Water Temperature Affects the Ontogenetic Development of Yellowtail Amberjack Seriola lalandi dorsalis (Gill 1863)
Yu G1,2*, Ma Z1,2, Hu J1,2, Liu Y1,2, Yang Q1,2 and Yang R1,2
1Tropical Aquaculture Research and Development Centre, South China Sea Fisheries, Research Institute, Chinese Academy of Fishery Sciences, China
2Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization, Ministry of Agriculture, Guangzhou, China
- Corresponding Author:
- Yu G
Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization
Ministry of Agriculture, Guangzhou, China
Tel: +86 20 8445 1448
Email: gyu0928@163.com
Received date: March 9, 2017; Accepted date: March 28, 2017; Published date: April 4, 2017
Citation: Yu G, Ma Z, Hu J, et al. Water Temperature Affects the Ontogenetic Development of Yellowtail Amberjack Seriola lalandi dorsalis (Gill 1863). Insights Aquac Cult Biotechnol. 2017, 1:1.
Copyright: © 2017 Yu G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The effect of temperature on survival, growth, jaw deformity, and point of no return (PNR) after food deprivation in newly hatched yellowtail amberjack Seriola lalandi dorsalis larvae were studied in experimental conditions. The performance of fed and un-fed yellowtail amberjack larvae were tested at 21, 23 and 25˚C for 24 days. In the fed treatment, fish survivals at 21 and 23˚C were significantly higher than that at 25˚C by 24 day post hatch (DPH). Fish length and dry weight at 25˚C were significantly higher than those reared at 21 and 23˚C. Temperature significantly affects the feeding incidence of fish larvae from 3 DPH to 5 DPH. In the unfed treatment, fish larvae reached PNR at 5, 6 and 8 DPH at 25, 23 and 21˚C, respectively. Higher temperatures increased fish ontogenetic development, but decreased the time to reach PNR. The high rate of jaw deformity occurred at high temperature by 24 DPH. Our results indicate that rearing temperature is a key factor affecting the ontogenetic development of yellowtail amberjack larvae and the optimum temperature for the first feeding larval is 21-23˚C. Temperatures above 25˚C are likely to cause mortality of yellowtail amberjack larvae in the first 10 DPH.
Keywords
Temperature; Yellowtail amberjack larvae; Growth; Survival; PNR; Jaw malformation
Introduction
Temperature is important for development of fish larvae because it can regulate metabolism and feeding behaviour [1,2]. Temperature directly influences the size of fish larvae at hatching, yolk absorption, growth, survival, feeding success and digestion [3-9]. In many cases, high mortality and abnormality of fish larvae are attributed to unsuitable temperature in the rearing system [10-13]. Within the temperature range of fish tolerance, the increase of temperature accelerates ontogenetic development, but a high temperature may reduce fish survival rates at the same time [14]. During ontogenetic development, the size at metamorphosis tends to increase at low temperature [15,16]. In contrast, at high temperatures, yolk-sac absorption of fish larvae is faster resulting in a shorter endogenous feeding period [9,17,18]. Within the range of optimal temperatures, food intake of fish usually increases with temperature increment, but falls dramatically at the low end of optimal temperatures [19]. Therefore, choosing appropriate temperature for rearing fish larvae is essential to achieve fast growth and high survival in finfish hatcheries.
In larval fish rearing, fish mortality in the early stage is closely related to food supply under favourable conditions because fish development depends on adequate nutrition uptake [20-23]. However, temperature can influence the duration of endogenous feeding and the ability of fish larvae to tolerate food deprivation because temperature directly regulates the rate of yolk absorption [24,25]. After yolk sac is depleted, fish larvae will rely on food from exogenous sources only. Therefore, the time at first feeding in fish larvae is crucial for their growth and survival.
Furthermore, when larvae are deprived of food after yolk-sac absorption, some fish may permanently lose their ability to feed from an external source [20,24]. Blaxter and Hempel [20] were the first to define the term of point-of-no-return (PNR) for herring as being 50% of larvae losing their ability of normal feeding after a period of food deprivation. This definition is also termed as “irreversible starvation” in larval fish biology [20]. During the onset of exogenous feeding, fish mortality is likely to occur if the provision of first feeding is beyond the PNR [20]. For this reason, it is necessary to examine the PNR at various temperatures for a newly introduced fish species in aquaculture.
Jaw malformation is another factor not only causing low fish survival but also reducing the market value of marine fish species [26,27]. Jaw malformations have been reported to be associated with poor nutrition [27-31] and environmental factors [27,32-34]. Lein et al. [10] suggested that temperature can be a primary rearing condition influencing jaw development because organ development and differentiation in fish are temperature-dependent. At low temperature, significant deformities of gill-cover and skeleton in gilt-head sea bream Sparus aurata [35] and cranial deformities in European sea bass, Dicentrarchus labrax [36] have been reported.
The yellowtail amberjack (Seriola lalandi dorsalis) belongs to the Carangidae family and is widely distributed throughout warm–temperate waters of the northern hemisphere. It has been introduced as a new species for aquaculture due to its fast growth, high flesh quality and suitability for cage culture for 20s. However, low survival and unreliable fingerling quality have greatly hindered the fingerling production of yellowtail amberjack in China. Up to present, the impact of temperature on survival and growth of yellowtail amberjack in the early stage is not known for this species. It is unclear if temperature could affect deformity in yellowtail amberjack, though jaw malformation has been a serious problem in the larval rearing of yellowtail kingfish [27,36], a Seriola lalandi subspecies distributing in the Southern Hemisphere waters. It is therefore necessary to examine the relationship between temperature and jaw deformity during the early stage of larval fish rearing. The objective of this study was to understand the impact of temperatures on the ontogenetic development of yellowtail amberjack. Specifically, we examined survival, growth and development of fish larvae at different temperatures. We further quantified the time required for fish larvae to reach the point of no return after yolk absorption and jaw malformation at different temperatures. The results of this study would provide insights into the understanding of role of temperature in regulating fish survival and the development of management strategies to improve production efficiency in finfish hatcheries.
Materials and Methods
Ethical approval
In this study, the handle of fish was carried out in strict accordance with the recommendation in the Animal Welfare of Chinese Academy of Fishery Sciences Animal Welfare Committee. The protocol, species and number of animals used in this study were approved by the South China Sea Fisheries Research Institute Animal Welfare Committee (Approved Number: A201601A01).
Experimental design and larval fish rearing
Fertilized eggs were obtained from a local hatchery in Lingshui Town, Hainan Province, China and transported to the Tropical Aquaculture Research and Development Centre, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. Upon arrival, all larvae hatched in 500 L fibreglass incubators at 23.5°C. The experimental design included three temperatures 21, 23 and °C with three replicates. A total of nine tanks were used in the temperature experiment. After hatching, the larvae were stocked into the 500 L fibreglass rearing tanks at a density of 60 larvae/L per tank. All rearing tanks were supplied with filtered seawater with a 6 μm filter in a flow-through system at a daily water exchange rate of 300% tank volume. Two air stones were used in each tank to maintain dissolved oxygen at saturation and to homogenize the distribution of microalgae, rotifers and Artemia nauplii. Light intensity at 2400 lx and a photoperiod of 14 h light and 10 h dark was used. Salinity was maintained at 36‰ throughout the experiment.
Rotifers (Brachionus plicatilis) were fed to the 3 days post hatching (DPH) larvae until 13 DPH at 10 rotifers/ml. The rotifers fed with microalgae (Nannocholoropsis sp.) were enriched with DHA Selco (INVE Aquaculture) for 12 h before adding into fish rearing tanks. Instant microalgae (Nannocholoropsis sp.) were also added into the larval rearing tanks as food for rotifers, and to create a green background for fish larvae. Artemia nauplii were enriched with DHA Selco (INVE Aquaculture) before they were introduced into the larval rearing tanks from 9 to 24 DPH at 5 Artemia/mL.
Fish sampling, growth and dry weight measurements
Upon egg arrival, 50 eggs were randomly collected in triplicate from the incubating tanks to measure the egg diameters. The average egg diameter was 1.40 ± 0.03 mm (n=50). Fish larvae were daily collected in triplicate from the rearing tanks from hatching to 6 DPH. Subsequently, the specimens were collected on 8, 11, 15, 19 and 24 DPH. Standard length, feeding incidence, and yolk sac size were measured on 10 fish per tank under a dissecting microscope on each sampling day. Ten fish from each tank were used to determine dry weight, and the larvae were first washed with distilled water before body weight analysis. Fish were dried in an oven at 60 °C for 48 h to obtain dry weight.
Mortality rates were recorded daily by counting dead fish on the bottom of each tank. Growth was determined by the absolute growth rate (AGR) in mm/day and by the specific growth rate (SGR) as %/day using the following equations [37].
AGR=(SLf–SLi)/Δt,
SGR=100 (LnSLf–LnSLi)/Δt,
Where SLf and SLi were the final and initial fish standard length (mm), respectively, and Δt was the time between sampling intervals. The volume of yolk sac (YSV, mm3) was calculated using the formula for an ellipsoidal volume: YSV=π/6 × L × H2, where L was the major axis and H the minor axis of the yolk sac [20]. The feeding incidences were calculated by the following formula:
Feeding incidence=100 × N1/N0,
Where N0 is the number of fish larvae, and N1 is the number of larvae with food in the gut [10].
Point of no return (PNR)
At each temperature, a total of 30,000 fish larvae were deprived of food and kept in a 500 L tank. All other rearing conditions were the same as those in the feeding experiment. Starting from 6 h after yolk sac exhaustion, 20 starved larvae were daily taken from the unfed tank and transferred into a 4 L beaker in triplicate to test the time required to reach the PNR. Larvae were provided with rotifers stained with neutral red (10 μL/mL) for 30 min at a density of 20 rotifers/ml. The fish gut was examined under a microscope after fish were feeding for 2 h. The percentage of feeding larvae that had been deprived of food for a given number of days was calculated in respect to the total number of larvae tested. The PNR was defined as a threshold time when 50% of the larvae were still alive but was unable to eat, despite food availability [20].
Jaw deformity analysis
At the end of the temperature trial, 50 larvae were randomly selected from each tank to examine jaw deformity. The jaw malformation was scored using the method by Battaglene and Cobcroft [32]. In brief, the “0” score stands for normal jaw; “0.5” for very minor malformation; “1” stands for minor malformation; “2” for moderate malformation; and “3” for severe malformation.
Statistical analysis
All data were expressed as mean ± SD, and tested using oneway ANOVA (PASW Statistics 18.0). When a significant treatment effect was found, Tukey’s test was performed for multiple range comparisons (P<0.05). The relationship between standard length (SL) and dry weight (DW) were calculated by the power regression DW=a × SLb (PASW Statistics 18.0). Values of the exponent b provide information on fish growth. When b=3, the increase in weight was isometric. When the value of b was >3, the weight increase was allometric (positive if b>3, negative if b<3) [38].
Results
Fish survival, growth, feeding and yolk depletion
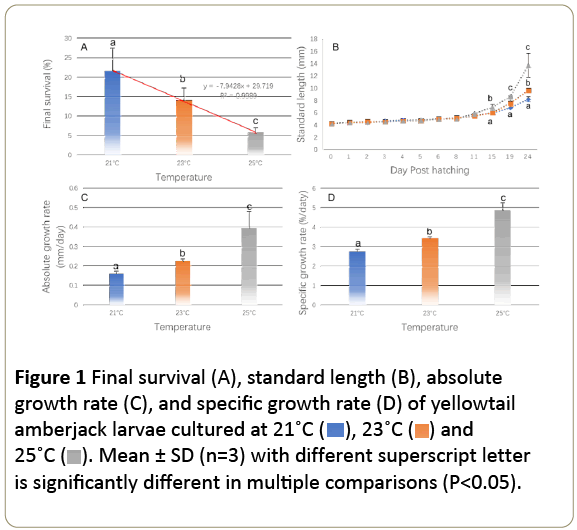
Fish survival was significantly affected by temperature (P<0.05, Figure 1A). The final survival of fish larvae decreased with the increasing of temperature (Figure 1) and the decreasing of survival followed a linear regression of y= –7.9428x + 29.719 (r2=0.9989, Figure 1A). At the end of this experiment, the highest survival was observed in fish reared at 21°C and lowest survival was observed at °C (P<0.05). Temperature significantly affected the growth of yellowtail amberjack larvae (P<0.05, Figure 1B-1D). Fish length increased from 4.18 ± 0.06 mm at hatching (0 DPH) to 8.3 ± 0.5 mm at 21°C, 9.8 ± 0.4 mm at 23°C, and 13.8 ± 1.9 mm at °C by 24 DPH, respectively. But before 11 DPH, no differences were observed between temperature treatments (P>0.05, Figure 1B). On 15 DPH, the standard length of fish at °C was significantly higher than at other temperatures (P<0.05). On 24 DPH, fish at °C were still bigger than those at 21°C and 23°C (P<0.05) and fish at 23°C were larger than those cultured at 21°C environment (P<0.05). The absolute and specific growth rates of yellowtail amberjack were significantly affected by rearing temperature (P<0.05). Within the rearing temperature of 21-°C, the increment of temperature increased both absolute and specific growth rates of fish larvae (Figure 1C and 1D). Larvae in the °C treatment exhibited the highest absolute (0.38 ± 0.07 mm/day) and specific growth rate (4.8 ± 0.60%/day). Furthermore, the absolute and specific growth rates of larvae at °C were almost two folds greater than the larvae at 21°C.
Figure 1: Final survival (A), standard length (B), absolute growth rate (C), and specific growth rate (D) of yellowtail amberjack larvae cultured at 21˚C (  ), 23˚C (
), 23˚C ( ) and 25˚C (
) and 25˚C ( ). Mean ± SD (n=3) with different superscript letter is significantly different in multiple comparisons (P<0.05).
). Mean ± SD (n=3) with different superscript letter is significantly different in multiple comparisons (P<0.05).
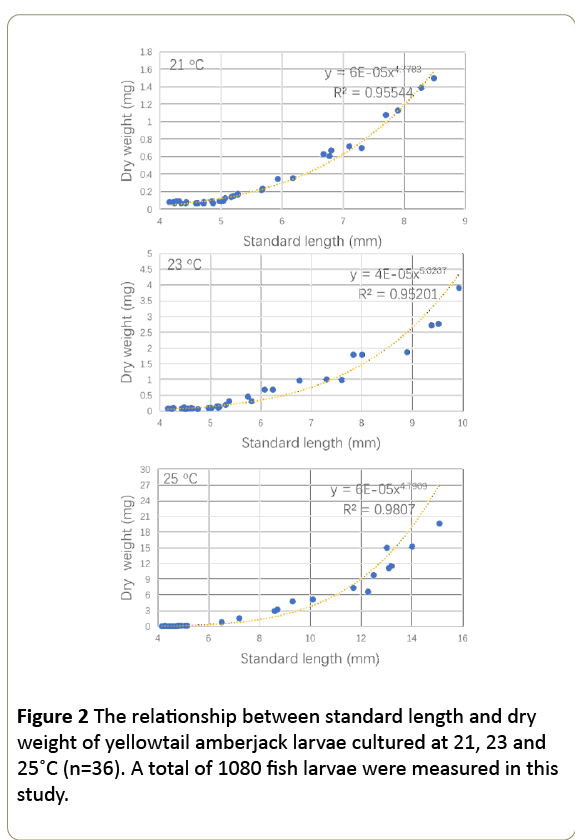
In this study, the standard length (x) and dry weight (y) relationships of yellowtail amberjack larvae were quantified by the regression equation of y=a × xb. The length-weight relationships of fish culture at 21, 23 and °C can be expressed at y21=6E-05 × 4.78 (r2=0.96), y23=4E-05 × 5.02 (r2=0.95) and y25=6E-05 × 4.79 (r2=0.98), respectively (Figure 2). The b values were 4.78–5.02, where a ranged from 4.0 × 10-5 to 6.0 × 10-5. The maximum b was observed at 23°C.
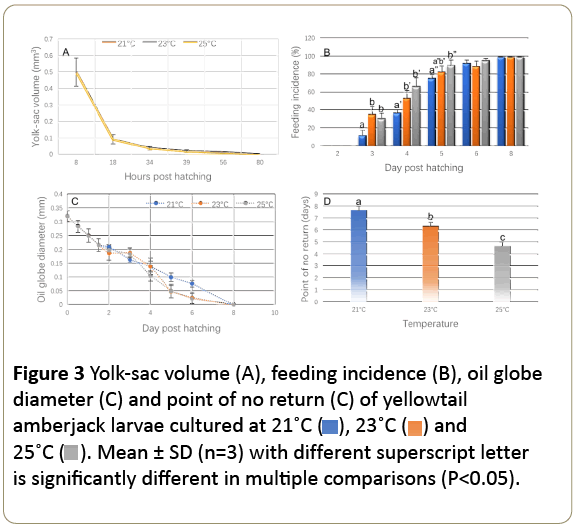
The volume of yolk sac in yellowtail amberjack larvae was 0.49 ± 0.09 mm3 at 8 h after hatch and decreased to 0.009 ± 0.00 mm3 at 80 h after hatch. Yolk-sac depletion was not significantly affected by the rearing temperatures (P>0.05, Figure 3A) and a rapid depletion was found between 8 h and 18 h. Feeding incidences of fish larvae were significantly affected by the rearing temperature from 2 DPH to 5 DPH (P>0.05, Figure 3B). The feeding incidence of fish larvae in 21°C group was significantly lower than those observed in 23 and °C (P<0.05) between 3 DPH and 5 DPH. Starting from 6 DPH, the feeding incidences of fish larvae were not significantly affected by the rearing temperature (P>0.05).
Figure 3: Yolk-sac volume (A), feeding incidence (B), oil globe diameter (C) and point of no return (C) of yellowtail amberjack larvae cultured at 21˚C ( ), 23˚C (
), 23˚C ( ) and 25˚C (
) and 25˚C ( ). Mean ± SD (n=3) with different superscript letter is significantly different in multiple comparisons (P<0.05).
). Mean ± SD (n=3) with different superscript letter is significantly different in multiple comparisons (P<0.05).
The time for fish larvae to reach the PNR depended on the rearing temperatures (Figure 3D). The PNR at 25, 23 and 21°C occurred on 5, 6 and 8 DPH, respectively. The average of feeding incidences at 21, 23 and °C was 4.46 ± 1.88% in starved fish and no significant differences were found between these treatments (P>0.05). On 5 DPH, feeding incidence in fish at °C was significantly higher than that at 21°C (P<0.05). The peaks of feeding incidence at 21 and 23°C occurred on 6 and 5 DPH, respectively.
Jaw deformities
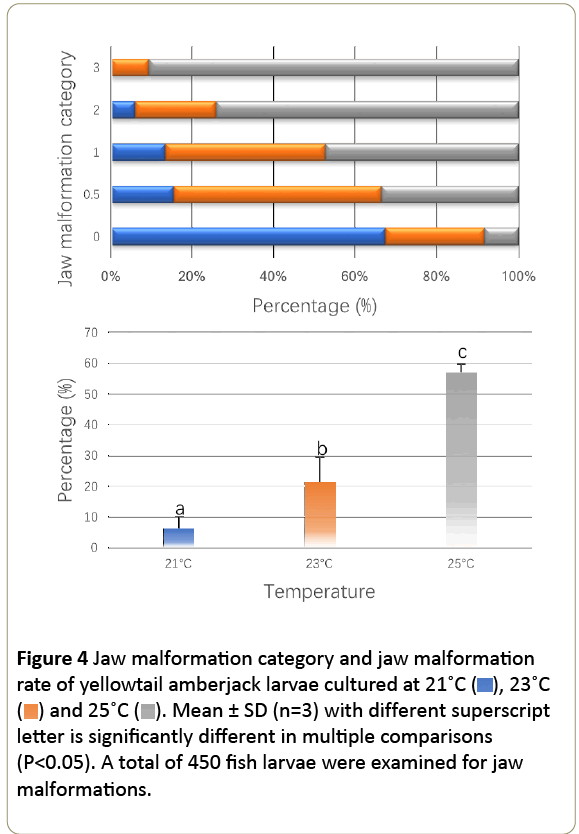
Temperature significantly affected the jaw malformation of yellowtail amberjack larvae (Figure 4). In this study, category 0.5 jaw malformations were 15.06%, 50.33% and 33.31% in 21, 23 and °C, respectively (Figure 4). In °C group, significantly higher category 1, 2 and 3 malformations were observed. In calculating the final malformation percentage, we excluded category 0.5, as most of malformations in this category was minor which cannot significantly affect fish feeding. Jaw malformation of fish larvae increased with the increasing of rearing temperature. By the end of this experiment, the highest jaw malformation was observed in °C group, and the lowest jaw malformation was recorded in the 21°C rearing group (P<0.05).
Figure 4: Jaw malformation category and jaw malformation rate of yellowtail amberjack larvae cultured at 21˚C ( ), 23˚C (
), 23˚C ( ) and 25˚C (
) and 25˚C ( ). Mean ± SD (n=3) with different superscript letter is significantly different in multiple comparisons (P<0.05). A total of 450 fish larvae were examined for jaw malformations.
). Mean ± SD (n=3) with different superscript letter is significantly different in multiple comparisons (P<0.05). A total of 450 fish larvae were examined for jaw malformations.
Discussion
Previous studies suggest that the temperature range of 17-24°C suits larvae of most temperate fish species [39-41]. Moran [42] reported that 18-24°C should be suitable for egg incubation and the first feeding yellowtail kingfish larvae. This present study scrutinised the influence of temperature on ontogeny of yellowtail amberjack during early life. Water temperature significantly affected growth, survival and jaw deformities of yellowtail amberjack larvae. Importantly, rearing temperature not only affected the time of yolk sac depletion, but also changed the window for the period when the initial feeding should start for the first feeding larvae.
Increasingly more evidence indicates that high fish mortality is associated with food availability when fish start first feeding [43-48]. Previous studies indicate that PNR is closely related to temperature, as low temperature extends the time for larvae to reach the PNR and high temperature shows the opposite [18,20,49]. In the present study, a similar trend was observed where high temperature shortened the time for PNR. Dou et al. [18] suggested that the insufficient time for the first feeding larvae to learn to take food before the onset of irreversible starvation (PNR) might be the potential cause for the mortality. Since higher temperature reduced the time for PNR, larvae of yellowtail amberjack have less time to find their feeding capability. This may explain why massive mortality rates occur earlier under higher temperature in the present study.
The effects of temperature on fish growth, food intake and metabolic activity are usually concurrent [50]. In the present study, the growth of newly hatched amberjack was not temperature dependent in their first 12 days of life. However, fish growth was visibly affected by temperatures after 15 DPH, and growth was accelerated when temperature elevated from 21 to °C. Previous studies have demonstrated that higher temperature can lead to increasing of metabolism, feeding and food assimilation in fish larvae such as Australian snapper Pagrus auratus [8], striped trumpeter Latris lineata [51], brown sole Pseudopleuronectes herzensteini [15], Atlantic halibut Hippoglossus hippoglossus [10] and yellowtail kingfish Serilola lalandi lalandi [41]. In yellowtail amberjack, the positive relationship between temperature and growth may be attributed to the improved digestive function of larvae after 15 DPH as Chen et al. [52] reported that the goblet cells and gastric glands present in the gut of yellowtail kingfish larvae after 15 DPH. However, this need further verify in this species.
The impact of temperature on fish growth could be detected from the length – weight regression equation that is to evaluate the impact of environmental variables on growth [53]. In the regression equation of length-weight relationship, the exponent b is a measure of relative logarithmic growth rates between length and weight, and the b value represents the increments of weight over length in the same period [54]. The range of b values for marine fish larvae in previous studies was 2.7 to 4.5 [53,55]. In the present result, the b value was 4.78 at 21°C, 5.02 at 23°C and 4.79 at °C, suggesting that weight gain of larvae at 21, 23 and °C is faster than other fish species.
The size of the yolk sac volume decreases as fish grow [10]. In the present study, yolk depletion was rapid from 8 to 18 h and the time of yolk sac exhaustion was 56-80 h after hatching. Despite temperature differences, the rate of yolk decline in yellowtail amberjack was not different between treatments. These results were similar to our previous studies on Seriola lalandi lalandi (unpublished data). Similarly, the rate of yolk utilization haddock was not temperature dependent in the temperature range tested [56].
Ambient water temperature can be a significant factor influencing the success of initial feeding in fish larvae [57]. Brett [5] suggested that the amount of food intake is concomitant with the temperature increase and peaks before reaching the supra-optimal temperature. In the present study, the feeding incidence was significantly affected by temperature in the first five days after hatching. A higher feeding incidence was observed in 23°C and °C group suggesting these two temperatures may stimulate the feeding of fish larvae in their early life.
Temperature is a key factor determining the tolerance of food deprivation in fish larvae since it directly affects fish metabolism, yolk absorption, feeding activity and food conversion efficiency [24,25,41]. Evidence indicates that mortality was strongly temperature-dependent in the larvae and juveniles of the Asian catfish, Pangasianodon hypophthalmus [58]. Similarly, high temperature caused poor digestion and high mortality in Japanese flounder larvae Paralichthys olivaceus [18]. In the present study, temperature increment lead to low fish survival especially in the first 10 days after hatching, though mortality was relative stable after 10 DPH. Similar mortality patterns were also found in striped trumpeter Latris lineata as its massive mortality occurred during the initial feeding period [59].
Jaw malformation is a major concern in fish culture because it affects fingerling quality for further growout [36,60,61]. Extreme temperatures and salinities can contribute to jaw malformation [62-64]. In the present study, the rate of jaw malformation in fish larvae increased with temperature rise. This result supports the early finding that jaw malformations are associated with environmental temperature [10,65] and increasing of rearing temperature could lead to high deformity in fish larvae [64]. It has been suggested that temperature can indirectly affect larval ontogeny by alteration of nutritional requirement of fish at different temperatures [66]. The increased fish metabolism at high temperature will lead to a high demand of energy and nutrition supply [67] and interrupt the balance between nutrient requirement and food intake leading to larval malnutrition [29,68,69]. These could explain the appearance of high jaw deformity rate at higher rearing temperature, though the mechanism warrants further exploration.
Conclusion
The present study demonstrated that temperature affected ontogenetic development of yellowtail amberjack larvae. At °C fish larvae had lower survival but higher growth rate, suggesting that this temperature is not suitable for early larval rearing. The optimum temperature for amberjack larvae should be 21-23°C since fish showed higher survival and low jaw deformity at this range. Consequently, we recommend that it is potential to use lower temperature in fish early development stage and a comparatively higher temperature in a later stage to achieve better production yield. The timing of temperature changes, however, requires further investigation.
Acknowledgement
This project was funded by Fishing Port Construction and Fishery Industry Development Special Funds of Guangdong Province (Marine Fishery Science and Technology Extension Direction - Science and Technology Research and Development Projects, A201601A01).
References
- Kestemont P, Baras E (2001) Environmental factors and feed intake: Mechanisms and interactions in food intake in fish (Houlihan D, et al eds), Blackwell Science, Cornwall, pp: 131-156.
- Ma Z, Qin JG, Nie Z (2012) Morphological changes of marine fish larvae and their nutrition need in larvae: Morphology, biology and life cycle (Pourali K, Raad VN eds), Nova Science Publishers, Inc., New York, USA, pp: 1-20.
- Jobling M (1997) Temperature and growth: Modulation of growth rate via temperature change. In: Global warming: Implications for freshwater and marine f (Wood CM, McDonald DG eds.), Cambridge University Press, Cambridge, pp. 225-253.
- Martinez-Palacios CA, Chavez-Sanchez MC, Ross LG (1996) The effects of temperature on food intake, growth and body composition of Cichasoma urophthalmu (Gunther) juveniles. Aquaculture Research 2.
- Brett JR (1979) Environmental factors and growth In Fish Physiology (Hoar WS ed), 8: 599-675. Academic Press, New York.
- Blaxter JHS (1988) Pattern and variety in development In: Fish physiology (Hoar WS, Randall DJ eds) 11: 1-58.
- Rombough PJ (1996) The effects of temperature on embryonic and larval development. In: Society for Experimental Biology Seminar Series 61: Global Warming Implications for Freshwater and Marine Fish. (Wood CM, McDonald DG eds), Cambridge University Press, pp: 177-223.
- Fielder DS, Bardsley WJ, Allan GL, Pankhurst PM (2005) The effects of salinity and temperature on growth and survival of Australian snapper, Pagrus auratus larvae. Aquaculture 250: 201-214.
- Bustos CA, Landaeta MF, Bay-Schmith E, Lewis R, Moraga X (2007) Effects of temperature and lipid droplet adherence on mortality of hatchery-reared southern hake Merluccius australis larvae. Aquaculture 270: 535-540.
- Lein I, Holmefjord I, Rye M (1997) Effects of temperature on yolk sac larvae of Atlantic halibut (Hippoglossus hippoglossus L.) Aquaculture 157: 123-135.
- Buckley LJ, Smigielski AS, Halavik TA, Laurence GC (1990) Effects of water temperature on size and biochemical composition of winter flounder Pseudopleuronectes americanus at hatching and feeding initiation. Fishery Bulletin 88: 419-428.
- Ludwig GM, Lochmann SE (2009) Effect of temperature on larval sunshine bass growth and survival to the fingerling stage. N Am J Aquac 71: 260-266.
- Ørnsrud R, Gil L, Waagbø R (2004) Teratogenicity of elevated egg incubation temperature and egg vitamin A status in Atlantic salmon, Salmo salar L. J Fish Dis 27: 213-223.
- Réalis-Doyelle E, Pasquet A, Charleroy DE, Fontaine P, Teletchea F (2016) Strong effects of temperature on the early life stage of a cold stenothermal fish species, brown trout (Salmo trutta L.). PLoS ONE 11: e0155487.
- Aritaki M, Seikai T (2004) Temperature effects on early development and occurrence of metamorphosis-related morphological abnormalities in hatchery-reared brown sole Pseudopleuronectes herzensteini. Aquaculture 240: 517-530.
- Martínez-Palacios CA, Barriga Tovar E, Taylor JF, Ríos Durán G, et al. (2002) Effect of temperature on growth and survival of Chirostoma estor estor, Jordan 1879, monitored using a simple video technique for remote measurement of length and mass of larval and juvenile fishes. Aquaculture 209: 369-377.
- Fukuhara O (1990) Effects of temperature on yolk utilization, initial growth and behaviour of unfed marine fish-larvae. Mar Biol 106: 169-174.
- Dou SZ, Masuda R, Tanaka M, Tsukamoto K (2005) Effects of temperature and delayed initial feeding on the survival and growth of Japanese flounder larvae. J Fish Biol 66: 362-377.
- Brett JR, Groves TDD (1979) Physiological energetics. In: Fish Physiology. Bioenergetics and Growth (Hoar WS eds), Academic Press, New York 8: 279-352.
- Blaxter JHS, Hempel G (1963) The influence of egg size on herring larvae (Clupea harengus L.). ICES J Mar Sci 28: 211-240.
- Kohno H, Ordonio-Aguilar RS, Ohno A, Taki Y (1997) Why is grouper rearing difficult? An approach from the development of the feeding apparatus in early stage larvae of the grouper, Epinephelus coioides. Ichthylogcal Research 44: 267-274.
- Hunt von Herbing I, Gallager SM (2000) Foraging behavior in early Atlantic code larvae (Gadus morhua) feeding on a protozoan (Balanion sp.) and a copepod nauplius (Pseudodiaptomus sp.). Mar Biol 136: 591-602.
- Houde ED (1989) Comparative growth, mortality and energetics of marine fish larvae: Temperature and implied latitudinal effects. Fish Bull 87: 471-495.
- Kamler E (1992) Early life history of fish: An energetics approach, Chapman and Hall, London.
- McGurk MD (1984) Effects of delayed feeding and temperature on the age of irreversible starvation and on the rates of growth and mortality of Pacific herring larvae. Mar Biol 84: 13-26.
- Barahona-Fernandes MH (1982) Body deformation in hatchery reared European sea bass Dicentrarchus labrax (L). types, prevalence and effect on fish survival. J Fish Biol 21: 239-249.
- Cobcroft JM, Pankhurst PM, Poortenaar C, Hickman B, Tait, M (2004) Jaw malformation in cultured yellowtail kingfish (Seriola lalandi) larvae. N Z J Mar Freshw Res 38: 67-71.
- Suzuki T, Srivastava AS, Kurokawa T (2000) Experimental induction of jaw, gill and pectoral fin malformations in Japanese flounder, Paralichthys olivaceus, larvae. Aquaculture 185: 175-187.
- Cahu C, Zambonino Infante J, Takeuchi T (2003a) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227: 245-258.
- Sandel E, Nixon O, Lutzky S, Ginsbourg B, Tandler A, et al. (2010) The effect of dietary phosphatidylcholine/phosphatidylinositol ratio on malformation in larvae and juvenile gilthead sea bream (Sparus aurata). Aquaculture 304: 42-48.
- Mazurais D, Glynatsi N, Darias MJ, Christodoulopoulou S, Cahu CL, et al. (2009) Optimal levels of dietary vitamin A for reduced deformity incidence during development of European sea bass larvae (Dicentrarchus labrax) depend on malformation type. Aquaculture 294: 262-270.
- 5Battaglene SC, Cobcroft JM (2007) Yellowtail kingfish juvenile quality: Identify timing and nature of jaw deformities in yellowtail kingfish and scope the likely causes of this condition. Final report of project 2007/718, the Australian Seafood CRC, pp: 150.
- Pankhurst PM, Hilder PE (1998) Effect of light intensity on feeding of striped trumpeter Latris lineata larvae. Mar Freshw Res 49: 363-368.
- Cobcroft JM, Battaglene SC (2009) Jaw malformation in striped trumpeter Latris lineata larvae linked to walling behaviour and tank colour. Aquaculture 289: 274-282.
- Georgakopoulou EK, Divanach P, Koumoundouros G (2010) Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 308: 13-19.
- Ma Z, Tan DAY, Qin JG (2014) Jaw deformities in the larvae of yellowtail kingfish (Seriola lalandi Valenciennes, 1833) from two groups of broodstock. Indian J Fish 61: 137-140.
- Hopkins KD (1992) Reporting fish growth: A review of the basics. J World Aquac Soc 23: 173-179.
- Morey G, Moranta J, Massuti E, Grau A, Linde M, et al. (2003) Weight-length relationships of littoral to lower slop fishes from the western Mediterranean. Fish Res 62: 89-96.
- Carton AG (2005) The impact of light intensity and algal-induced turbidity on first-feeding Seriola lalandi larvae. Aquaculture Research 36: 1588-1594.
- Hilton Z, Poortenaar CW, Sewell MA (2008) Lipid and protein utilisation during early development of yellowtail kingfish (Seriola lalandi). Mar Biol 154: 855-865.
- Ma Z (2014) Food ingestion, prey selectivity, feeding incidence and performance of yellowtail kingfish Seriola lalandi larvae under constant and varying temperatures. Aquaculture International 22: 1317-1330.
- Moran D (2007) Size heterogeneity, growth potential and aggression in juvenile yellowtail kingfish (Seriola lalandi Valenciennes). Aquaculture Research 38: 1254-1264.
- Shan X, Quan H, Dou S (2008) Effects of delayed first feeding on growth and survival of rock bream Oplegnathus fasciatus larvae. Aquaculture 277: 14-23.
- Shan XJ, Cao L, Huang W, Dou SZ (2009) Feeding, morphological changes and allometric growth during starvation in miiuy croaker larvae. Environ Biol Fishes 86: 121-130.
- Gisbert E, Conklin DB, Piedrahita RH (2004) Effects of delayed first feeding on the nutritional condition and mortality of California halibut larvae. J Fish Biol 64: 116-132.
- Strussmann CA, Takashima F, Inst O (1992) larval growth and point-of-no-return in fish. In: Growth Determinants in Aquaculture - Third France - Japan Conference in Oceanography. Inst Oceanographique, Paris 18: 27-39.
- Naas KE, Naess T, Harboe T (1992) Enhanced first feeding of halibut larvae (Hippoglossus hippoglossus L.) in green water. Aquaculture 105: 143-156.
- Daniels M, Berlinsky DL, Hodson RG, Sullivan CV (1996) Effects of stocking density, salinity and light intensity on growth and survival of southern flounder Paralichthy lethostigma larvae. J World Aquac Soc 27: 153-159.
- Yin MC, Blaxter JHS (1987) Feeding ability and survival during starvation of marine fish larvae reared in the laboratory. J Exp Mar Bio Ecol 105: 73-83.
- Jobling M (1994) Fish Bioenergetic, Chapman and Hall, London.
- Choa BY, Carter CG, Battaglene SC (2010) Effects of temperature regime on growth and development of post-larval striped trumpeter (Latris lineata). Aquaculture 305: 95-101.
- Chen BN, Qin JG, Kumar MS, Hutchinson W, Clarke S (2006) Ontogenetic development of the digestive system in yellowtail kingfish Seriola lalandi larvae. Aquaculture 256: 489-501.
- Laurence GC (1979) Larval length-weight relations for seven species of northwest Atlantic fishes reared in the laboratory. Fish Bull 76: 890-895.
- Le Cren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol 20: 201-219.
- Pepin P (1995) An analysis of the length-weight relationship of larval fish: Limitations of the general allometric model. Fish Bull 93: 419-426.
- Martell DJ, Kieffer JD, Trippel EA (2005) Effect of temperature during early life history on embryonic and larval development and growth in haddock. J Fish Biol 66: 1558-1575.
- Jordaan A, Kling LJ (2003) Determing the optimal temperature range for Atlantic cod (Gadus morhua) during early life In The Big Fish Bang. Proceedings of the 26th Annual larval fish conference (Browman HI, Skiftesvik AB eds.). The Institute of Marine Research, pp: 46-62.
- Baras E, Raynaud T, Slembrouck J, Caruso D, Cochet C, Legendre M (2011) Interactions between temperature and size on the growth, size heterogeneity, mortality and cannibalism in cultured larvae and juveniles of the Asian catfish, Pangasianodon hypophthalmus (Sauvage). Aquaculture Research 42: 260-276.
- Trotter AJ, Pankhurst PM, Morehead DT, Battaglene SC (2003) Effects of temperature on initial swim bladder inflation and related development in cultured striped trumpeter (Latris lineata) larvae. Aquaculture 221: 141-156.
- Von Westernhagen H (1988) Sublethal effects of pollutants on fish eggs and larvae. In: Fish Physiology (Hoar WS & Randall DJ eds.) Volume XI The Physiology of Developing Fish Part A: Eggs and Larvae. Academic Press. INC, San Diego.
- Ma Z, Zheng P, Guo H, Zhang N, Jiang S, et al. (2016) Jaw malfromation of hatchery reared golden pompano Trachinotus ovatus (Linnaeus 1758) larvae. Aquaculture Research, 47: 1141-1149.
- Von Westernhagen H (1974) Incubation of garpike eggs (Belone belone Linne) under controlled temperature and salinity conditions. J Mar Biol Assoc U.K 55: 945-957.
- Alderdice DF, Velsen FPJ (1971) Some effects of salinity and temperature on early development of Pacific herring (Clupea pallasi). Journal of the Fisheries Research Board of Canada 28: 1545-1562.
- Yang Q, Ma Z, Zheng P, Jiang S, Qin JG, et al. (2016) Effect of temperature on growth, survival and occurrence of skeletal deformity in the golden fish. Indian J Fish 63, 74-82.
- Bolla S, Holmefjord I (1988) Effect of temperature on development of Atlantic halibut. Aquaculture 74: 355-358.
- Georgakopoulou EA, Kaspiris A, Divanach PP, Koumoundouros G (2007) Temperature effects on cranial deformities in European sea bass, Dicentrarchus labrax (L.) J Appl Ichthyol 23: 99-103.
- Rombough PJ (1997) The effects of temperature on embryonic and larval development. In Warming: Implications for Freshwater and Marine Fish (Wood CM, MacDonald DG eds). Cambridge University Press, Cambridge, pp. 177-223.
- Cahu CL, Infante JLZ, Barbosa V (2003b) Effect of dietary phospholipid level and phospholipid: Neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. Br J Nutr 90: 21-28.
- Kanazawa A, Teshima S, Inamori S, Iwashita T, Nagao A (1981) Effects of phospholipids on growth, survival rate and incidence of malformation in the larval ayu. Memoirs of the Faculty of Fisheries, Kagoshima University 30: 301-309.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences