Toxicological Effects and Consumption Safety of Cowpea Seeds Treated with the Extracts of Plumbago Zeylanica
Otitoju Lawrence Kunle*, Ajatta MO and Alamuoye NO
Department of Food Science and Technology, Bamidele Olumilua University of Education, Ekiti State Nigeria
- *Corresponding Author:
- Otitoju Lawrence Kunle, Department of Food Science and Technology, Bamidele Olumilua University of Education, Ekiti State Nigeria, Tel: 08030641884; E-mail: otitojulawrencekunle@gmail.com
Received date: December 18, 2021, Manuscript No. IPJPSAR-21-12052; Editor assigned date: December 20, 2021, PreQC No. IPJPSAR-21-12052 (PQ); Reviewed date: January 03, 2022, QC No. IPJPSAR-21-12052; Revised date: February 21, 2022, Manuscript No. IPJPSAR-21-12052 (R); Published date: March 01, 2022, DOI: 10.36648/IPJPSAR/6.3.74
Citation: Kunle OL, Ajatta MO, Alamuoye NO (2022) Toxicological Effects and Consumption Safety of Cowpea Seeds Treated with the Extracts of Plumbago Zeylanica. J Plant Sci Agri Res Vol:6 No:3
Abstract
This study investigated the toxic effects of the plant extract and powder in stored cowpea seeds on the liver and kidney of albino rats. Eighteen albino rats were randomly grouped into six (Groups 1-6) of three animals each. The root bark oil extract of P. zeylanica was administered orally to the animals. Group 1 serve as the control, while Group 2, 3, 4, 5 and 6 received 5%, 10%, 15%, 20% and 25% oil extract respectively for 24 hours. Table 1 showed the kidney activities of urea, total bilirubin, direct bilirubin, and creatinine of the animals treated with 5%, 10%, 15%, 20% and 25% P. zeylanica’s root bark oil extract respectively (P>0.05). The powder and oil extract of P. zeylanica root bark tested on the liver and kidney of albino rat were found to be non-toxic, since there was no significant difference between the control and the animal treated with the powder and the ethanolic oil extracts. This study therefore showed that the powder and ethanolic oil extract of P. zeylanica would provide alternative to synthetic insecticides in the management of Callosobruchus maculatus’s infesting cowpea seeds in Nigeria.
Keywords
Plumbago zeylanica; Cowpea; Ethanolic and toxic
Introduction
Toxicology is the science of the adverse effect of some chemicals or food on living organisms [1]. The discipline is often divided into several major areas. The descriptive toxicologist performs toxicity tests to obtain information that can be used to evaluate the risk that exposure to foreign substance poses to man and the environment [2]. The mechanistic toxicologist attempts to determine how chemical exert deleterious effects on living organisms. Such studies are useful for the development tests for the prediction of risks, to facilitate the search for safe or chemical and for rational treatment of the manifestations of toxicity [3]. The regulatory toxicity judges of a drug or other chemical has low enough risks to be made available for its intended purpose. The National Agency for Food and Drug Administration and Control (NAFDAC) regulate drugs, medical devices, and cosmetic as well as food additives. The Federal Environment protection Agency (FEPA) is responsible for the regulation of pesticides, toxic chemicals, hazardous wastes and toxic pollutants, water and air. The occupational Safety and Health Administration (OSHA) determine whether employers are providing working conditions that are safe for employees.
All substances are poisons [4]. The right dose differentiates a poison and a remedy. The duration of animal exposure to toxicants is usually divided into four categories viz: (i) acute, (ii) sub-acute, (iii) sub-chronic and (iv) chronic [5]. Acute is exposure to toxic chemical for less than or equal to 24 hours and this is usually referred to as a single administration. Sub-acute involves repeated exposure for one month or less. In sub-chronic, the exposure is repeated for 1-3months while chronic involves repeated exposure to toxicant for a very long period [6]. First effects of chemicals produced in laboratory animals when properly quantified, apply to toxicity in man. When calculated on the basis of dose per unit of body surface, toxic effects in man are usually encountered in the same range of concentration as are those in experimental animal. On the basis of body weight, man is generally more vulnerable than experimental animals by a factor of about ten. Such information is used to select dosages for clinical trials of candidate therapeutic agents and to attempt to set limits on permissible exposure to environmental toxicants. The second main principle is that exposure of experimental animals to toxic agents in high doses is a necessary and valid method to discovered possible hazards to man. This principle is based on the quantal dose-response concept [7]. As a matter of fact, the number of animals used in experiments on toxic materials will usually be small compared with the size of human populations potentially at risk. This study is therefore sought to assess the toxic effects of the plant extract and powder on kidney and liver of albino rats.
Literature Review
- zeylanica-commonly known as “Chitraka” has been recognized in different trendy system of medicines and treatment of various diseases of human begins in the form of paste and powder. The plant mainly contains Naphthoquinones and steroidal compounds. Different parts of this plant are traditionally claimed to be used for the treatment of ailments including antifungal, anti-tumor, disease of heat, rheumatic pains, liver diseases, fever, diabetes and kidney diseases, fever, diabetes and kidney diseases to his agent. P. zeylanica is also known as Ceylon leadworth, cluta, Chtra and Chitamoona. It is one of the common plants used in india traditional system of medicine. The family plumbaginaceae consists of ten genus and 280 species. The genus Plumbago includes three species namely, P. indica, P. capensis and P. zeylanica which are distributed in several parts of india. Among the species, P. zeylanica grows in all districtof plains in Andhra Pradesh, Karnataka, and Maharashtra etc. common wild or in cultivation due to its more therapeutic uses [8].
Other pharmacological uses are laxative, expectorant, astrigent, arbortifacient and in dysentery treatment [9]. Tincture of root bark is used as anti-periodic. The leaves are used as aphrodisiac and in scabies. Its roots are used in traditional system of medicine to cure various ailments like body pains, headache, fever and inflammation because of its anti-oxidant properties [10].
The major phytochemicals in the stem bark are saponins, alkaloids, tannins, flavonoids and cardiac glycosides [11]. In addition, it has also been suggested that it also contains macro elements such as calcium, magnesium, sodium, potassium, phosphorus, iron, zinc, manganese, copper and cobalt to varying degrees [12]. Alkaloids are medicinally useful, possessing analgesic, antispasmodic and bactericidal effects. Tannins promote healing [13]. Cardiac steroids are widely used in treating congestive heart failure. Flavonoids lower risk of heart diseases, saponins also promote wound healing [14]. Though reported the insecticidal efficacy of P. zeylnicaethanoil oil extract in the management of Plodia interpunctella (hubner) (Lepidoptera; pyralidae), we present the toxicological and histopathological effects of P. zeylanica root bark powder which is scarce in literature.
Materials and Methods
Toxicological investigation of P. zeylanica root barks oil extract.
Experimental animalsAdult male albino rats weighing 150-160 g were used for this experiment and were purchased from the breeding colony of the Department of Biochemistry, Federal University of Technology, Akure, Nigeria. The rats were maintained at 25oC on 24 hour light/dark cycle with free access to food and water. They were made to acclimatize with new environment for two week prior to the commencement of the experiment.
Animals grouping and extract administrationAfter a week of acclamatization, they were randomly selected and grouped into sizesEighteen albino rats were randomly grouped into six (Group 1-6) of three animals each. The root bark oil extract of P. zeylanica was administered orally to the animals using a cannula. Group 1 serve as the control group, and received 3.8 ml Dimethylsulphide saline, while Group 2, 3, 4, 5 and 6 received 5%, 10%, 15%, 20% and 25% oil extract respectively for 24 hours i.e. a concentration of 5% was prepared by diluting 0.5ml of plant oil extract in 9.5 ml of dimethyl sulphide saline, 10% concentration was made by 1.0ml of plant extract in 9.0 ml of dimethyl sulphide saline. Also 15%, 20% and 25% concentration were obtained by diluting 1.5 ml, 2.0 ml and 2.5 ml of the plant extract with 8.5 ml, 8.0 ml and 7.5 ml of the Dimethyl sulphide saline respectively. Cage side examinations were performed to detect overt signs of toxicity (salivation, lacrimation, and convulsion, loss of hair, stress, behavioral abnormalities and dead rats). After 24 hours the animals were sacrificed by cervical dislocation.
Preparation of serum
The procedure described by Yakubu et al. was adopted for the preparation of serum. The animals were sacrificed by cervical dislocation and the blood collected by direct heart punctured into EDTA sample bottles and spinned at 3000 rmp for 20 mins. The serum was carefully aspirated with Pasteur pipette into sample bottles for the various biochemical assays.
Biochemical assays
Determination of Aspartate Amino Transferase (AST) activity
This was carried out according to the method as described by the manufacturer’s manual (Randox Laboratories Ltd) [15]. Briefly, the blank and samples were set up in duplicates. 50 μl of serum was pipette in the sample tubes and 100 μl of buffer (containing 100 mM phosphate buffer pH 7.4, 100 mM L-aspartate, and 2 nM α-oxogluterate) was pipette into both sample and blank. The mixture were thoroughly mixed and incubated for 30 minutes at 37°C. 250 μl of Reagent 2 (2, 4-dinitrophenylhyhrazine) were added into all, 50 μl of distilled water were added to the mixture in blank tubes. The tubes were mixed thoroughly and incubated for exactly 20 minutes at 25°C. 250 μl of sodium hydroxide was then added to each tube and mixed. The absorbances were read against the blank after 5 min at 546 nm wave length.
The AST activity is measured by monitoring the concentration of oxaloacetate hydrazone formed with DNPH. The equation for the reaction is given below:
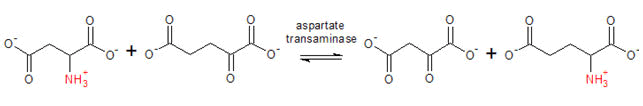
This was carried out according to the method as described by the manufactures manual (Randox Laboratories Ltd). The blank and sample test tubes were set up in duplicates. 50 μl of serum was pipette into the sample tubes. To these were added 250 μl buffer solution containing phosphate buffer, L-alanine and α-oxogutarate. The mixtures were thoroughly mixed and incubated for exactly 30 min at 37°C and pH 7.4, 250 μl of Reagent 2 (2, 4-dinitrophenylhyhrazine) was added to both tubes while 50 μl of distilled water was added to the sample and blank tubes. The tubes were mixed thoroughly and incubated for exactly 20 minutes at 25°C. About 250 μl of sodium hydroxide solution was then added to each tube and mixed. The absorbance was read against the blank after 5 min at 540 nm wave length [16].
Determination of plasma Alkaline Phosphatase (ALP) activityPrinciple
The quantity of ALP in plasma was determined using colorimetric method according to the recommendation of Deutschens Gesellschaft fur Klinische Chemie, DGKL (1972). The principle of the method is as shown below:
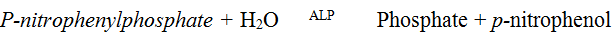
Method
The blank and sample test tubes were set up in duplicates. 50 μl of serum was pipette into the sample tubes. 50 μl of distilled water was pipette into the blank tubes. 250 μl of substrate (Diethanolamine buffer, Magnesium chloride, p-nitrophenyl phosphate) were pipette into each respectively, which was then mixed and the initial absorbance taken at 405 nm. The stop watch was started and the absorbance of the sample and the blank read again three more times at one minute intervals.
Determinattion of creatininePrinciple: Creatinine in alkaline solution reacts with picric acid to form a coloured complex. The amount of the complex formed is directly proportional to the creatinine concentration.
Method: The standard blank sample test tubes were set up in duplicates 1000 μl of serum was pipette into the sample tubes. To there were added 1000 μl working reagent containing RIa (picric acid) and RIb (sodium hydroxide) and mixed. 1000 μl of standard was pipetted into the standard tube. The mixture were thoroughly mixed after 30 seconds, the absorbance A1 of the standard and sample read while 2 minutes later absorbance A2 of standard and sample were also read at 492 nm wave length.
Calculation
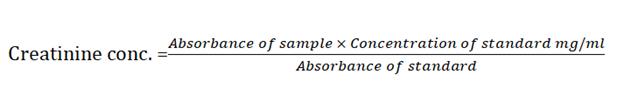
Urea in serum is hydrolyzed to ammonia in the presence of urease. The ammonia is then measured photometrically by bretholet’s reaction.
Urea + H2O Urea 2NH3 + CO2
NH3 + hypochloride + Phenol Indophenols
(Blue compounds)
Method
The blank, standard and sample test tubes were set up. 5 μl of serum was pipette into the sample tubes. Sodium nitroprusside 50 μl of Reagent 1 (containing urease and Sodium nitropuside) was pipette into both samples, blank and standard tubes, and then 15 μl of standard was pipette into both samples, blank and standard tubes. The tubes were mixed thoroughly and incubated at 37°C for 10 min. after 10 mins, 1250 μl of Reagent 2 (phenol) and 1250 μl Reagent 3 (diluted sodium hypochloride and sodium hydroxide) were pipette into both the sample, standard and blank. The tubes were mixed and incubated at 37°C for 15 min; the absorbance was read against the blank and standard at 546 nm wavelength.
Determination of Total bilirubinPrinciple
Colorimetric method based on that described by Jendrassik and Grof. Total bilirubin is determined in the presence of caffeine, which release albumin bound bilirubin, by the reaction with diazotized sulphanic acid [17].
Method
The blank and sample test tubes were set up in duplicates. 100 μl of Reagent 1(sulphanilic acid and hydrochloric acid) were added into both sample and blank tubes. 25 μl of Reagent 2 (sodium nitrite) were added to the mixture in the sample tubes. Then, reagent 500 μl of Reagent 3 (caffeine and sodium benzoate) was also added into the mixture in both sample and blank tubes [18]. The tubes were mixed and incubated at 25°C for 10 min. after 10 min, 500 μl of Reagent 4 (sodium hydroxide and tartrate) was added into the mixture in both blank and sample tubes. The tubes were also mixed and incubated for 10 min at 25°C, the sample or absorbance was read against the blank and standard at 546 nm wavelengths. The total bilirubin concentration was then calculated using the formula:
Total bilirubin (mg/dl)=10.8 x ATB (578 nm).
Determination of direct bilirubinPrinciple
Direct (conjugate) bilirubin reacts with diazotized sulphanic acid in alkaline medium to form a blue coloured complex.
Method
The blank and sample test tubes were set up in duplicates. 100 of Reagent 1(sulphanilic acid and hydrochloride acid) were added into both sample and blank tubes. 25 of Reagent 2 (sodium nitrite) were added to the mixture in the sample tubes. 100 of 0.9% sodium chloride were also added to the mixture in both sample and blank tubes. The tubes were incubated for 10 min at 25°C. The sample or absorbance was read against blank at 540 nm wavelength.
Toxicological investigation of P. zeylanica powderFeed formulation and treatment group
The diets were freshly formulated according to the modified method of Oboh (2005) and were kept air tight containers and stored at 4°C until needed for use.
- Group I–control rats, fed with basal diet (18% corned starch, 18% rice grain,50% skinned milk, 4% minerals & vitamin primates and 10% vegetable oil);
- Group II–rats fed diet supplemented with 0.5 g zeylanica root bark powder
- Group III–rats diet supplemented with 1.0 g zeylanica root bark powder;
- Group IV–rats diet supplemented with 1.5 g zeylanica root bark powder;
- Group V–rats diet supplemented with 2.0 g zeylanica root bark powder;
- Group VI–rats diet supplemented with 2.5 g zeylanica root bark powder;
After two week’s acclimatization, another eighteen albino rats were randomly grouped in six (Group I–VI) of three animal each.
Group I was fed with Basal diet,
Group II was fed with Basal diet containing 0.5 g P. zeylanica root bark powder, Group III was fed with Basal diet containing 1.0 g P. zeylanica root bark powder, Group IV was fed with Basal diet containing 1.5 g P. zeylanica root bark powder, Group V was fed with Basal diet containing 2.0 g P. zeylanica and Group VI was fed with Basal diet containing 2.5 g P. zeylanica root bark powder, the experiment lasted for seven days and cage side examination were perform daily to overt signs of toxicity. Cage side examinations were performed to detect overt signs of toxicity (salvation, lacrimation, and convulsion, loss of hair, stress, behavioural abnormalities and dead rats) [19]. After 24 hours the animals were sacrified by cervical dislocation.
Preparation of SerumThe procedure described by Yakubu et al. [20]. Was adopted for the preparation of serum. The animals were sacrificed by cervical dislocation and the blood collected by direct heart punctured into EDTA sample bottles and spinned at 3000 rpm for 20 mins. The serum was carefully aspirated with pasture pipette into sample bottles for the various biochemical assays.
Biochemical assaysThe serum were analyzed using the available biochemical assays (Alkaline phosphatase (ALP), Asparate Amino Transferase (AST), Alanine Amino Transferace (ALT), urea, total bilirubin, direct bilirubin and creatinine) as described above in 3.6.2.1–3.6.2.7.
Data analysis
All data on adult and larval mortality were corrected using Abbot (1925) Formula. Thus;
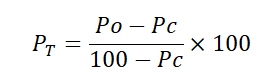
- PT=corrected mortality (%)
- PO=observed mortality (%)
- PC=control mortality (%)
Data were subjected to analysis of variance (ANOVA) and treated means were separated using the New Duncan’s Multiple Range Test. The ANOVA was using SPSS 16.0 software.
Results
Effect of P. zeylanica’s root bark oil extract on liver function of albino ratsTable 1 present the effect of P. zeylanica’s root bark oil extract on liver function parameter of albino rats. There was no significant alteration (P>0.05) in the liver biochemical parameters: Aspirate Amino Transferase (AST), Alanine Amino Transferase (ALT), and alkaline phosphatase in roots treated with 5%, 10%, 15%, 20% and 25% of P. zeylanica root bark oil extract in comparism with rats treated with dimethyl sulphide saline in control
Effects of P. zeylanica’s root bark on kidney functions of albino ratsTable 2 showed the effects of P. zeylania’s root bark oil extract on kidney function parameter of albino rats. The kidney activities of urea, total bilirubin, direct bilirubin, and creatinine of the animals treated with 5%, 10%, 15%, 20% and 25% P. zeylanica’s root bark oil extract respectively were not significantly different (P>0.05) with rate in control groups [21-23].
Table 1: Liver function parameters of albino rats administered with oil extract of P. zeylanica’s root bark.
| GROUPS | CONCENTRATION OF EXTRACTS (%) | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|---|
| I | 0 | 26.67 ± 0.33a | 48.67 ± 2.97a | 101.00 ± 1.00a |
| II | 5 | 25.00 ± 1.52a | 47.67 ± 3.75a | 100.00 ± 0.57a |
| III | 10 | 25.00 ± 3.33a | 48.67 ± 3.70a | 101.30 ± 2.33a |
| IV | 15 | 26.67 ± 0.33a | 49.33 ± 3.33a | 101.00 ± 1.00a |
| V | 20 | 26.67 ± 0.33a | 48.67 ± 0.33a | 102.4 ± 0.33a |
| VI | 25 | 26.67 ± 0.33a | 49.33 ± 0.89a | 102.7 ± 0.33a |
Each value is a mean standard error of three replicates. Means followed by the same letter along the column are not significantly different (P>0.05) using New Duncan’s Multiple Range Test.
Note
ALT=Alanine amino transferase
AST=Aspartate amino transferas
ALP=Alkaline phosphatase
Table 2: Kidney function parameters of albino rats administered oil extract of P. zeylanica root bark.
| GROUPS | CONCENTRATION OF EXTRACTS (%) | UREA (mlmol) | TOTAL BILIRUBIN (µmol/l) | DIRECT BILIRUBIN (µmol/l) | CREATININE (µmol/l) |
|---|---|---|---|---|---|
| I | 0 | 2.28 ± 0.00a | 16.33 ± 0.67a | 335.7 ± 0.88a | 90.07 ± 0.00a |
| II | 5 | 2.30 ± 0.20a | 16.33 ± 0.67a | 335.7 ± 0.88a | 90.75 ± 0.68a |
| III | 10 | 2.30 ± 0.20a | 17.00 ± 1.15a | 336.0 ± 0.00a | 90.09 ± 13.0a |
| IV | 15 | 2.30 ± 0.20a | 17.67 ± 0.67a | 336.3 ± 0.33a | 90.09 ± 13.0a |
| V | 20 | 2.30 ± 0.20a | 17.67 ± 0.67a | 336.7 ± 0.33a | 90.77 ± 13.1a |
| VI | 25 | 2.33 ± 0.23a | 17.33 ± 1.45a | 336.7 ± 0.33a | 90.09 ± 13.0a |
Each value is a mean standard error of three replicates. Means followed by the same letter along the column are not significantly different (P>0.05) using New Duncan’s Multiple Range Test.
Effects of P. zeylanica’s root bark powders on liver biochemical parameters of albino ratsTable 3 revealed the effects P. zeylanica’s root bark powders on liver biochemical parameter of albino rats, which showed no significant different (P>0.05) in serum level of both Aspartate Amino Transferase (AST), Alanine Amino Transferase (ALT) and Alkaline Phosphate (ALP) for the rats in group I, II, III, IV and V when compared with the control rats (Group 1).
Effect of P. zeylanica’s root bark on kidney biochemical parameter of albino ratsThe effects of P. zeylanica’s root bark powders parameters of albino rats were presented on Table 4. The kidney activities of urea, total bilirubin, direct bilirubin, creatinine of the animals fed with basal diet (Group 1), and animals fed with basal diet containing 0.5 g 1.0 g, 1.5 g, 2.0 g and 2.5 g of P. zeylanica’s root bark powders were not significantly different (P>0.05).
Table 3: Biochemical parameters of Liver of Albino rats fed with P. zeylanica’sroot bark powder.
| GROUP | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|
| I | 21.67 ± 1.33a | 66.00 ± 0.00a | 20.50 ± 1.50a |
| II | 22.67 ± 2.03a | 67.00 ± 11.00a | 19.17 ± 1.59a |
| III | 21.67 ± 2.96a | 66.00 ± 0.00a | 18.00 ± 2.31a |
| IV | 21.33 ± 1.20a | 67.00 ± 11.00a | 19.67 ± 0.67a |
| V | 22.67 ± 2.03a | 67.00 ± 11.00a | 20.33 ± 1.33a |
| VI | 22.67 ± 2.03a | 66.00 ± 0.00a | 20.83 ± 0.16a |
Each value is a mean standard error of three replicates. Means followed by the same letter along the column are not significantly different (P>0.05) using New Duncan’s Multiple Range Test.
Table 4: Biochemical parameters of kidney of Albino rats fed with P. zeylanica’s root bark powder.
| GROUPS | UREA (µmol/l) | TOTAL BILIRUBIN (µmol/l) | DIRECT BILIRUBIN (µmol/l) | CREATININE (µmol/l) |
|---|---|---|---|---|
| I | 8.06 ± 0.31a | 20.97 ± 1.23a | 271.00 ± 2.87a | 120.1 ± 30.00a |
| II | 8.24 ± 0.32a | 21.00 ± 0.60a | 264.00 ± 2.00a | 120.1 ± 30.00a |
| III | 8.14 ± 0.09a | 22.22 ± 1.05a | 270.00 ± 3.79a | 121.2 ± 30.00a |
| IV | 8.79 ± 0.23a | 20.97 ± 1.23a | 268.30 ± 2.33a | 120.1 ± 30.20a |
| V | 7.70 ± 0.32a | 20.37 ± 1.85a | 266.70 ± 0.67a | 121.1 ± 30.0a |
| VI | 8.41 ± 0.09a | 20.98 ± 1.85a | 266.00 ± 0.00a | 120.1 ± 30.1a |
Each value is a mean standard error of three replicates. Means followed by the same letter along the column are not significantly different (P>0.05) using New Duncan’s Multiple Range Test.
Keys
Group 1–Group I–normal control rats, fed with basal diet (18% corned starch, 18% rice grain, 50% skinned milk, 4% minerals & vitamin primates and 10% vegetable oil);
Group II–rats fed with basal diet with 0.5 g P. zeylanica’s root bark powder;
Group III–rats fed diet supplemented with 1.0 g P. zeylanica’s root bark powder;
Group IV–rats fed diet supplemented with 1.5 g P. zeylanica’s root bark powder;
Group V–rats fed diet supplemented with 2.0 g P. zeylanica’s root bark powder;
Group VI–rats fed diet supplemented with 2.5 g P. zeylanica’s root bark powder
Effect of P. zeylanica root bark Powder on Body Weight of Rats Mean values for body weights of rats fed with basal diet and basal diet plus P. zeylanica root bark powder at various doses for 30 days period is presented in Table 5. Measurement of the body weight was used to evaluate the health status of the rats during the experimental period. There was no significant difference (p<0.05) in the body weights of rats from the start until the end of the experimental period in all groups apart from rat fed with basal diet plus 10% P. zeylanica root bark powder that show weight loss compared with the normal control rats (basal diet only), rat fed with basal diet plus 1% and 4% P. zeylanica root bark powder.
Table 5: Change in Body Weight of Rats Fed with Basal Diet and Basal Diet plus A. booneiStem Bark Powder.
| Groups | Initial weight (g) | Final weight (g) | Weight gain/loss (%) |
|---|---|---|---|
| I | 164.23 ± 9.21a | 176.67 ± 10.37b | 7.61b |
| II | 165.67 ± 9.52a | 175.23 ± 9.21b | 5.73b |
| III | 167.53 ± 9.43a | 171.67 ± 9.52b | 2.51b |
| IV | 163.33 ± 9.19a | 157.13 ± 9.79a | -3.80a |
| V | 164.13 ± 9.21a | 176.67 ± 10.37b | 7.61b |
| VI | 162.50 ± 9.13a | 171.67 ± 9.52 b | 2.51b |
Each value is a mean + standard error of six replicates. Means followed by the same letter along the column are not significantly different (P>0.05) using New Duncan Multiple Range Test.
Discussion
Assessment of liver and kidney function is very important in toxicity evaluation of drugs and plants extract as organs are necessary for the survival of all organisms [24]. Asparaste Amino Transferase (AST) and Alanine Amino Transferase (ALT) are markers of liver damage and can thus be used to assess liver cytolysis with ALT being a more sensitive biomarker of hepatotoxicity than AST [25]. The reduction in the activity of the ALT and AST from the liver without the corresponding increase in the serum enzyme could be due to inhibition of the enzyme activity by components of the extract [26]. In the present study, the toxicity of ethanolic oil extract of P. zeylanicaroot bark at 5, 10, 15, 20% and 25%kg body weight did not induce any damage to the liver after 24 hrs oral route in albino rats compared with albino rats in the control because the oil extract caused no significant increase in the level of AST and ALT this agreed with work of [27]. In which the toxicity of Anacadium occidentaleobserved produced no toxic effect in mice treated with aqueous extracts of A. occidentaleat doses of 2 g–6 g/kg body weight by oral route. While reported no damage in the liver since there is no significant increase in serum level of AST and ALT, this was observed in the experimental studies on the hypolipidemic and haematological properties of aqueous leaf extract of Cleistopholis patensin rats. Alkaline Phosphatase (ALP) is a marker enzyme for the plasma membrane and often employed to assess the integrity of the plasma membrane [28]. The increase in the ALP activity in the serum during both the treatment and recovery periods could be consequences of leakage of the enzyme from other tissues apart from small intestine and kidney or a reduced rate of clearance of the enzyme from the serum [29]. The observed normal Alkaline Phosphatase (ALP) activities in the liver following the administration of 5%, 10%, 15%, 20% nd 25% oil extract of C. patensroot bark will not hinder the transportation of the required molecules across the plasma membrane with the rat in the control group. This was similar to the findings of Brown et al.in the toxicity of Azadirachta indicaseeds and peels of Citrus sinesison the liver enzymes of albino rats. The oil extract of C. patensroot bark did not show any significant effect nor caused liver injury, since there is no significant increase in the serum level of ALP compare with the control group. This agreed with the work of Assy et al., [30]. Which observed that there was no change in the serum level of ALP in the anti-hyper lipidemia activity of T. violacearhizome extract.
Kidney is to excrete the waste products of metabolism and to regulate the body concentration of water and salt. When there is compromise of the normal glomerular function substances normally cleared by the kidneys such as urea, creatinine, total bilirubin and direct bilirubin accumulate in the biological fluid. One of the objectives of this study was designed to investigate the toxicity of P. zeylanicaroot bark oil by assessment of the urea, creatinine, total bilirubin and direct bilirubin. Urea is a byproduct from protein breakdown. About 90% of urea produced is excreted through the kidney [31]. Meanwhile, the creatinine is a waste product from a muscle creatnine, which is used during muscle contraction. Creatinine is commonly measured as an index of glomerular function [32]. Therefore, damage to the kidney will make the kidney inefficient to excrete both urea and creatinine and causes their accumulation in the serum. The urea, creatinine, total bilirubin of the rats administered with 5, 10, 15, 20 and 25% concentration of oil extract of P. zeylanicaroot bark compared with the rats in the control groups after 24 hr of oral route, showed that there is no significant change of urea, creatinine, total bilirubin and direct bilirubin serum, levels of the rats, therefore, level ofserum urea, creatinine, total bilirubin and direct bilirubin indicate no kidney damage similar observation have been made by Bamisaye et al.in normal rats treated with the extract of Morinda lucida,caster seed oil and powder Nigellasativafor 7 days, 30 days and five weeks respectively.
These results suggested that the evidence of normal AST, ALT, ALP, urea, creatinine, total bilirubin and direct bilirubin level in the serum, the ethanolic oil extract of P. zeylanica root bark does not alter the liver and kidney function [33-38].
Conclusion
The powder and oil extract of P. zeylanica root bark tested on the liver and kidney of albino rat and were found to be non-toxic, since there was no significant difference between the control and the animal treated with the powder and the ethanolic oil extracts.
Therefore, both the powder and the oil extract of P. zeylanica root bark could be recommended for use to protect stored cowpea seeds and can also be integrated with other pest management procedure. Additionally, the cowpea seeeds are safe for consumption after post-harvest treatment since the root bark and oil were found to be non-toxic to mammals.
References
- Olaleye MT (2003) Phytochemical and toxicological investigation of per sea Americana and Hibiscus Sab dariffa A Ph D Thesis Federal University of technology Akure Nigeria 145
- Hodgson E, Mailman RP, chamber JB (1998) Dictionary of Toxicology (2nd Edition), Nature Pub Group, London Macmillan.
- Abbot WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265-267
- Adebayo AH, Abolaji AO, Opata TK, Adegbenro KL (2010) Effects of ethanolic leaf Extract of Chrysophyllum albidium G on biochemical and heamatological Parameters of albino rats. Afr J Biotechnol 9:2145-2150
- Adedire CO, Ajayi TS (1996) Assessment of the insecticidal properties of some plants extracts as grains protectants against the maize weevil Sitophilus zeamais motschulsky. Nigerian J Entomol 13: 95-141
- Adedire CO, Lajide L (2003) Ability of extracts of ten tropical plants species to protect maize grains against Infestation by the maize weevils.Sitophilus zeamais, during storage. Nigeria J Exp Biol 4:1745-179
- Ahmed ME, Abd El-Salam (2010) Fumigant toicity of seven essential oils against the cowpea weevil Callosobruchus maculates (F) and the rice weevil Sitophilus oryzae (L) Egypt J Biol Sci 2:1-6
- Akanji MA, Olagoke OA, Oloyede OB (1993) Effects of chronic consumption of Metabisulphite on the integrity of rat cellular system. J Toxicol 81:173-179
[Crossref] [Google Scholar] [Pubmed]
- Akinkurolere RO, Adedire CO, Odeyemi OO (2006) Laboratory evaluation of the toxic properties of forest Auto-manes difformis, against pulse beetle, Callosobruchus maculatus (Coleopteran: Bruchidae). Insect Sc 13:25-29
- Akinneye JO (2003) Biology and control of the yam moth, Euzopheroides vapidella (Mann) Lepidoptera:Pyralidae) Federal University of Technology Akure M Tech Thesis pp65
- Akinneye JO, Adedire CO, Arannilewa SA (2006) Potential of cleisthopholis patens (benth)as maize protectant against the stored product moth Plodia interpunctella (Hubher) (Lepidoptera: Pyralidae). Afr J Biotechnol 5:2510-2515
- Akinneye JO, Ashamo MO (2009) Insecticidal activity of cleistopholis paterns (Benth) Engl and Diels (Annonacae) against the stored product moth Ephestical cautella (Walker) (Lepidoptera: Pyralidae) in stored cocoa (Theobroma cacao L.) Bean. J Entomol 26:63-66
- Akinneye JO (2011) Aspects of Biology and Control of Tropical Warehouse moth E cautella (Walker) (Lepidoptera): Pyralidae) in coacoa beans PhD Thesis Federal University of Tecnology Akure Nigeria 59
- Arafa HM (2005) Curcumin attenuates diet induced hypercholesterolemia in rats. Med Sci Monit 11:BR228-BR234
- Arannilewa ST, Ekrakene, JO Akinneye (2006) Laboratory evaluation of four medicinal plants as protectants against the maize weevil Sitophilus zeamais (Moth) Afr J Biotechnol 5:2032-2036
- Arther FH, Philips TW, Hui YH, Bruinsma BL, Gorham JR et al. (2003) Stored-product insect pest management and control 1:341-58
- Ashamo MO, Akinneye JO (2004) Toxicity of powder of some tropical plants against yam moth Euzopheroides vapidella (Mann) (Lepidoptera: Pyralidae). Nigeria J Exp Biol 5:63-68
- Assy N, Kaita K, Mymin D, Levy C, Rosser B, et al. (2000) Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci 45:1929-1934
[Crossref] [Google Scholar] [Pubmed]
- Bamisaye FA, Odutua AA, Minari JB, Dairo JO, Oluba OM, et al. (2013) Evaluation of hypoglycemic and toxicological effects of leaf extracs of Morinda lucida ihyperglycemic albino rats. Int J Biosci Biochem Bioinform 3:37-43
- Ileke KD, Oni MO (2011) Toxicity of some plant powers to maize weevil, Sitophilus zeamais(motschulsky) Coleoptera: curculionidae) on stored what grains. Afr J Agric Res 6:3043-3048
- Kawakami F (1999) Current research of alternatives to methyl bromide and its reduction in Japanese plant quarantine Research Bulletin of the Plant Protection service Japan 35:109-120
- Keita SM, Vincent C, Schmit J, Armason JT, Belanger A (2001) Efficacyof oil of Ocimum basilicum L and O gratissimumL Applied as an insecticidal fumigant and powder to control Callosobruchus maculatus (Fabr). J Stored Prod Res 37:33
[Crossref] [Google Scholar] [Pubmed]
- Khan MA (1981) repellents against stored product Insects. Anzeiger fur schaedlingskunde Pflanzenschutz umwelts chutz 54:70-77
- Khoshnoud H, Ghiyasi M, Amimia R, Fard SS, Tajbakhsh M et al. (2008) The potential of using insecticidal properties of medicinal plants against pest. Pak J Biol Sci 11:1380-1384
[Crossref] [Google Scholar] [Pubmed]
- Lal NES (1992) A laboratory study of the comparative toxicity of products from three spices to the maize weevil. Post-Harvest Biol Technol 2:61-64
- Lale NES (1995) An overview of the use of plant products in the management of stored product coleoptera in the tropiss Post-harvest news and information 6:69N-75N
- Loschiavo SR, Okumura GT (1979) A survey of stored product insects in Hawaii USA Proc Hawaii. Entomol Soc 23:95-118
- Mayne PD (2005) Clinical Chemistry in Diagnosis and treatment. 6th Ed, Lloid~Luke (Medical Books) Ltd, England
- Mbata GN, Philips TW (2001) Effects of temperature and exposure time on mortality of stored-product insects exposed to low temperature. J Econ Entomol 94: 1302-1307
[Crossref] [Google Scholar] [Pubmed]
- Morah SC, Mbata GN (1982) Assessment of the relative susceptibility of some maize varieties to post-harvest infestation by the maize weevil, Sitophilus zeamais (Motsch). Rep Nigerian Stored Prod Res Inst 1982 Tech Rep 5:63–68
- Okonkwo E, Okoye W (1996) The efficacy of four seed powders and the essential oils as protectants of cowpea and maize grains against infestation by callosobruchus maculates (Fabricius) (Coleoptera: Curculionidea) in Nigeria. Int J Pest Manag 42:143-146
- Olorunnisola OS, Amao S, Ehigie LO, Ajayi AF (2008) Anti-Hyperglycemic and Hypolipidemic Effect of Ethanolic Extracts of Chrysophylum Albidun Seed Cotyledon in Alloxan induced-diabetic Rats. Res J Appl Sci 3:123-127
- Olorunnisola OS, Bradley G, Afolayan AJ (2011a) Enthomological information on plants used for the management of cardiovascular diseases in Nkonkobe Municipality South Africa J Med Plant Res 5:4256-4260
- Oni MO, Ileke KD (2008) Insecticidal efficacy of oil extracts of Balanites aegyptiaca seeds and cashew nuts against callosobruchus maculatus fabr Coleoptera Bruchidae). Afr J Agric Res. 8(25):3285-3288.
- Ratnasooriya WD, Dharmasiri MG (2000) Effect of Terminalia catappa seeds on sexual behaviours and fertility of male rat. Asian J Androl 2:213-226
[Google Scholar] [Pubmed]
- Reitman S, Frankel S (1957) A colorimetric method for determination of serum as pertate aminotransferase (AST) and alanine aminotransferase (ALT). Am J Clin Pathol 28:56-58
- Yakubu MT, Akanji MA, Oladiji AT (2007) Haematological evaluation in male aibino rats following chronic administration of aqeuos extracts of Fadogia agrestis stem. Pharmacogn Mag 3:34-38
- Yalamanchill TS, Punukollu DT (2000) Activity of essential oils from Curcuma domestica leaf against Callosobruchus chinesis. J Stored Prod Res 37:207-209
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
