individual’s daily dietary supplement regimen.
While we cannot make definitive claims on the success of ReBuilder due to the small number of subjects in this pilot, we can say that the improvement in cognitive function that we have seen in subjects taking ReBuilder warrants further study in a larger AD cohort with biomarker assessment. In future clinical studies, advanced imaging technologies [33,115-120] should be used to verify that ReBuilder has effects on structural outcomes such as amyloid plaques, Tau tangles, and neuronal loss prevention with time.
References
- Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E (2017) Deaths: Final data for 2015. In: National vital statistics reports.
- https://www.alz.org/media/Documents/alzheimers-facts-and-figures-infographic.pdf
- Weuve J, Hebert LE, Scherr PA, Evans DA (2014) Deaths in the United States among persons with Alzheimer's disease (2010-2050). Alzheimers Dement 10: e40-e46.
- Cipriani G, Dolciotti C, Picchi L, Bonuccelli U (2011) Alzheimer and his disease: a brief history. Neurol Sci 32: 275-279.
- Perl DP (2010) Neuropathology of Alzheimer's disease. Mt Sinai J Med 77: 32-42.
- Li Y, Wang K, Zhou K, Guo W, Dai B, et al. (2018) Novel D-A-D based near-infrared probes for the detection of beta-amyloid and Tau fibrils in Alzheimer's disease. Chem Commun 54: 8717-8720.
- Song C, Deng P, Que L (2018) Rapid multiplexed detection of beta-amyloid and total-tau as biomarkers for Alzheimer's disease in cerebrospinal fluid. Nanomedicine 14: 1845-1852.
- Bettcher BM, Johnson SC, Fitch R, Casaletto KB, Heffernan KS, et al. (2018) Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of alzheimer's disease pathology and neuronal damage. J Alzheimers Dis 62: 385-397.
- Bisht K, Sharma K, Tremblay ME (2018) Chronic stress as a risk factor for Alzheimer's disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol Stress 9: 9-21.
- Businaro R, Corsi M, Asprino R, Di Lorenzo C, Laskin D, et al. (2018) Modulation of inflammation as a way of delaying alzheimer's disease progression: the diet's role. Curr Alzheimer Res 15: 363-380.
- Parbo P, Ismail R, Sommerauer M, Stokholm MG, Hansen AK, et al. (2018) Does inflammation precede tau aggregation in early Alzheimer's disease? A PET study. Neurobiol Dis 117: 211-216.
- Ravichandran S, Michelucci A, Del Sol A (2018) Integrative computational network analysis reveals site-specific mediators of inflammation in alzheimer's disease. Front Physiol 9: 154.
- Santos LE, Ferreira ST (2018) Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease. Neuropharmacology 136: 350-360.
- Selles MC, Oliveira MM, Ferreira ST (2018) Brain inflammation connects cognitive and non-cognitive symptoms in alzheimer's disease. J Alzheimers Dis 64: S313-S327.
- Lourenco CF, Ledo A, Dias C, Barbosa RM, Laranjinha J (2015) Neurovascular and neurometabolic derailment in aging and Alzheimer's disease. Front Aging Neurosci 7: 103.
- Luo H, Han G, Wang J, Zeng F, Li Y, et al. (2016) Common aging signature in the peripheral blood of vascular dementia and alzheimer's disease. Mol Neurobiol 53: 3596-3605.
- Gauthier S, Zhang H, Ng KP, Pascoal TA, Rosa-Neto P (2018) Impact of the biological definition of Alzheimer's disease using amyloid, tau and neurodegeneration (ATN): what about the role of vascular changes, inflammation, Lewy body pathology? Transl Neurodegener 7: 12.
- Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, Diamandis T, Borlongan MC, et al. (2013) Vascular damage: a persisting pathology common to Alzheimer's disease and traumatic brain injury. Med Hypotheses 81: 842-845.
- Luca M, Luca A, Calandra C (2015) The role of oxidative damage in the pathogenesis and progression of alzheimer's disease and vascular dementia. Oxid Med Cell Longev 2015: 504678.
- Muhammad S, Bierhaus A, Schwaninger M (2009) Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer's disease. J Alzheimers Dis 16: 775-785.
- Walker DG, Dalsing-Hernandez JE, Lue LF (2008) Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer's disease. Microvasc Res 75: 411-419.
- Caruso A, Nicoletti F, Mango D, Saidi A, Orlando R, et al. (2018) Stress as risk factor for Alzheimer's disease. Pharmacol Res 132: 130-134.
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, et al. (2018) Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol 14: 450-464.
- Collin F, Cheignon C, Hureau C (2018) Oxidative stress as a biomarker for Alzheimer's disease. Biomark Med 12: 201-203.
- Donley GAR, Lonnroos E, Tuomainen TP, Kauhanen J (2018) Association of childhood stress with late-life dementia and Alzheimer's disease: the KIHD study. Eur J Public Health.
- Gerakis Y, Hetz C (2018) Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer's disease. FEBS J 285: 995-1011.
- Hoeijmakers L, Lesuis SL, Krugers H, Lucassen PJ, Korosi A (2018) A preclinical perspective on the enhanced vulnerability to Alzheimer's disease after early-life stress. Neurobiol Stress 8: 172-185.
- Justice NJ (2018) The relationship between stress and Alzheimer's disease. Neurobiol Stress 8: 127-133.
- Mecocci P, Boccardi V, Cecchetti R, Bastiani P, Scamosci M, et al. (2018) A long journey into aging, brain aging, and alzheimer's disease following the oxidative stress tracks. J Alzheimers Dis 62: 1319-1335.
- Mravec B, Horvathova L, Padova A (2018) Brain under stress and alzheimer's disease. Cell Mol Neurobiol 38: 73-84.
- Youssef P, Chami B, Lim J, Middleton T, Sutherland GT, et al. (2018) Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer's disease. Sci Rep 8: 11553.
- Yuede CM, Timson BF, Hettinger JC, Yuede KM, Edwards HM, et al. (2018) Interactions between stress and physical activity on Alzheimer's disease pathology. Neurobiol Stress 8: 158-171.
- Rijpma A, van der Graaf M, Meulenbroek O, Olde Rikkert MGM, Heerschap A (2018) Altered brain high-energy phosphate metabolism in mild Alzheimer's disease: A 3-dimensional (31)P MR spectroscopic imaging study. Neuroimage Clin 18: 254-261.
- Xie F, Peng F (2017) Radiopharmaceuticals for assessment of altered metabolism and biometal fluxes in brain aging and alzheimer's disease with positron emission tomography. J Alzheimers Dis 59: 527-536.
- Hartmann T, van Wijk N, Wurtman RJ, Olde Rikkert MG, Sijben JW, et al. A nutritional approach to ameliorate altered phospholipid metabolism in Alzheimer's disease. J Alzheimers Dis 41: 715-717.
- Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, et al. (2014) Altered arginine metabolism in Alzheimer's disease brains. Neurobiol Aging 35: 1992-2003.
- Whiley L, Sen A, Heaton J, Proitsi P, Garcia-Gomez D, et al. (2014) Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging 35: 271-278.
- Bustamante HA, Gonzalez AE, Cerda-Troncoso C, Shaughnessy R, Otth C, et al. Interplay between the autophagy-lysosomal pathway and the ubiquitin-proteasome system: a target for therapeutic development in alzheimer's disease. Front Cell Neurosci 12: 126.
- Colacurcio DJ, Pensalfini A, Jiang Y, Nixon RA (2018) Dysfunction of autophagy and endosomal-lysosomal pathways: Roles in pathogenesis of Down syndrome and Alzheimer's Disease. Free Radic Biol Med 114: 40-51.
- Congdon EE (2018) Sex differences in autophagy contribute to female vulnerability in alzheimer's disease. Front Neurosci 12: 372.
- Cordero JG, Garcia-Escudero R, Avila J, Gargini R, Garcia-Escudero V (2018) Benefit of oleuropein aglycone for alzheimer's disease by promoting autophagy. Oxid Med Cell Longev 2018: 5010741.
- Ntsapi C, Lumkwana D, Swart C, du Toit A, Loos B (2018) New insights into autophagy dysfunction related to amyloid beta toxicity and neuropathology in alzheimer's disease. Int Rev Cell Mol Biol 336: 321-361.
- Reddy PH, Yin X, Manczak M, Kumar S, Pradeepkiran JA, et al. (2018) Mutant APP and amyloid beta-induced defective autophagy, mitophagy, mitochondrial structural and functional changes and synaptic damage in hippocampal neurons from Alzheimer's disease. Hum Mol Genet 27: 2502-2516.
- Song GL, Chen C, Wu QY, Zhang ZH, Zheng R, et al. Selenium-enriched yeast inhibited beta-amyloid production and modulated autophagy in a triple transgenic mouse model of Alzheimer's disease. Metallomics.
- Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, et al. (2018) Autophagy and alzheimer's disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci 10: 04.
- Fakhoury M (2018) Microglia and astrocytes in alzheimer's disease: implications for therapy. Curr Neuropharmacol 16: 508-518.
- Criscuolo C, Fontebasso V, Middei S, Stazi M, Ammassari-Teule M, et al. Entorhinal cortex dysfunction can be rescued by inhibition of microglial rage in an alzheimer's disease mouse model. Sci Rep 7: 42370.
- Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci 28: 8354-8360.
- Grimm A, Friedland K, Eckert A (2016) Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer's disease. Biogerontology 17: 281-296.
- Birnbaum JH, Wanner D, Gietl AF, Saake A, Kundig TM, et al. (2018) Oxidative stress and altered mitochondrial protein expression in the absence of amyloid-beta and tau pathology in iPSC-derived neurons from sporadic Alzheimer's disease patients. Stem Cell Res 27: 121-130.
- Majd S, Power JHT (2018) Oxidative stress and decreased mitochondrial superoxide dismutase 2 and peroxiredoxins 1 and 4 based mechanism of concurrent activation of AMPK and mTOR in alzheimer's disease. Curr Alzheimer Res 15: 764-776.
- Manczak M, Kandimalla R, Yin X, Reddy PH (2018) Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum Mol Genet 27: 1332-1342.
- Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 323: 170-182.
- Assefa BT, Gebre AK, Altaye BM (2018) Reactive astrocytes as drug target in alzheimer's disease. Biomed Res Int 2018: 4160247.
- Chun H, Lee CJ (2018) Reactive astrocytes in Alzheimer's disease: A double-edged sword. Neurosci Res 126: 44-52.
- Cornejo F, Vruwink M, Metz C, Munoz P, Salgado N, et al. (2018) Scavenger Receptor-A deficiency impairs immune response of microglia and astrocytes potentiating Alzheimer's disease pathophysiology. Brain Behav Immun 69: 336-350.
- Gomez-Arboledas A, Davila JC, Sanchez-Mejias E, Navarro V, Nunez-Diaz C, et al. (2018) Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer's disease. Glia 66: 637-653.
- Liu CY, Yang Y, Ju WN, Wang X, Zhang HL (2018) Emerging roles of astrocytes in neuro-vascular unit and the tripartite synapse with emphasis on reactive gliosis in the context of alzheimer's disease. Front Cell Neurosci 12: 193.
- Ruffinatti F, Tapella L, Gregnanin I, Stevano A, Chiorino G, et al. (2018) Transcriptional remodeling in primary hippocampal astrocytes from an Alzheimer's disease mouse model. Curr Alzheimer Res.
- Zhuo JM, Pratico D (2010) Acceleration of brain amyloidosis in an Alzheimer's disease mouse model by a folate, vitamin B6 and B12-deficient diet. Exp Gerontol 45: 195-201.
- Martin ISM, Barato VP, Oliva SL, Rodriguez M, Yurrita LC, et al. (2018) Body composition, dietary, and gustatory function assessment in people with alzheimer's disease. Am J Alzheimers Dis Other Demen 2018: 1533317518782173.
- Hutton CP, Lemon JA, Sakic B, Rollo CD, Boreham DR, et al. (2018) Early intervention with a multi-ingredient dietary supplement improves mood and spatial memory in a triple transgenic mouse model of alzheimer's disease. J Alzheimers Dis 64: 835-857.
- Ruan Y, Tang J, Guo X, Li K, Li D (2018) Dietary fat intake and risk of alzheimer's disease and dementia: a meta-analysis of cohort studies. Curr Alzheimer Res 15: 869-876.
- Peters DG, Pollack AN, Cheng KC, Sun D, Saido T, et al. (2018) Dietary lipophilic iron alters amyloidogenesis and microglial morphology in Alzheimer's disease knock-in APP mice. Metallomics 10: 426-443.
- Hsu HW, Bondy SC, Kitazawa M (2018) Environmental and dietary exposure to copper and its cellular mechanisms linking to alzheimer's disease. Toxicol Sci 163: 338-345.
- Nichols CD (2006) Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther 112: 677-700.
- Pandey UB, Nichols CD (2011) Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev 63: 411-436.
- Matsagas K, Rizza C, Goertzel B, Benford G, Villeponteau B (2018) Multipath natural product supplement suppresses dementia symptoms in Amyloid-beta and tau transgenic Drosophila. J Biol Med Res 2.
- Aldarmaa J, Liu Z, Long J, Mo X, Ma J, et al. (2010) Anti-convulsant effect and mechanism of Astragalus mongholicus extract in vitro and in vivo: protection against oxidative damage and mitochondrial dysfunction. Neurochemical Res 35: 33-41.
- Auyeung KK, Mok NL, Wong CM, Cho CH, Ko JK (2010) Astragalus saponins modulate mTOR and ERK signaling to promote apoptosis through the extrinsic pathway in HT-29 colon cancer cells. Int J Mol Med 26: 341-349.
- Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW, et al. (2012) Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int J Mol Sci 13: 1747-1761.
- Liu J, Zhang JF, Lu JZ, Zhang DL, Li K, et al. (2013) Astragalus polysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. Acta Pharmacologica Sinica 34: 137-145.
- Liu Y, Liu F, Yang Y, Li D, Lv J, et al. (2014) Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. International journal of biological macromolecules. 68: 209-214.
- Zhang L, Yang Y, Wang Y, Gao X (2011) Astragalus membranaceus extract promotes neovascularisation by VEGF pathway in rat model of ischemic injury. Die Pharmazie 66: 144-150.
- Zou F, Mao XQ, Wang N, Liu J, Ou-Yang JP (2009) Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta pharmacologica Sinica 30: 1607-1615.
- Durairajan SS, Liu LF, Lu JH, Chen LL, Yuan Q, et al. (2012) Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiology of aging 33: 2903-2919.
- Habtemariam S (2011) The therapeutic potential of Berberis darwinii stem-bark: quantification of berberine and in vitro evidence for Alzheimer's disease therapy. Natural product comm 6: 1089-1090.
- Ji HF, Shen L (2011) Berberine: a potential multipotent natural product to combat Alzheimer's disease. Molecules 16: 6732-6740.
- Ji HF, Shen L (2012) Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer's disease. Scientific World J 2012: 823201.
- Panahi N, Mahmoudian M, Mortazavi P, Hashjin GS (2013) Effects of berberine on beta-secretase activity in a rabbit model of Alzheimer's disease. Arch Med Sci 9: 146-150.
- Bonesi M, Loizzo MR, Conforti F, Passalacqua NG, Saab A, et al. (2013) Berberis aetnensis and B. libanotica: a comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer's disease and antioxidant activity. J Pharm Pharmacol 65: 1726-1735.
- Chang J, Rimando A, Pallas M, Camins A, Porquet D, et al. (2012) Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer's disease. Neurobiol Aging 33: 2062-2071.
- Joseph JA, Fisher DR, Cheng V, Rimando AM, Shukitt-Hale B (2008) Cellular and behavioral effects of stilbene resveratrol analogues: implications for reducing the deleterious effects of aging. J Agr Food Chem 56: 10544-10551.
- Robb EL, Stuart JA (2014) The stilbenes resveratrol, pterostilbene and piceid affect growth and stress resistance in mammalian cells via a mechanism requiring estrogen receptor beta and the induction of Mn-superoxide dismutase. Phytochemistry 98: 164-173.
- Sato D, Shimizu N, Shimizu Y, Akagi M, Eshita Y, et al. (2014) Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Bioscience, biotechnology, and biochemistry 78: 1123-1128.
- Lin VC, Tsai YC, Lin JN, Fan LL, Pan MH, et al. (2012) Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J Agri Food Chem 60: 6399-6407.
- Cho HS, Kim S, Lee SY, Park JA, Kim SJ, et al. (2008) Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology 29: 656-662.
- Di X, Yan J, Zhao Y, Zhang J, Shi Z, et al. (2010) L-theanine protects the APP (Swedish mutation) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience 168: 778-786.
- Egashira N, Hayakawa K, Osajima M, Mishima K, Iwasaki K, et al. (2007) Involvement of GABA(A) receptors in the neuroprotective effect of theanine on focal cerebral ischemia in mice. J Pharmacol Sci 105: 211-214.
- Siamwala JH, Dias PM, Majumder S, Joshi MK, Sinkar VP, et al. (2013) L-theanine promotes nitric oxide production in endothelial cells through eNOS phosphorylation. J Nutr Biochem 24: 595-605.
- Takeda A, Sakamoto K, Tamano H, Fukura K, Inui N, et al. (2011) Facilitated neurogenesis in the developing hippocampus after intake of theanine, an amino acid in tea leaves, and object recognition memory. Cell Mol Neurobiol 31: 1079-1088.
- Unno K, Fujitani K, Takamori N, Takabayashi F, Maeda K, et al. (2011) Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Rad Res 45: 966-974.
- Wu Z, Zhu Y, Cao X, Sun S, Zhao B (2014) Mitochondrial toxic effects of Abeta through mitofusins in the early pathogenesis of Alzheimer's disease. Mol Neurobiol 50: 986-996.
- Kaminska B, Ciereszko R, Kiezun M, Dusza L (2013) In vitro effects of genistein and daidzein on the activity of adrenocortical steroidogenic enzymes in mature female pigs. J Physiol Pharmacol 64: 103-108.
- Li Y, Chen H, Hardy TM, Tollefsbol TO (2013) Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PloS one 8: e54369.
- Palacios-Gonzalez B, Zarain-Herzberg A, Flores-Galicia I, Noriega LG, Aleman-Escondrillas G, et al. (2014) Genistein stimulates fatty acid oxidation in a leptin receptor-independent manner through the JAK2-mediated phosphorylation and activation of AMPK in skeletal muscle. Biochimica Biophysica Acta 1841: 132-140.
- Forlenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF (2012) Does lithium prevent Alzheimer's disease? Drugs & aging 29: 335-342.
- Sudduth TL, Wilson JG, Everhart A, Colton CA, Wilcock DM (2012) Lithium treatment of APPSwDI/NOS2-/- mice leads to reduced hyperphosphorylated tau, increased amyloid deposition and altered inflammatory phenotype. PloS one 7: e31993.
- Nunes MA, Viel TA, Buck HS (2013) Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease. Curr Alzheimer Res 10: 104-107.
- Trujillo-Estrada L, Jimenez S, De Castro V, Torres M, Baglietto-Vargas D, et al. (2013) In vivo modification of Abeta plaque toxicity as a novel neuroprotective lithium-mediated therapy for Alzheimer's disease pathology. Acta Neuropathologica Comm 1: 73.
- Forlenza OV, De-Paula VJ, Diniz BS (2014) Neuroprotective effects of lithium: implications for the treatment of Alzheimer's disease and related neurodegenerative disorders. ACS Chem Neurosci 5: 443-450.
- Nery LR, Eltz NS, Hackman C, Fonseca R, Altenhofen S, et al. (2014) Brain intraventricular injection of amyloid-beta in zebrafish embryo impairs cognition and increases tau phosphorylation, effects reversed by lithium. PloS one 9: e105862.
- Sofola-Adesakin O, Castillo-Quan JI, Rallis C, Tain LS, Bjedov I, et al. (2014) Lithium suppresses Abeta pathology by inhibiting translation in an adult Drosophila model of Alzheimer's disease. Fron Aging Neurosci 6: 190.
- Wallace J (2014) Calcium dysregulation, and lithium treatment to forestall Alzheimer's disease - a merging of hypotheses. Cell Calcium 55: 175-181.
- Zhao L, Gong N, Liu M, Pan X, Sang S, et al. (2014) Beneficial synergistic effects of microdose lithium with pyrroloquinoline quinone in an Alzheimer's disease mouse model. Neurobiol Aging 35: 2736-2745.
- Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, et al. (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Res 1281: 117-127.
- Cardoso BR, Ong TP, Jacob-Filho W, Jaluul O, Freitas MI, et al. (2010) Nutritional status of selenium in Alzheimer's disease patients. British J Nutr 103: 803-806.
- Lakshmi BV, Sudhakar M, Prakash KS (2015) Protective effect of selenium against aluminum chloride-induced Alzheimer's disease: behavioral and biochemical alterations in rats. Biological Trace Element Res 165: 67-74.
- Bernick C, Cummings J, Raman R, Sun X, Aisen P (2012) Age and rate of cognitive decline in Alzheimer disease: implications for clinical trials. Arch Neurol 69: 901-905.
- Kim J, Basak JM, Holtzman DM (2009) The role of apolipoprotein E in Alzheimer's disease. Neuron 63: 287-303.
- Alvarez XA, Alvarez I, Aleixandre M, Linares C, Muresanu D, et al. (2018) Severity-related increase and cognitive correlates of serum VEGF Levels in Alzheimer's Disease ApoE4 Carriers. J Alzheimers Dis 63: 1003-1013.
- Lin YT, Seo J, Gao F, Feldman HM, Wen HL, et al. (2018) APOE4 causes widespread molecular and cellular alterations associated with alzheimer's disease phenotypes in human iPSC-derived brain cell types. Neuron 98: 1294.
- Theendakara V, Peters-Libeu CA, Bredesen DE, Rao RV (2018) Transcriptional effects of ApoE4: relevance to Alzheimer's disease. Mol Neurobiol 55: 5243-5254.
- Uddin MS, Kabir MT, Al Mamun A, Abdel-Daim MM, Barreto GE, et al. (2018) APOE and Alzheimer's disease: evidence mounts that targeting APOE4 may combat alzheimer's pathogenesis. Mol Neurobiol.
- Abdel Razek A, Mazroa J, Baz H (2014) Assessment of white matter integrity of autistic preschool children with diffusion weighted MR imaging. Brain Dev 36: 28-34.
- Canu E, Sarasso E, Filippi M, Agosta F (2018) Effects of pharmacological and nonpharmacological treatments on brain functional magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment: a critical review. Alzheimers Res Ther 10: 21.
- Fantoni ER, Chalkidou A, JT OB, Farrar G, Hammers A (2018) A systematic review and aggregated analysis on the impact of amyloid pet brain imaging on the diagnosis, diagnostic confidence, and management of patients being evaluated for alzheimer's disease. J Alzheimers Dis 63: 783-796.
- Giulietti G, Torso M, Serra L, Spano B, Marra C, et al. Whole brain white matter histogram analysis of diffusion tensor imaging data detects microstructural damage in mild cognitive impairment and alzheimer's disease patients. J Magn Reson Imaging.
- Razek AA, Abdalla A, Ezzat A, Megahed A, Barakat T (2014) Minimal hepatic encephalopathy in children with liver cirrhosis: diffusion-weighted MR imaging and proton MR spectroscopy of the brain. Neuroradiol 56: 885-891.
- Shigemoto Y, Sone D, Imabayashi E, Maikusa N, Okamura N, et al. (2018) Dissociation of tau deposits and brain atrophy in early alzheimer's disease: a combined positron emission tomography/magnetic resonance imaging study. Front Aging Neurosci 10: 223.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences