Clostridium perfringens Induced Autism Disorders Counter Act by Using Natural Bee Pollen in vitro
Hanan M. Al-Yousef1*, Manal M. Alkhulaifi2, Huda S. Al-Salem3 and Syed Rizwan Ahamad4
1 Department of Pharmacognosy, College of Pharmacy, King Saud University, Saudi Arabia
2 Department of Botany and Microbiology, College of Science, King Saud University, Saudi Arabia
3 Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Saudi Arabia
4 Central Laboratory, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Saudi Arabia
- *Corresponding Author:
- Hanan M. Al-Yousef
Department of Pharmacognosy
College of Pharmacy
King Saud University, Saudi Arabia.
Tel: 96611-8056803
E-mail: halyousef@ksu.edu.sa
Received Date: March 24, 2018; Accepted Date: April 27, 2018; Published Date: May 05, 2018
Citation: Al-Yousef HM, Alkhulaifi MM, Al-Salem HS, Ahamad SR (2018) Clostridium perfringens Induced Autism Disorders Counter Act by Using Natural Bee Pollen in vitro. J Biol Med Res. Vol.2 No.1:8
Abstract
Background: Autism is usually accompanied by many symptoms such as abdominal pain, constipation/diarrhea, due to deregulation of physiological microflora. Recently, regression of the autistic symptoms after oral administration of antibiotic such as vancomycin was detected, that leads to the disappearance of the symptoms and eliminate toxins produced by bacteria such as Clostridium spp.
Methods: In the present study we are identified the chemical compositions of non-polar fractions (PGp and PGc) from natural bee pollen by using GC/MS against AD induced by Clostridium perfringens in vitro.
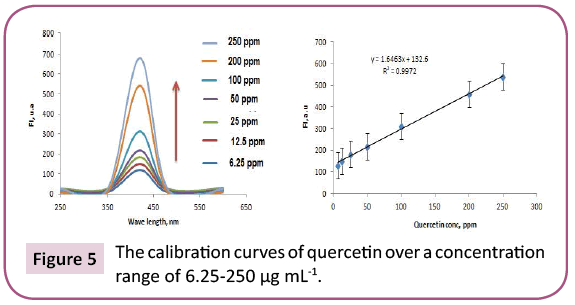
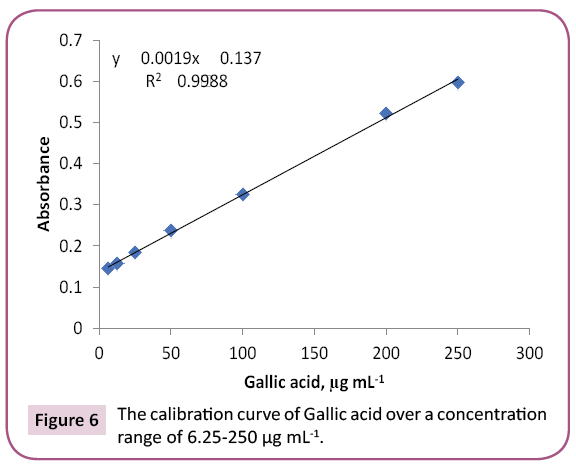
Results: 23 and 14 chemical components representing 84.07% and 92.92% for PGp and PGc respectively by GC/MS. Total phenolic and proanthocyanidine contents were determined in both PGc and PGe fractions by Folin -Ciocalteau and vanillin-sulfuric acid assays respectively and expressed in term of quercetin equivalent (y=1.6463x + 132.6, R2=0.9972) as mg QUE/g fraction or gallic acid equivalent (y=0.0019x + 0.137, R2=0.9988) as mg GAE/g fraction and as (+) - catechin equivalent (% CT w/w) in bee pollen (y=0.0013x + 0.3998, R2=0.9997).
Conclusion: The results of the present study revealed the capacity of natural bee pollen to enhance the recovery from AD induced by C. perfringens in vitro. This is due to the diversity of different chain of fatty acids as well as phenolic/ proanthocyanidin compounds that found in bee pollen fractions.
Keywords
Bee pollen; Autism disorders; Clostridium perfringens; GC\MS
Introduction
Autism disorders (AD) are developmental disorders which characterized by social defects and communication impairments with a dissemination of 1 per 50 children in the US. The incidence of AD in 2010 was 1.47% in the US (DDMNS, 2014) which was apparently increasing with time, evoking global concern about the causes of autism [1-3]. The studies have a lot of hypotheses concerning various environmental factors as well as genes involving for its higher prevalence and concerned neuropathology, respectively [4-6]. Although there has been promotion in determining a genetic cause in AD, recent studies of twins suggest there is a stronger environmental component than previously believed [7,8].
The corner stone in autism is based on the increasing incidences of AD and on the gastrointestinal disorders (GID) in children. 90% of children with AD complained from GID such as gastroesophageal reflux, constipation, diarrhea, vomiting and nutrition issues [9- 11]. A direct relation between the severity of autism and GID has been identified [12,13]. Knowing of the GI pathophysiology in AD perhaps helps for the early determination of AD-related GI pathology also for guiding the treatment of GI symptoms and might be AD. There is strong considerable evidence that GID are linked to intestinal dysbiosis (a state of imbalance in the gut microbial). Gut microbes play a significant role in modulating metabolism and development of the immune system in human. The biochemical pathways of gut-brain intervention give a basis for the effect of normal microbes in gut on development, neurochemistry and brain function [14-18]. Changes in the gut microbe composition are associated with variations in nervous system and behavior normal functions [19,20]. For the last several years an escalating number of studies show the changes of gut microbes in children with AD.
Involvement of the microbial in the pathogenesis of autism was obvious in 1998 that Clostridium tetani neurotoxin ascends along the vagus nerve from the GI tract to the CNS trigger autism symptoms [21]. Afterward, the potential effect of vancomycin on the GI symptoms in autistic children convinced the involvement of gut bacteria [22]. Approving of dysbiosis in autistic children and health improvement after probiotics use only support the evidence that the composition of gut microbes is strongly associated with changes that happened in normal functioning of the nervous system and behavioral in AD [23,24]. Numerous bacterial species have been established to be involved in children with autism specially if they had higher population of Desulfovibrio spp. than the control children [21,23,25]. The incidence of bacteria was also higher in autistic children compared to neurotypical children. e.g., they had more Clostridia species [26-28].
One of the most important theories of AD contains the etiopathogenetic role of certain bacteria such as Clostridia , Desulfovibrio and Bifidobacterium [29]. Special concerns were paid to the bacteria manifested as being involved in descript of AD (Lactobacillus, Bifidobacterium, Clostridia , and Desulfovibrio). Many anaerobic bacteria are pathogenic to humans and their virulence (secreted toxins) is mainly produced by different Clostridium species [30]. These are not invasive bacteria, but their toxins may exert harmful effects at a distance from microbes. Bolte reported that a subgroup of children diagnosed with AD might be suffering from Clostridium tetani colonization of the GIT and that the neurological symptoms were manifested as result of in vivo induction of tetanus neurotoxin. Parracho et al. showed that the fecal flora of AD patients was enriched with Clostridium histolyticum (Clostridium clusters I and II) than that of healthy children; these groups are considered as toxin-producers. Furthermore, higher concentrations of 3-(3-hydroxyphenyl)- 3hydroxypropionic acid (a compound produced by Clostridium sp.) have been noticed in the urine of autistic children. The authors have suggested that this might be a probable metabolite of a tyrosine analog which able to deplete brain catecholamines and causing typical autism related disorders [31].
Furthermore, acute and sub-acute orally administered propionic acid (PA) showed manipulates in biochemical parameters. The change in neurotransmitters, cytokines and pro-apoptotic markers noticed might be due to oxidative stress caused by PA. Bee pollen, due to the biological properties of its constituents, has been identified to exhibit potential free radical scavenging and antioxidant activities [32]. Now-a-days, numerous researchers are giving attention to gut dysbiosis that includes excessive proliferation of specific microbes and lack of others, as a potential cause for several disorders such as autism. Interestingly, it has been reported that bee pollen can be used safely to ameliorate neuroinflammation, oxidative stress, poor detoxification, and abnormal gut microbes as mechanisms involved in the etiology of AD. By these examples, our goal is to study one of the pathological events as well as etiological causes participated in AD. We aimed to elucidate the chemical constituents of non-polar fractions (pet. ether and dichloromethane) of bee pollen by using GC/MS and the importance of these fractions on the inhibition of gut Clostridium perfringens in vitro which possibly in combination with other factors leading to developing the disease.
Material and Methods
Bee pollen was purchased from local market Wadi Al-Nahil in Riyadh, Saudi Arabia in June 2017 under trade name of bee pollen, 100% natural bee pollen first elite. It is imported for Wadi Al-Nahil one of the largest marketing companies in Saudi Arabia (www.wadialnahil.net).
Solvents
All solvents used were ethanol 95%, petroleum ether 40–60°C, and dichloromethane, methanol which were distilled prior to use. Analytical grade solvents were used for extraction processes.
Chemicals
All chemicals were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Precoated Si gel plates (Merck, Kieselgel 60 F254, 0.25 mm) were used (TLC). Vanillin-sulfuric acid, and Folin-Ciocalteu assays; on a Reflotron® Plus Analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Shinoda’s test, purple coloration revealed on the presence of flavonoids. To 10% methanolic solution of the fractions, a few drops of 10% FeCl3 solution were added. A dark-green coloration indicated the presence of PAC [33]. The PAC content was also detected by the red color developed after spray with vanillin-HCl reagent [34]. Si gel TLC plates were developed with EtOAc-MeOH-H2O (30:5:4). Spraying a TLC plate with 1% AlCl3 in methanol yellow spots were observed and indicated the presence of many flavonoids [35]. The brownish red color observed on the TLC plate, after spraying with 5% vanillin-HCl, disclosed the presence of PAC.
Extraction procedure
Bee pollen grain (PG) (1250 g) extracted with 95% alcohol at room temperature for 48h, till exhaustion. The extracts were combined and filtered. The filtrate was evaporated to dryness under reduced pressure, using rotary vacuum at 45°C, to yield a dark yellow gummy extract (383.0 g, yield 30. 64% w/w). From this total extract was dissolved only 80.0 g in 30% methanol in distilled water (1.0 L). Hydro-methanolic solution was successively fractionated with petroleum ether (3 ×0.5 L), CH2Cl2 (3 × 0.5 L), EtOAc (4 × 0.5 L) and n-butanol saturated with water (4 × 0.5 L). Each fraction was concentrated under reduced pressure to give solvent-free residues : pollen grain petroleum ether, (PGp 25 g); dichloromethane, (PGc 15.0 g); ethyl acetate, (PGe 10.0 g); and butanol, (PGb 10.0 g), respectively. Remaining aqueous layer was concentrated to yield aqueous, (PGa 19 g) for further experiments.
Experimental parameters
GC/MS: The GC-MS analysis was performed in a Perkin Elmer Clarus 600 gas chromatograph inked to a mass spectrometer (Turbomass) available at Central Laboratory, College of Pharmacy, King Saud University, Riyadh. An aliquot of 1 μL of PGp and PGc fractions were injected into the GC column Elite - 5MS of 30 m long, 0.25 μm film thickness, 0.25 mm internal diameters
Capillary column using the following temperature program
Experimental procedure: 1 μL of PGp and PGc fractions were injected into the system with the split mode (split ratio 1:20). To perform the analysis Perkin Elmer GC-MS coupled with Clarus 600 T mass spectrometer (USA) was used. The system was composed of an auto-sampler unit, auto injector unit and a gas chromatograph Clarus 600 coupled with a single quadrupole mass spectrometer. For the analysis of the obtained data, TurboMass Solution Software version was used in GC/MS Analysis The samples were separated on Elite 5 MS (30 m × 0.25 mm i.d., 0.25 μm film thickness) capillary CG column (Perkin Elmer, USA). Analyses were performed with the helium as a carrier gas at a constant pressure mode (65.2 kPa). The separation was carried out in a gradient temperature program. The oven temperature was maintained at 40°C for 2 min, ramped to 150°C at 5°C/ min for 2 min and again increased at a rate of 5°C/min to 280°C, ramped with the grade 5°C /min and held for 2 min. The total run time was 54 min. The injector, ion source and interface temperature were set at 280°C, 220°C and 200°C, respectively. The mass spectra of components were in range of 40-600 m/z range were recorded by the positive electron impact ionization mass spectrometer, with ionization voltage of 70 eV.
Identification of components by GC-MS
The components were identified based on GC retention time and by matching with Wiley 2006 library as well as by comparing the fragmentation patterns of their mass spectra with those reported in the literatures by Adams, Mclafferty and Staffer. The identified components were found to be sterols, FAs, alkanes and alcohols compounds. A total of 23 and 14 detectable peaks were selected from PGp and PGc fractions respectively.
Detection of total phenolic contents (TPC)
Instrumentation: The developed spectrofluorimetric approach was performed using a Spectro fluorophotometer model RF- 5301pc (Shimadzu, Japan) with matched 1 cm quartz cell. A xenon 150 W lamp was used with two automatic monochromators for excitation and emission. The detection was carried out using R 450-01 photomultiplier.
Preliminary study: Total phenols in bee pollen fractions were detected by using Folin-Ciocalteu assay according to a method described by Singleton and Rossi with modifications [36]. PGc and PGe were sonicated in methanol followed by centrifugation and each supernatant was separated, evaporated to dryness, and then dissolved in methanol to reach up a stock solution of 1 mg / ml. Aliquots (1.0 mL) of the diluted fraction or standard solutions (gallic acid and quercetin) in methanol were mixed with 2.5 mL 0.2 N Folin–Ciocalteu reagent. After 5 min, the reaction mixture was neutralized with sodium NaCO3 (2 mL, 7.5% w/v) solution. After incubation (2 h/RT), absorbance “A” of the resulting blue color was measured. To detect the maximum excitation and emission wavelengths, 10 μg mL-1 samples of standard quercetin/gallic acid were dissolved in methanol and scanned spectrofluorimetrically. The maximum wavelengths were recorded at λex=350 and λem=420. A standard calibration graph was constructed by plotting the relative fluorescence intensity versus different quercetin/gallic acid concentrations. The study displayed a linear calibration graph over a concentration range of 6.25 -250 μg mL-1 quercetin/gallic acid (Figures 5 and 6).
Analytical detection of quercetin, gallic acid in natural extracts:
The proposed spectrofluorimetric method was evaluated by detecting the target analyte in its bee pollen fractions. PGc and PGe were investigated. The total concentration of quercetin and gallic acid in each fraction was calculated using the flowing equation; T=C ×V/M. Where, C is the concentration of quercetin/ gallic acid obtained from the calibration graph, V is the volume of the fraction and M is the weight of the fraction. The recorded results were 0.032, and 0.012 mg as well as 0.069 and 0.085 mg for the quercetin and gallic acid of PGc and PGe fractions, respectively. All measurements were performed in triplicate. TPC were expressed in the term of mg quercetin/gallic acid equivalent (mg QUE/GAE/g fractions, the results were found in Tables 3 and 4.
Detection of Catechin using Vanillin and sulfuric acid
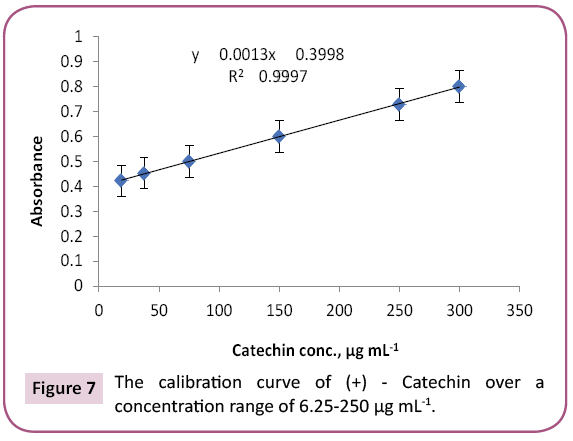
Proanthocyanidin (PAC) in PGc and PGe fractions were determined using vanillin-sulfuric acid assay according to a method described by Sun with little modifications [37]. To 1 ml of (+) ‑ catechin solutions (18.75, 37.5, 75, 150, or 300 μg/ ml methanol), 1 ml of each PGc and PGe (test samples in methanol), 2.5 ml of 1% vanillin solution in methanol and 2.5 ml of 3.6 N H2SO4 in methanol were added in a test tube. After incubation (30°C/20 min), absorbance of each reaction mixture was measured at 500 nm and calculated using the equation: A=(As – Ab) − (Ac – A0). As, Ab, Ac and Ao are absorbance of solutions with the test sample, without the test sample, without vanillin and with only H2SO4, respectively. PAC content was calculated from a calibration curve prepared by using absorbance of catechin dilutions (Figure 7) and the above equation as mg (+) ‑ catechin equivalent per g of each fraction (Table 5).
Anti-bacterial activity
Anti-bacterial assay: Tested anaerobic bacteria Clostridium perfringens ATCC#13124 was cultured on neomycin anaerobic blood agar in anaerobic jar for 48 hrs. at 37°C. The anaerobic conditions were provided using anaerobic gas pack (Thermo Scientific™ Oxoid™ AnaeroGen™) during incubation and confirmed using anaerobic indicator strips (Thermo Scientific™ Oxoid™).
Anti-bacterial extracts preparation: Samples were dissolved in Dimethyl sulfoxide (DMSO) in 200 mg /ml DMSO ratio, then filtered using syringe filters (0.22 μm) into sterilized tubes. Samples were stored at 5°C till used.
Anti-bacterial susceptibility assay: Antibacterial susceptibility test was evaluated using agar well diffusion method. The bacteria were suspended and adjusted to 0.5 McFarland Equivalence Standard. And then streaked heavily on BHI agar media using a sterile cotton swab. The wells were made using sterilized glass Pasteur pipette. Each well was filled with 100 μL of the sample extract. The plates were then incubated at 37°C for 48hrs in anaerobic conditions using anaerobic jar and anaerobic gas pack. Bacterial resistance to antibiotic was determined by measuring the diameter of the inhibition zone measure by (mm). Each extract was analyzed in triplicates [38].
Results and Discussion
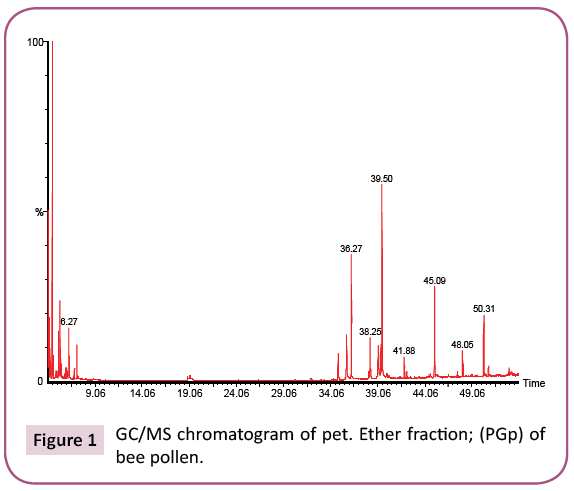
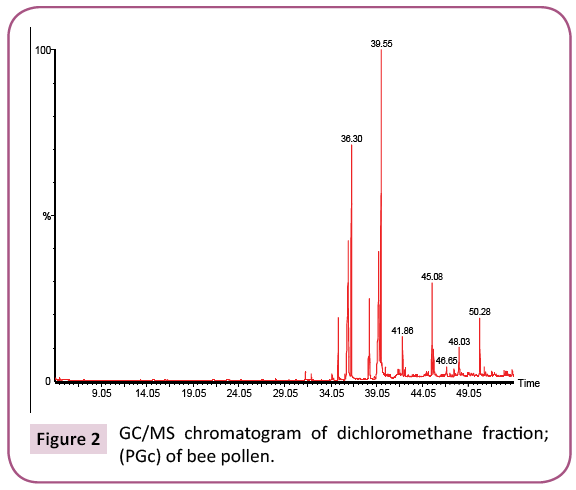
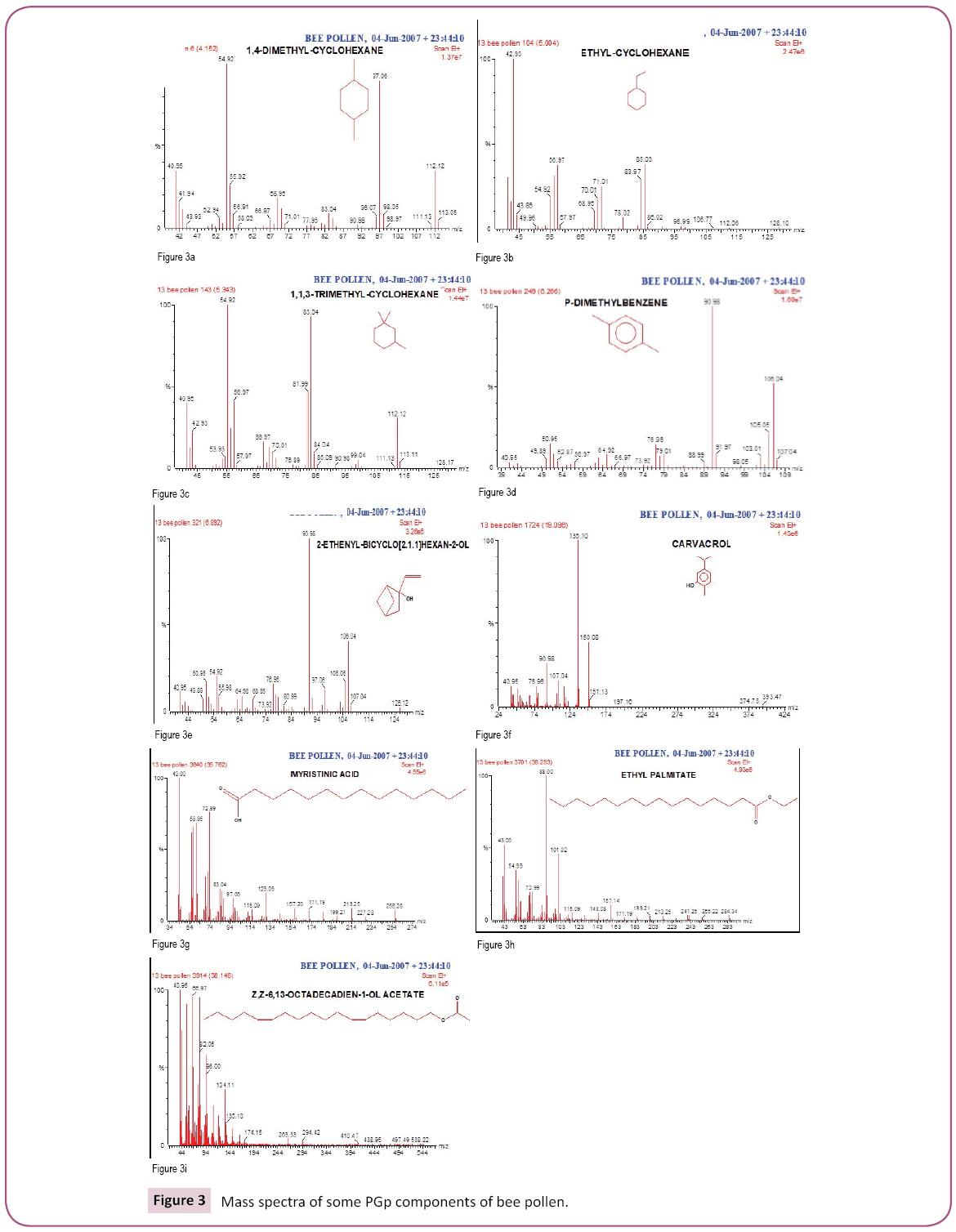
This is the first time to study bee pollen purchased from Riyadh market. There are no previous GC and GC/MS as well as anti- Clostridium perfringens studies on these pollen grains. Analysis of the two bee pollen fractions (Figures 1 and 2) resulted in 23 and 14 components representing 84.07% and 92.92% for PGp and PGc respectively, their retention indices and area percentages (concentrations) are summarized in Tables 1 and 2. The major compounds of GPp and PGc were ethyl ester of hexadecanoic acid (18, 18.34%), 11,14,17-Eicosatrienoic acid (20, 12.74%) and 1,4-Dimethyl-benzene (12, 12.59%) as well as ethyl ester of hexadecanoic acid (6, 29.16%), hexadecanoic acid (5, 27.05%) and nonacosane (14, 11.72%) respectively. However, the minor components of these two different fractions were 1,1,2-trimethyl cyclohexane (8, 0.36%), methyl ester of 9,12-octadecanoic acid (19, 0.37%) and 1-ethyl-4-methyl cyclohexane (13, 0.59%) as well as 11,14,17-ecosatrienoic acid (4, 0.41%), ethyl ester of 9,12- octadecadienoic acid (10, 0.57%) and heptacosane (13, 0.75%) respectively (Figures 3 and 4).
| S.No | Name | RT | Area (%) | RI |
|---|---|---|---|---|
| 1 | 1,4-Dimethyl-Cyclohexane | 4.15 | 4.61 | 751 |
| 2 | 2,6-Dimethyl-1-Heptanol | 4.93 | 1.2 | 753 |
| 3 | 4-Methyl-Octane | 5 | 0.96 | 764 |
| 6 | Ethyl-Cyclohexane | 5.34 | 7.25 | 795 |
| 7 | 1,1,3-Trimethyl-Cyclohexane | 5.45 | 1.75 | 797 |
| 8 | 1,1,2-Trimethyl-Cyclohexane | 5.53 | 0.36 | 799 |
| 9 | 2,3-Dimethyl-Heptane | 5.89 | 1.81 | 815 |
| 10 | Ethyl Benzene | 6.01 | 2.74 | 820 |
| 11 | 2-Methyl-Octane | 6.14 | 0.91 | 825 |
| 12 | 1,4-Dimethyl-Benzene | 6.27 | 12.59 | 829 |
| 13 | 1-Ethyl-4-Methyl-Cyclohexane | 6.8 | 0.59 | 845 |
| 14 | Bicyclo [2.1.1] Hexan-2-Ol | 6.88 | 2.25 | 852 |
| 15 | 5-Methyl-2-(1-Methylethyl)-Phenol | 19.1 | 1.41 | 1307 |
| 16 | Methyl Ester ofHexadecanoic Acid | 34.86 | 4.4 | 1885 |
| 17 | 9-Octadecenoic Acid | 35.76 | 5.67 | 1917 |
| 18 | Ethyl Ester ofHexadecanoic Acid | 36.27 | 18.34 | 1935 |
| 19 | Methyl Ester Of 9,12 Octadecanoic Acid | 38.15 | 0.37 | 2003 |
| 20 | 11,14,17-Eicosatrienoic Acid | 39.5 | 12.74 | 2052 |
| 21 | Ethyl Ester of Heptadecanoic Acid | 40.02 | 0.56 | 2121 |
| 22 | Octacosane | 41.88 | 2.8 | 2137 |
| 23 | Eicosane | 47.51 | 0.76 | 2341 |
Table 1: Chemical composition of the pet ether fraction (PGp) of bee pollen.
| S.No. | Name | RT | Area % | RI |
|---|---|---|---|---|
| 1 | Tetradecanoic acid | 31.33 | 0.77 | 1757 |
| 2 | Ethyl ester of pentadecanoic acid | 31.93 | 0.81 | 1779 |
| 3 | Methyl ester of hexadecanoic acid | 34.87 | 8.3 | 1885 |
| 4 | 11,14,17-eicosatrienoic acid | 35.62 | 0.41 | 1912 |
| 5 | Hexadecanoic acid | 35.98 | 27.05 | 1923 |
| 6 | Ethyl ester of hexadecanoic acid | 36.3 | 29.16 | 1936 |
| 7 | Methyl ester of 9,12-octadecenoic acid | 38.15 | 0.82 | 2003 |
| 8 | 9,12,15-octadecatrienoic acid | 38.26 | 5.56 | 2007 |
| 9 | 11,14,17-eicosatrienoic acid | 39.29 | 1.71 | 2044 |
| 10 | ethyl ester of 9,12-octadeca dienoic acid | 39.41 | 0.57 | 2049 |
| 11 | Heneicosane | 41.86 | 4.47 | 2137 |
| 12 | 1-(2'-hydroxycyclohexyl)-2-hydroxycycloxane | 42.12 | 0.82 | 2158 |
| 13 | Heptacosane | 47.49 | 0.75 | 2731 |
| 14 | Nonacosane | 45.08 | 11.72 | 3015 |
Table 2: Chemical composition of the dichloromethane fraction (PGc) of bee pollen.
From our data, we observed that more number of compounds extracted from GPp than PGc; this might be due to the compounds in PGc fraction are heat labile which might be decomposed when exposure to heat. On the other hand, the GC/MS has given a high recovery of the compounds from PGp fraction; this might be due to stability of compounds in this fraction. The major compounds of both fractions (GPp and PGc) were ethyl ester of hexadecanoic acid (18, 18.34%) and (6, 29.16%) respectively. Hexadecanoic acid (Palmitic acid) is the most common saturated FA found in animals, plants and microorganisms. Palmitic acid is a common saturated FA found in fats and waxes including olive oil, palm oil and body lipids. Palmitic Acid is a saturated long-chain FA with a 16-carbon backbone [39]. Palmitic acid is found naturally in palm oil and palm kernel oil, as well as in butter, cheese, milk and meat. Octadecanoic acid ester (Stearic acid); Fats and oils rich in stearic acid are more abundant in animal fat (up to 30%) than in vegetable fat (typically < 5%). In general, the applications of stearic acid exploit its bifunctional character, with a polar head group that can be attached to metal cations and a nonpolar chain that confers solubility in organic solvents. Stearic acid undergoes the typical reactions of saturated carboxylic acids, a notable one being reduction to stearyl alcohol and esterification with a range of alcohols [40,41].
The purple color observed by Shinoda's test determined the presence of flavonoids, which were noticed as dark green and yellow spots on Si gel TLC after FeCl3 and AlCl3 spry reagents, respectively. Also, red and dark green colors observed by using vanillin/HCl and FeCl3 reagents respectively, which indicated the presence of PAC in bee pollen fractions. The TPC in bee pollen was estimated by Folin - Ciocalteu assay and expressed in term of quercetin equivalent (y=1.6463x + 132.6, R2=0.9972) as mg QUE/g fraction or gallic acid equivalent (y=0.0019x + 0.137, R2=0.9988) as mg GAE/g fraction (Tables 3 and 4) (Figures 5 and 6). As a part of TPC, the PAC was determined by Vanillin - sulfuric acid assay and expressed as (+) - catechin equivalent (% CT w/w) in bee pollen (y=0.0013x + 0.3998, R2=0.9997). The results in Table 5 and Figure 7 indicated that bee pollen is rich in PAC, CT 0.124 mg /0.0051 g and 0.192 mg/0.0059 g for PGc and PGe respectively. On the bases of our results, it was predicted that the protection activity of bee pollen against AD may be explained by many characteristics of bee pollen. The fractions contained high number of polyphenols, represented mainly by flavonoids and proanthocyanidins which conducted high antioxidant potential through their direct free radical scavenging as well as antibacterial activities in vitro.
| Fractions | Volume of fraction taken (mL) |
Calculated amount QUE mg / 4 mL |
|---|---|---|
| PGc | 4 | 0.032 ± 0.001 |
| PGe | 4 | 0.012 ± 0.02 |
Table 3: Total Quarcetin content of bee pollen fractions as determined by Folin-Ciocalteu's assay.
| Fractions | Volume of fraction taken (mL) |
Calculated amount, T mg / 4 ml. |
Calculated amount GAE mg / 4 Ml |
|---|---|---|---|
| PGc | 4 | 0.089 mg x 4 mL / 5.1 mg | 0.069 ± 0.003 |
| PGe | 4 | 0.013 mg x 4 mL / 5.9 mg | 0.085 ± 0.004 |
Table 4: Total gallic acid content of Bee pollen fractions as determined by Folin-Ciocalteu's assay.
| Fractions | Conc. of CT /Volume of fraction taken (ml.) |
Calculated amount mg / 4 ml. |
Total content CT (w/w) |
|
|---|---|---|---|---|
| 1 ml. | 4 ml. | |||
| PGc | 36 µg/mL catechin | 144 µg/mL catechin | 5.1 mg per 4 ml | 0.124 mg/0.0051 g fraction |
| PGe | 48 µg/mL catechin | 192 µg/mL catechin | 5.9 mg per 4 ml | 0.192 mg/ 0.0059 g fraction |
Table 5: Total (+)-catechin (CT) content of bee pollen fraction detected by Vanillin/sulfuric assay.
Antibacterial screening for the PGp, PGc, PGe, and PGb fractions of bee pollen was investigation against anaerobic bacteria Clostridium perfringens (ATCC#13124) at a dose of 200 mg / ml tested by determination of the inhibition zone and showed potent inhibitory activities against anaerobic bacteria Clostridium perfringens by 16.50 mm, 17.25 mm, 16.90 mm, 17.62 mm respectively when compare to lincomycin, clindamycin and vancomycin by 14 mm, 9 mm and 16 mm respectively (Table 6).
| Bee pollen fractions (200mg/ml) | Zone of inhibition (mm)* |
|---|---|
| PGp | 16.50 |
| PGc | 17.25 |
| PGe | 16.90 |
| PGb | 17.62 |
| Lincomycine (2µg) | 14.00 |
| Clindamycine (2µg) | 9.00 |
| Vancomycine (5µg) | 16.00 |
| *triplicate | |
Table 6: Inhibition zone by different bee pollen fractions against anaerobic bacteria Clostridium perfringens.
The short - chain fatty acids (FAs) such as hexanoic acid, medium- chain FAs such as octanoic acid and long-chain FAs such as palmitic acid, were established for antimicrobial activity against Streptococcus mutans, Streptococcus gordonii, Streptococcus sanguis, Candida albicans, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum and Porphyromonas gingivalis [42]. This data assessed that the FAs exhibited inhibition patterns against oral microorganisms. As a group the FAs such as hexanoic, octanoic and lauric acids were much less effective toward C. albicans than the oral bacteria. However, formic acid, capric and lauric acids were widely inhibitory for the bacteria. The results indicate that the antimicrobial activity of short-chain FAs, medium-chain FAs, long-chain FAs might influence on the ecology of the microbial in the oral cavity through at least two significant pathways. First, the agents released exogenously as therapeutic adjuncts might be packaged to promote a microbial-regulatory environment in the sub-gingival sulcus. Second, it could be the intrinsic nature of these FA inhibitors in participating to the characteristics of the microbial biofilms, their evolution and emergence of species within the biofilms [42].
The most substantial target of antimicrobial FAs is the cell membrane. They enhance membrane fluidity, which will result in leakage of the intracellular substances and death of the cell. Other targets pertaining to protein synthesis, which might be inhibited by analogues of the myristic acid, FA metabolism, also topoisomerase activity which might be inhibited through others acetylenic FAs. Carolina et al. have reported that different saturated FAs of varying chain length such as palmitic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid with potent activity against fungi including human pathogens and spoilage organisms [43,44].
Conclusion
This is the first study that investigates the Clostridium perfringens in vitro by using bee pollen wide word. Many studies have been conducted in the past that elevated Clostridia incidence in children with AD. In the current study, we demonstrated that FAs and their derivatives hold high potential antibacterial agents that may lead to new antibacterial drugs. The knowledge about the various mechanisms of action show that the cell membrane is an important target for these substances, however, many enzymes as well as metabolic pathways are also targets for these substances. In conclusion, we observed that a potent inhibitory effect of all fractions of bee pollen on production of anaerobes bacteria Clostridium perfringens in comparison with many antibiotic standards. This may be due to the present of numerous FA and TPC and PAC compounds in bee pollen that may trigger to inhibit growth of bacteria and intern prevent release of the toxins. The results of this study suggest that bee pollen can serve as an adjuvant therapy in the treatment of AD ailments in addition to its regular use in the management and prevention of such symptoms. In this study, we recommend that complete work on bee pollen towards AD in vivo should be conducted.
References
- Principal Investigators, Centers for Disease Control and Prevention (2014) Prevalence of autism spectrum disorder among children aged 8 years autism in 11 sites.Developmental Disabilities Monitoring Network Surveillance Year, 2010.USA. MMWR Surveill Summ 63: 1-21.
- Rutter M (2005) Incidence of autism spectrum disorders: Changes over time and their meaning. Acta Paediatr 94: 2-15.
- Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR (2013) Changes in prevalence of parent-reported autism spectrum disorder in school-aged USA. children: 2007 to 2011–2012. Natl Health Stat Report 65: 1-12.
- Zeidán-Chuliá F, Rybarczyk-FilhoJL, Salmina AB, De Oliveira BH, Noda M, et al. (2013) Exploring the multifactorial nature of autism through computational systems biology: calcium and the Rho GTPase RAC1 under the spotlight. Neuromolecular Med 15: 364-383.
- Zeidán-Chuliá F, Gursoy UK, Könönen E, Gottfried CA (2011) Dental look at the autistic patient through orofacial pain. Acta Odontol Scand 69: 193-200.
- Casanova MF (2007) The neuropathology of autism. Brain Pathol 17: 422-433.
- Anderson GM (2012) Twin studies in autism: what might they say about genetic and environmental influences. J Autism Dev Disord 42: 1526-1527.
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, et al. (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68: 1095-1102.
- Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M (2012) Gastrointestinal conditions in children with autism spectrum disorder: Developing a research agenda. Pediatrics 130: S160–S168.
- Babinská, K, Slobodníková L, Jánošíková D, Lakatošová S, BakošJ, et al. (2012) The effects of probiotic administration on gastrointestinal functions in children with autism. Act Nerv Super Rediviva 54: 82.
- Adams JB, Johansen LJ, Powell LD, Quig D (2011) Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11: 22.
- Adams RP, Adams RP (2005) Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Publishing Corporation, Illinois, USA.
- Buie T, Campbell DB, Fuchs III GJ, Furuta GT, Levy J, et al. (2010) Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 125: S1–S18.
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J (2010) Mood and gut feelings. Brain Behav Immun 24: 9-16.
- Mulle JG, Sharp WG, Cubells JF (2013) The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep 15: 337.
- CryanJF, O'Mahony SM (2011) The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23: 187-92.
- Diaz Heijtz, R, Wang S, Anuar F, Qian Y, Bjorkholm B, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc NatlAcadSci USA 108: 3047-3052.
- Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6: 306-14.
- Mayer EA (2011) Gut feelings: The emerging biology of gut-brain communication. Nat Rev Neurosci 12: 453-66.
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, et al. (2009) A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog 1: 6.
- Bolte ER (1998) Autism and Clostridium tetani. Med Hypotheses 51: 133-44.
- Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, et al. (2000) Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 15: 429-35.
- Parracho HM, Bingham MO, Gibson GR, McCartney AL (2005) Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54: 987-91.
- CritchfieldJW, Van Hemert S, Ash M, Mulder L, Ashwood P (2011) The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract 2011: 161358.
- Song Y, Liu C, Finegold SM (2004) Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 70: 6459-6465.
- Finegold SM (2011) Desulfovibrio species are potentially important in regressive autism. Med Hypotheses 77: 270-274.
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, et al. (2010) Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16: 444-453.
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, et al. (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35: S6–S16.
- Heberling CA, Dhurjati PS, Sasser M (2013) Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: Links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses 80: 264-70.
- PopoffMR, Bouvet P (2009) Clostridial toxins. Future Microbiol 4: 1021-1064.
- Shaw W (2010) Increased urinary excretion of a 3-(3-hydroxyphenyl)-3hydroxypropionic acid (HPHPA):An abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 13: 135-143.
- Al-Salem HS, Bhat RS, Al-Ayadhi L, El-Ansary A (2016) Therapeutic potency of bee pollen against biochemical autistic features induced through acute and sub-acute neurotoxicity of orally administered propionic acid. BMC Complement Altern Med 16: 120.
- Evans WC (2002) Trease and Evans Pharmacognosy. Saunders, London, UK.p. 223.
- Bate-Smith EC, Lerner NH (1954) Leuco-anthocyanins 2. Systematic distribution of leuco-anthocyanins in leaves. Biochem J 58: 126-132.
- Medic-Saric M, Jasprica I, Mornar A, Males Z (2008) Application of TLC in the isolation and analysis of flavonoids. In: WaksmundzkaHajnos M, Sherma J, Kowalska T (eds) Thin Layer Chromatography in Phytochemistry. CRC Press, Taylor and Francis Group.New York, USA. pp. 405-424.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J EnolVitic 16: 144-158.
- Sun B, Ricardo-da-Silva JM, Spranger I (1998) Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 46: 4267-4274.
- Aleksandra T, Veronika H, Silvia L, Jan B, Barbora V, et al. (2015) Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav 138: 179-187.
- Anneken DJ, Both S, Christoph RM, Fieg G, Steinberner U, Westfechtel A (2006) Fatty Acids.In: Elvers B (eds.) Ullmann's Encyclopedia of Industrial Chemistry.
- National Institute of Standards and Technology (2014) Octadecanoic acidNIST Chemistry WebBook:NIST Standard reference database number 69 [https://webbook.nist.gov] Accessed on: June 15, 2014.
- Tsenga, WJ, Mo Liua D, Hsub CK (1999) Influence of stearic acid on suspension structure and green microstructure of injection-molded zirconia ceramics. Ceram Int 25: 191–195.
- Huang CB, Alimova Y, Myers TM, Ebersole JL (2011) Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 56: 650-654.
- Carolina HP, Johan LF, KockP, Vuyisile ST (2011) Antifungal free fatty acids: A Review. In: Vilas AM (eds.)Science against microbial pathogens: Communicating current research and technological advances, pp. 61-71.
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, et al. (2011) Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6: e24585.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences