ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Evaluation of Antimicrobial Activity in C.Tinctoriusl Safflower Florets (C.Tinctoriusl)
Ayesha Sultana1*, Paul Marx and SY Anwar
Department of Genetics and Biotechnology, Osmania University, Hyderabad, Telangana, India
- *Corresponding Author:
- Ayesha Sultana
Department of Genetics and Biotechnology,
Osmania University, Hyderabad, Telangana,
India,
Tel: 09642986263;
E-mail: ayeshaasra68@yahoo.com
Received date: April 30, 2022, Manuscript No. AJPSKY-22-13380; Editor assigned date: May 03, 2022, PreQC No. AJPSKY-22-13380 (PQ); Reviewed date: May 18, 2022, QC No. AJPSKY-22-13380; Revised date: July 01, 2022, Manuscript No. AJPSKY-22-13380 (R); Published date: July 11, 2022, DOI: 10.36648/2249-7412/22.12.6.225
Citation: Sultana A, Marx P, Anwar SY (2022) Evaluation of Antimicrobial Activity in C.Tinctoriusl Safflower Florets (C.Tinctoriusl). Asian J Plant Sci Res Vol:12 No:8.
Abstract
Antibacterial activity of various extracts was studied against gram positive bacteria like, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and gram negative bacteria like E.coli and Klebsiella pneumonia. These studies revealed that the methanolic extract of safflower florets showed varying degree of antibacterial activities ex-cept aqueous extract may be due to the presence of secondary metabolites, the highest zone of inhibition with 21 mm diameter in bacillus whereas lowest 13 mm diameter in Pseudomonas.
Keywords
Staphylococcus aureus; Escherichia coli; Chloramphenicol
Introduction
During the last 40 years, at least a dozen potent drugs have been derived from flowering plants including Dioscorea species derived diosgenin from which all an ovulatory contraceptive agents have been derived; reserpine and other anti-hypertensive and tranquilizing alkaloids from Rauwolfia species; pilocarpine to treat glaucoma and dry mouth, derived from a group of South American trees (Pilocarpus species) in the citrus family; two powerful anti-cancer agents from the Rosy Periwinkle (Catharanthus roses); laxative agents from Cassia species and as a cardio tonic agent to treat heart failure from Digitalis species [1]. In addition to the regular metabolites that are present in each of the medicinal plants, however in addition to the natural metabolites C. tincturesis additionally blessed with two important pigments that are present in the florets of safflower and these two pigments have an immense medicinal properties. Safflower, Carthamus tinctorius L. is a thistle herb belonging to the family Asteraceae. Safflower plants are 30-150 cm tall with globular flower heads (Capitula) and commonly, brilliant yellow, orange or red flowers. It is one of humanity’s oldest crops cultivated in India mainly for oil from the seeds and adyes from the flowers. Though, safflower flowers have been used in preparations of ayurvedic medicines in India and also merit mention in European and Japanese pharmacopoeia’s, the interest in this crop has been rekindled in the last few years as the medicinal use of these flowers in china, has become more widely known. China has a significant area under safflower plantation, but is grown almost exclusively for its flowers, which are harvested for use in traditional medicines. Safflower flowers are used in china for the treatment of many illnesses as well as in the preparation of “tonic tea” [2].
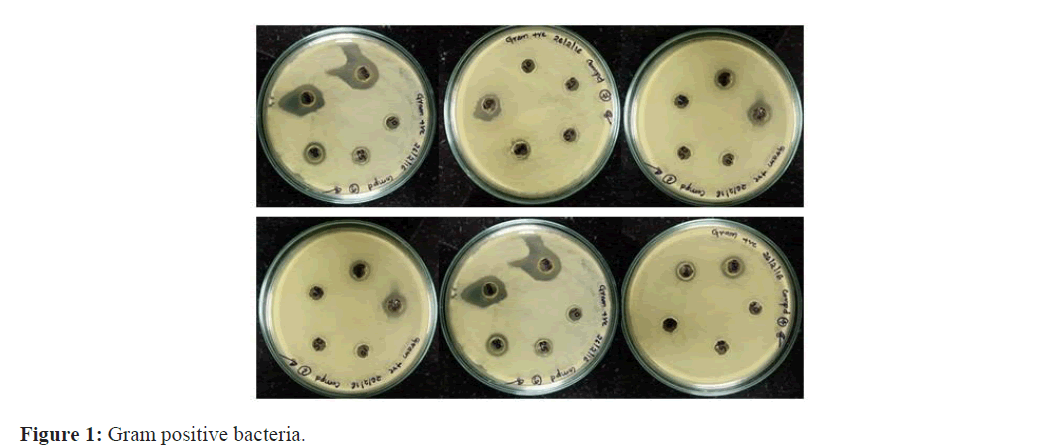
Many investigations have found that safflower pigments had a lot of pharmacological effects. It could inhibit the conglomeration of hematoblast efficaciously and exhibit anti-inflammatory, anti-allergic and antimicrobial, anti-cancer activities. Safflower was successfully used as sole food for late-pregnant dairy cows (Figure 1).
Materials and Methods
Escherichia coli, Streptococcus and Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, were obtained from the department of microbiology, Osmania University, Hyderabad. Chloramphenicol 10 ug/ml used as a standard.
Culture and maintenance of test microorganisms for antimicrobial studies:
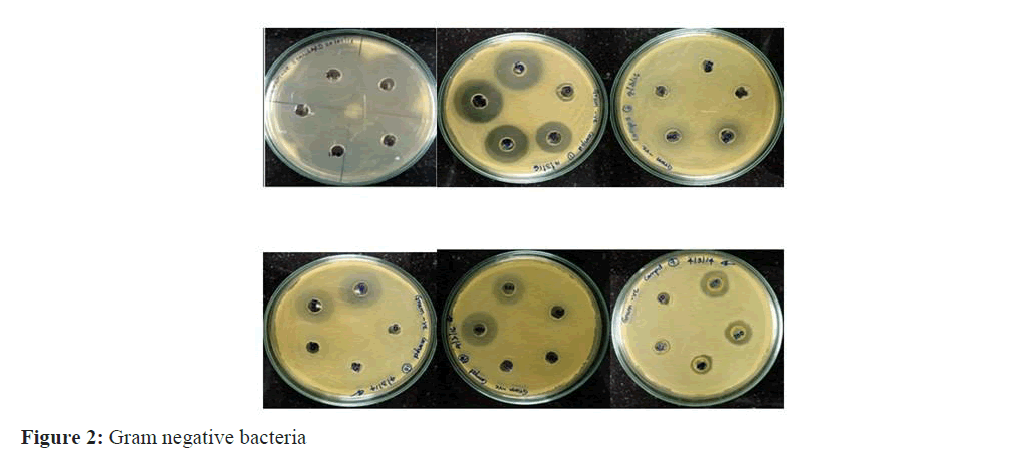
Bacterial cultures of Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumonia and Pseudomo- nas aeruginosa were obtained from the microbiology laboratory. All the bacterial strains were maintained on nutrient agar (NA, Hi-Media) at 37°C at room temperature [3]. Bacteria were inoculated in nutrient broth and incubated at 37°C for 24 hours for doing the test. Mueller-Hinton Agar (MHA, HI Media) for testing the antibacterial activity respectively (Figure 2).
Composition of nutrient agar media
1. Peptone-5 gr
2. Beef extract/yeast extract-3 gr
3. Agar-15 gr
4. Nacl-5 gr
5. Distilled water-1 liter
6. PH adjusted to neutral i.e. 7.2-7.4
7. Preparation of the Media
8. Dissolved 28 gr of the powder in 1liter of distilled water.
9. Heat this mixture while stirring to fully dissolve all components
10. Autoclave the dissolved mixture at 121 degrees Celsius for 15 minutes.
11. Once the nutrient agar has been autoclaved, allow it to cool but not solidify.
12. Pour nutrient agar into each plate and leave plates on the sterile surface until the agar has solidified.
13. Replace the lid of each Petri dish and store the plates in a refrigerator.
Muller hinton agar media compositionComposition of MHA
Ingredients in gram/litre
Beef extract 2.00 gm
Acid hydrolysate of casein 17.50 gm
Starch 1.50 gm
Agar 17.00 gm
pH 7.3
Preparation of MHA•Suspend 38 gm of the medium in one liter of distilled water.
• Heat with frequent agitation and boil for one minute to completely dissolve the medium.
• Autoclave at 121°C for 15 minutes. Cool to room temperature.
•Pour cooled Mueller Hinton agar into sterile petri dishes on a level, horizontal
• Surface to give uniform depth.
• Allow to cool to room temperature.
• Check for the final pH 7.3 ± 0.1 at 25°C.
• Store the plates at 2-8°C.
Antimicrobial activity by agar well diffusion method
Approximately 20 ml of sterile MHA was poured into sterile petri plates and allowed to set. Plates were then seeded with 0.5 ml of a 24 h old bacterial culture and using a sterile glass (L) rod made a lawn culture. The plates were al- lowed to dry. For doing agar well diffusion method, wells are made on the plate with the aid of a sterile hole puncture (8.0 mm diameter). Place 10 μl, 25 μl, 50 μl, 100 μl and 150 μl of the methanolic and aqueous extracts were poured into respective wells. The plates thus prepared were left at room temperature for ten minutes, allowing the diffusion of the extracts into the agar. Then the plates with bacterial culture along with safflower floral extracts were placed in the incubator at 37°C for 24-48 h. After incubation the plates were observed for the antimicrobial activity of the safflower extract and observed for inhibitory zone surrounding the well [4]. The zone of inhibition was measured and expressed in millimeters in diameter (Figure 3).
Results
Evaluation of antimicrobial activity of Carthamin
The methanol (Carthamin) and aqueous (Carthamidin) extracts of six genotypes belonging to same families were evaluated for antimicrobial activity against three Gram-positive bacteria: Staphylococcus aureus, Pseudomonas aeruginosa; Bacillus. Subtilis two gram-negative bacteria: Escherichia coli K. pneumonia. The in vitro antimicrobial activity was performed by agar plate hole diffusion method. The extractive yield was more in methanol than aqueous extracts. The most susceptible Bacterium was Bacillus subtilin and the most resistant was Pneumoniae. Finally the result revealed that the aqueous extract (Carthamidin) does not processes any antimicrobial activity where methanolic extract (Carthamin) showing maximum antimicrobial activity against gram negative bacteria compared to gram posi-tive. The highest zone of inhibition with 21 mm diameter in Bacillus whereas lowest 13 mm diameter in Pseudomonas. Shown in Table: 1-3
| Conct(ug) | Standard (chloromphecal) | E.coli | Kleb | Pseudo | Staph | Bacillus |
|---|---|---|---|---|---|---|
| 10 | 5 mm | - | - | - | - | - |
| 25 | 9 | 8 mm | 5 mm | 6 mm | ||
| 50 | 11 | 11 mm | 11 mm | 9 mm | 10 | 6 |
| 100 | 18 | 14 mm | 15 | 13 mm | 14 | 14 |
| 150 | 23 | 18 mm | 24 | 15 mm | 18 | 14 |
Table 1: Nari-6.
| Conct(ug) | Standard (chloromphecal) | E. coli | Kleb | Pseudo | Staph | Bacillus |
|---|---|---|---|---|---|---|
| 10 | 5 | - | - | - | - | - |
| 25 | 9 | - | - | 6 mm | - | - |
| 50 | 11 | 7 mm | 8 mm | 10 mm | 10 mm | 6 mm |
| 100 | 18 | 13 mm | 15 mm | 13 mm | 17 mm | 14 |
| 150 | 23 | 20 mm | 24 mm | 17 mm | 18 mm | 16 mm |
Table 2: Pbns-12.
| Conct(ug) | Standard (chloromphecal) | E. coli | Kleb | Pseudo | Staph | Bacillus |
|---|---|---|---|---|---|---|
| 10 | 5 | - | 8 mm | - | - | - |
| 25 | 9 | - | 11 | - | - | - |
| 50 | 11 | 4 mm | 13 | 10 mm | 9 mm | 8 mm |
| 100 | 18 | 9 mm | 16 | 13 mm | 16 mm | 15 mm |
| 150 | 23 | 19 mm | 19 | 20 mm | 17 mm | 19 mm |
Table 3: SSF-658.
Discussion
Plants have an almost limitless ability to synthesize aromatic substances, most of the phytochemical are secondary metabolites, of which at least 12,000 have been Isolated, which was estimated to be less than 10% of the total. In many cases, these substances serve as plant defense mechanisms against predation by microorganisms, insects, and herbivores. Some of the phytochemicals such as terpenoids give plants their odors; others (quinines and tannins) are responsible for plant pigment [5]. Many compounds are responsible for plant flavor like the terpenoid capsaicin from chili peppers. Useful antimicrobial phytochemicals can be divided into several categories-phenolic and polyphenols, terpenoids and essential oils, alkaloids, lectins and polypeptides. The antimicrobial activities of the methanolic extracts of petals of safflower florets were tested against the pathogens by plate hole diffusion method and the antibacterial activities of the methanol and aqueous extracts were compared with the standards. The zone of inhibition of the antimicrobial activity for all the extracts is shown in the plates and also given in Table: 4-6.
| Conct(ug) | Standard (chloromphecal) | E.coli | Kleb | Pseudo | Staph | Bacillus |
|---|---|---|---|---|---|---|
| 10 | 5 | - | - | - | - | - |
| 25 | 9 | - | 4 | 3 | - | - |
| 50 | 11 | 5 mm | 11 mm | 7 mm | 6 mm | 9 mm |
| 100 | 18 | 9 mm | 17 mm | 8 mm | 13 mm | 12 |
| 150 | 23 | 15 mm | 23 mm | 13 mm | 19 mm | 13 mm |
Table 4: A-1
| Conct(ug) | Standard (chloromphecal) | E.coli | Kleb | Pseudo | Staph | Bacillus |
|---|---|---|---|---|---|---|
| 10 | 5 | - | - | - | - | - |
| 25 | 9 | - | 6 | - | - | - |
| 50 | 11 | 7 mm | 10 mm | 4 mm | 8 mm | -mm |
| 100 | 18 | 9 mm | 15 mm | 8 mm | 15 mm | 15 |
| 150 | 23 | 16 mm | 22 mm | 13 mm | 19 mm | 17 mm |
Table 5: CO-1 CO-1
| Conct(ug) | Standard (chloromphecal) | E.coli | Kleb | Pseudo | Staph | Bacillus |
|---|---|---|---|---|---|---|
| 10 | 5mm | - | - | - | - | - |
| 25 | 9 | 8 mm | 5 mm | 6 mm | - | - |
| 50 | 11 | 11 mm | 11 mm | 9 mm | 10 mm | 6 mm |
| 100 | 18 | 14 mm | 15 mm | 16 mm | 14 mm | 14 |
| 150 | 23 | 19 mm | 21 mm | 17 mm | 18 mm | 16 mm |
Table 6: Manjira
Acknowledgment
The authors are grateful to Professor SY Anwar for botanic identification, professor Rawoof and professor Roja rani, DOR (Director of Oilseed Research) Hyderabad for providing safflower varieties and all genetics and microbiology department Hyderabad. For providing the micro-organisms and testing antimicrobial activity.
References
- Mathekaga ADM, Meyer JJM (1998) Antibacterial activity of South African helichrysum species. South Afr J Bot 64: 293-295.
[Crossref], [Google Scholar]
- Kaneria M, Baravalia Y, Vaghasiya Y, Chanda (2009) Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India. Indian J Pharm Sci 71: 406-412.
[Crossref], [Google Scholar]
- Nidhal S, Kamel M, Salem E, Giuseppe M, Sana A, et al. (2014) Evaluation of antibacterial, antifungal, and antioxidant activities of safflower natural dyes during flowering. Biomed Res Int 5: 25-35.
[Crossref], [Google Scholar]
- Nagesh KS, Shanthamma C (2009) Antibacterial activity of curculigo orchioidesrhizome extract on pathogenic bacteria. African Journal of Microbiology Research 3: 005-009.
- Kalimuthu K, Vijayakumar S, Senthilkumar RR, Suresh Kumar M (2010) Antimicrobial activity of the biodiesel plant, Jatropha Curcas L. InternationalJournal of Pharma and Bio Sciences 1: 1-57.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences