ISSN : 2573-4466
Insights in Enzyme Research
The Measurements Of The Cholinesterase Activity Of Brain And Plasma In Rabbits By Using Modified Michel And Ellman Assays
Khalil Abdullah Khalil1,* and Kasim Sakran Abass2
1Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine,University of Mosul, Mosul, Iraq
2Department of Public Health, College of Veterinary Medicine, University of Kirkuk, Kirkuk, Iraq
- *Corresponding Author:
- Khalil Abdullah Khalil
Department of Physiology, Biochemistry
and Pharmacology, College of Veterinary
Medicine, University of Mosul, Mosul, Iraq
Tel: +9647702360141
E-mail: abdullahkhalil863@gmail.com
Received Date: June 06, 2017 Accepted Date: August 18, 2017 Published Date: August 25, 2017
Citation: Khalil KA, Abass KS. The Measurements of the Cholinesterase Activity of Brain and Plasma in Rabbits by Using Modified Michel and Ellman Assays. Insights Enzyme Res. 2017, 1:2.
Abstract
This study deals with the measurement of acetylcholinesterase activities in the plasma and brain of 8 rabbits using modified Michael and Ellman methods. The highest values of acetylcholinesterase activities in plasma and brain were 0.37 and 0.25, respectively, when the modified Michel method was employed. In the case of using Ellman method, the highest values of cholinesterase activities in plasma and brain were 0.89 and 0.43, respectively. In order to determine the efficiency and legitimacy of the measured result, the coefficient of variation was calculated. For the modified Michael method, the coefficients of variation in plasma and brain were 17.78 % and 19.37%, respectively. However, in the case of using Ellman method, the coefficients of variation in plasma and brain were 21.72% and 41.21%, respectively. These results were consistent with those reported in the previous studies.
Keywords
Cholinesterase; Rabbits; Plasma; Brain; Ellman method; Michel method
Introduction
Accurate measurement of cholinesterase activity is very important due to the important role played by this enzyme in breaking down the neurotransmitter acetylcholine and converting it to choline and acetic acid [1]. Also, when the body is exposed to the toxic organophosphorus compounds, cholinesterase would associate with these compounds [2], leading to the accumulation of acetylcholine at the postsynaptic cleft sites [3,4]. The level of acetylcholine can be enhanced by using cholinesterase inhibitory compounds in order to increase the amount of neurotransmitter acetylcholine in the cholinergic synaptic areas [5,6]. There are two main types of cholinesterase. The first type is A-esterases, which is a type of arylesterase. It is mainly found in the liver and plasma within the High Density Lipoprotein (HLD) and it is able to resist the poisonous organophosphorus compounds [7]. The resistance is more effective in mammals instead of birds [8]. Some examples of A-esterases are paraxonase and diisopropyl fluorophosphatase (DFPase). The association between Dephosphorylation and A-esterase is more rapid as compared to B-esterase [9]. The second type of Cholinesterase is B-esterase which is mainly found in tissues and it is inhibited by organophosphorus compounds. Some important subtypes of B-esterase [10] are specific acetylcholinestrase EC3.1.1.7/acetylcholine acetylhydrolase [11], neurotoxic esterase, Carboxylesterase EC 3.1.1.1 [12] and non-specific pseudocholinesterase EC3.1.1.8/Acylcholine acylhydrolase/Butyrylcholinestrase [13,14]. Acetylcholinesterase exhibits irritation ability in the Cholinergic synapses, liver and plasma within the HLD, neuromuscular junction in musclotendinous, neuron body, neuron axis, central nervous system (spinal cord and brain), blood serum, red blood cells, platelets in the pancreas, T-lymphocytes, motor end plate and Neural fibers [15,16]. The physiological function of Cholinesterase is the breakdown of acetylcholine into choline and acetic acid [17] as indicated in the following process:
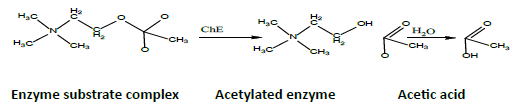
In this process, acetylcholine is serving as a neurotransmitter in neuromuscular tissue [18]. Many of the functions of cholinesterase citrate found in the body are unknown, but it is thought to protect the body from cholinesterase inhibitors and it is believed to be associated with cell growth and regulation of Hemopoiesis [19]. One of the popular methods in measuring cholinesterase activity is Ellman method [20]. The method has been used to measure the cholinesterase activity in the blood and tissues of humans and animals [21]. One of the colorimetric methods depends on Thiocholine, where the decomposition of acetylthiocholine via DTNB reaction (dithionitrobenzoate) would produce 5-thio2-nitrobenzoate. [22] argued that this method was simple and could be replicated. By using Ellman method, the Cholinesterase activity has been measured by [23,24]. In spite of the fact that this method is extensively used, the experimental results are inconsistent [22] due to the variations in experimental conditions [25,26]. Electrometric (modified Michel) method can be used to measure the cholinesterase activity as well, by degrading acetylcholine into choline and acetic acid (using cholinesterase). This method was adopted by Michel [27] to measure the cholinesterase activity in blood plasma and red blood cells in humans.
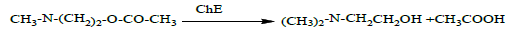
The low pH condition observed in reaction solution is attributed to the separation of hydrogen atoms from acetic acid as shown in the following reaction:

This method works based on the change in pH at constant temperature (25 C°) after a specific reaction time (i.e. 1 hour). This change represents the effectiveness of Cholinesterase (Δ pH / hour). Operating temperature affects the rate of reaction as well. The temperature change of 1°C results in 5.5% and 3% changes in the cholinesterase activities within plasma and red blood cells, respectively [28]. Since the basic Michael method is mainly used to measure the cholinesterase activity in humans, this method has been modified and applied in different animals [29,30]. The cholinesterase activities in blood plasma and red blood cells vary among animals. For example, cholinesterase is more active in blood plasmas of horses and mice than those of goats and sheep. An improved Michael method has been proposed to measure the cholinesterase activities in various animals [31,32]. [33] applied the improved Michael method to measure the cholinesterase activities in the bloods of cows, sheep and goats. The modification involved increasing the temperature to (37°C). The number blood samples were increased and the incubation period was ranging between 15-45 minutes. [29] adjusted the sample size to make the method suitable for rapid laboratory work. Their method has been adopted in studying the cholinesterase activity in sheep [34], mice [35] and rabbits [36]. Other modifications include the shortening of measurement period [31]. This modification was applied in rats [34], whereby Acetylthiocholine was used as a substrate [35]. According to [37], the electromechanical method is generally reliable, accurate, simple and does not require complex devices (requires only Memmert and pH-meter).
Materials and Methods
In this study, we used local rabbits (buck and doe), i.e. Lepus cuniculua domestica aged between 3-4 months. Their weights ranged from 800 g to 1200 g, which were available from the local markets in Kirkuk city. The animals were kept in a room for 20 days at temperature ranging from 27°C to 35°C and humidity level ranging from 25% to 30%. The room was subjected to 12 light hours per day and the animals were housed in 80 x 80 x 80 cm cages for breeding. The rabbits were provided by the Faculty of Veterinary Medicine / University of Kirkuk and the cages were cleaned and sterilized twice a week. The floor of the cages was sprayed with wood sawdust and foods were given to the rabbits (i.e. Corn 25%, animal protein 10%, dried milk 10%, food salt 1%) [38]. The foods were put in special pots attached to the cage walls in order to prevent contamination by wood splitting. In this study, sodium heparin solution (5000 IU/ml), DTNB (Dithio nitrobenzoate), sodium barbital, potassium phosphate hydrogen and acetylcholine iodide 99.9% were used as substrates in the Michael method. On the other hand, acetylthiocholine iodide, sodium chloride and sodium bicarbonate were used as substrates in Ellman method.
Samples Collection and preparation
Blood samples were collected from the ear veins of rabbits. The heparin anticoagulation (1:10 dilution) was used to rinse the glass test tubes before the blood samples were collected. Blood plasma was separated by a centrifuge operating at 3,000 rpm for 15 minutes. After separating the plasma, they were transferred to the dry and clean glass tubes for testing purpose. Subsequently, rabbit brains were extracted and placed inside some dry and clean plastic bags. The brain tissue was homogenized in a phosphatebuffered solution (pH = 8.1; concentration is 3 ml / 100 mg of tissue weight) for one minute by using a gynecological device. Samples were kept in glass test tubes for testing purpose and the test tubes were kept in crushed ice.
Enzyme measurement
Michel Method
This method involves the following steps.
1. Place 3 mL of distilled water in a glass container of capacity 10 mL.
2. Add 0.2 mL of plasma sample or tissue homogenize.
3. Add 3 mL of phosphate solution. The pH was measured by using a pH meter (pH-1).
4. Add 0.12 mL of acetylcholine iodide solution of 7.5%.
5. Transfer the mixture to exact water bath at 37°C and incubate for 30 min.
6. Measure the pH values (pH-2) on the mixtures after the mixtures are removed from the incubator.
7. Calculate the difference between pH-1 and pH-2, i.e. the change of pH within 30mins. The difference indicates the level of cholinesterase activity in the sample [39,40], i.e.
ΔpH/30 min=pH1-pH2-(pH of blank*)
*Blank contains all solutions except plasma or tissue sample.
Ellman method
This method can be accomplished via using spectrophotometer. Acetylthiocholine iodide was used as substrate (1 mM final concentration of acetylthiocholine iodide) for measuring cholinesterase activities. Substrate solutions were prepared and used on the same day (with ice). This method involves the following steps:
1) Measure the enzyme by using spectrophotometer. The measurement time is 5 min and the working temperature is fixed at 25°C. In each case, the rate of absorbance increase can be corrected by subtracting the rate observed in a reagent blank (i.e. without sample).
2) Calculate the cholinesterase activities by using an extinction coefficient of 13.6 mM−1 cm−1 for 5-thio-2- nitrobenzoate [41,42].
3) For plasma: Cholinesterase activity=(Change in absorption × Micromol × Reaction size ml)/ (Min × 13.6 × Sample size ml.)=Micro mol/min/ml.
4) For brain: Cholinesterase activity=(Change in absorption x Micromol x Reaction size ml)/ (Min × 13.6 × Sample size ml × tissue weight gm.)=Micro mol/min/g.
Experiments
For the purpose of standardizing the electrometric and Ellman methods in measuring the cholinesterase activity and demonstrating their efficiencies and legitimacies in measuring the inhibitory activity, various experiments were conducted. These measurements were conducted on blood plasma and brain due to the major reliance on models in the studies of cholinesterase inhibitors [43].
Measure the cholinesterase activity in blood plasma and brain tissue
Blood samples (plasma) and brain tissues were collected from 8 rabbits in order to measure the cholinesterase activity. The normal rate was calculated and other statistical data [39] related to the cholinesterase activity (e.g. standard error, standard deviation, 95% confidence interval and range) were measured using Michael and Ellman methods.
Determination of the measurement of cholinesterase activity in blood plasma and brain tissue
Blood plasma samples and brain tissue were collected from 5 rabbits. The samples were mixed separately in order to obtain plasma and brain pools. Cholinesterase activity was measured in 11 Aliquot parts of plasma and brain tissue. The coefficient of variation can be written as [39,44]:
Coefficient of variation=(Standard deviation/rate) × 100.
Statistical analysis
The results were analyzed using the Sigma Plot program to obtain the required statistical variables, regression equation as well as correlation coefficients of various measurement methods and samples. In the case of two groups, the test results were analyzed via Student’s-t-test [45].
Results
Measure the cholinesterase activity in blood plasma and brain tissue
The individual values, the mean and the standard error, the 95 % confidence interval as well as the rest of the statistical data of the cholinesterase activity in the blood plasma and the brain of eight rabbits and on the basis of the reaction size 0.2 ml for the modified Michael method and 20 mL for the Ellman method and the highest cholinesterase activity (0.37) and in the brain (0.25) for the modified Michael method (Table 1). From, the highest cholinesterase activities (based on the value of change in absorption/min) in plasma and brain are 0.89 and 0.43, respectively, when the Ellman method is used.
| Samples | (ΔpH/30 min) Michel method | (ΔAbsorbance/min) Ellman method | ||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| 1 | 0.22 | 0.07 | 0.55 | 0.12 |
| 2 | 0.30 | 0.08 | 0.56 | 0.42 |
| 3 | 0.37 | 0.25 | 0.57 | 0.19 |
| 4 | 0.37 | 0.18 | 0.69 | 0.32 |
| 5 | 0.32 | 0.21 | 0.64 | 0.14 |
| 6 | 0.30 | 0.15 | 0.48 | 0.43 |
| 7 | 0.35 | 0.13 | 0.89 | 0.20 |
| 8 | 0.29 | 0.14 | 0.77 | 0.17 |
| Mean | 0.315 | 0.151 | 0.644 | 0.215 |
| SE | 0.0176 | 0.0217 | 0.0476 | 0.0433 |
| SD | 0.0499 | 0.0613 | 0.135 | 0.122 |
| Range | 0.15 | 0.18 | 0.410 | 0.31 |
| Con. interval | 0.0416 | 0.0512 | 0.1125 | 0.1023 |
Table 1: Cholinesterase activity in plasma and brain in rabbits measured in modified Michael and Elman methods.
Determination accuracy of measurement of the activity of cholinesterase activity in plasma and brain in rabbits by using modified Michel and Ellman methods
The coefficients of variation were found to be 17.78 % and 19.37 % in plasma and brain, respectively, when Michel method was used (see Table 2), Meanwhile, for Ellman method, the coefficients of variation in plasma and brain were 21.72 % and 41.21 %, respectively (see Table 3).
| Samples | (Δ pH/30 min) Michel method | (Δ Absorbance/min) Ellman method | ||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| 1 | 0.22 | 0.07 | 0.55 | 0.12 |
| 2 | 0.30 | 0.08 | 0.56 | 0.42 |
| 3 | 0.37 | 0.25 | 0.57 | 0.19 |
| 4 | 0.37 | 0.18 | 0.69 | 0.32 |
| 5 | 0.32 | 0.21 | 0.64 | 0.14 |
| 6 | 0.30 | 0.15 | 0.48 | 0.43 |
| 7 | 0.35 | 0.13 | 0.89 | 0.20 |
| 8 | 0.29 | 0.14 | 0.77 | 0.17 |
| Mean | 0.315 | 0.151 | 0.644 | 0.215 |
| SE | 0.0176 | 0.0217 | 0.0476 | 0.0433 |
| SD | 0.0499 | 0.0613 | 0.135 | 0.122 |
| Range | 0.15 | 0.18 | 0.410 | 0.31 |
| Con. interval | 0.0416 | 0.0512 | 0.1125 | 0.1023 |
Table 2: Accuracy of measurement of cholinesterase activity when using Michel method (? pH/30 min) in blood plasma and brain in rabbits.
| Sample | Number of models | Mean | St. Deviation | Coefficient variation % |
|---|---|---|---|---|
| Plasma | 11 | 0.21 | 0.0363 | 17.78 |
| Brain | 11 | 0.191 | 0.037 | 19.37 |
| Plasma | 11 | 0.418 | 0.0908 | 21.72 |
| Brain | 11 | 0.296 | 0.122 | 41.21 |
Table 3: Accuracy of measurement of cholinesterase activity using Ellman method. (Δ Absorbance/min) in blood plasma and brain in rabbits.
Regression analysis
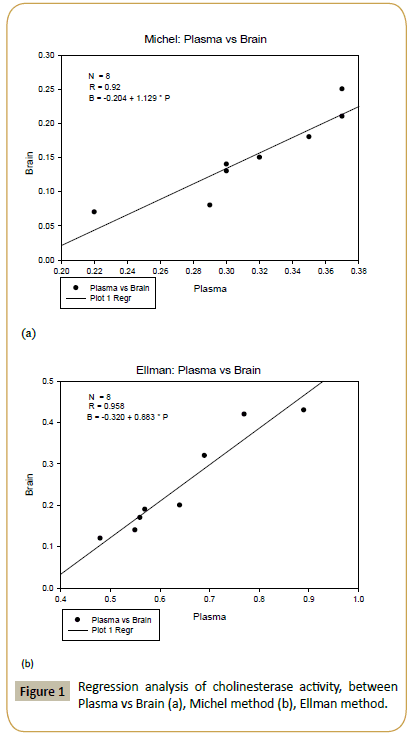
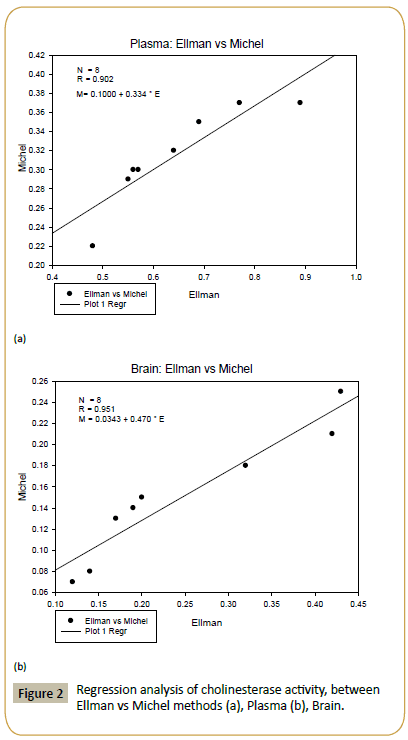
In the case of Michel method, the correlation coefficient is R=0.92 (see Figure 1a). However, for Ellman method, the correlation coefficient is higher, i.e. R=0.958 (see Figure 1b). The correlation coefficient between the modified Michael method and the Ellman method can be determined as well. From Figure 2a, the correlation coefficient in the plasma samples is R=0.902. For brain samples, the correlation coefficient is R=0.951 (see Figure 2b).
Figure 1: Regression analysis of cholinesterase activity, between Plasma vs Brain (a), Michel method (b), Ellman method.
Discussion
The Cholinesterase activities in blood, tissue and brain can be readily measured. Measuring the cholinesterase activities in blood and tissue is useful in determining the exposure status of cholinesterase inhibitors and detecting the toxicity of these compounds [46].
For Michael method, a water bath and a pH meter are required. On the other hand, for Ellman method, one requires a water bath and a spectrophotometer [47]. The modified Michael method was used in animals because the original method was used to measure the cholinesterase activity in humans [31]. Many variations of Michael method have been proposed, which differ in terms of buffer solution, strength, sample size, incubation time and operating temperature [33,47]. Most measurements in the current study were performed by using Michael's modified method and Ellman method.
In the first experiment, we found that the highest cholinesterase activities (i.e. Δ pH / 30 min using Michael method) in plasma and brain are 0.37 and 0.25, respectively (see Table 1). The measurements were taken based on the volume involved in the reaction mixture of plasma samples (i.e. 0.2 ml), In the brain, the use of a fixed size of 0.2 ml placenta taken from (100 mg tissue/3 ml of buffer phosphates is 8.1 pH). For the Ellman method, the highest cholinesterase activities in plasma and brain are 0.89 and 0.43, respectively. on the basis of the volume involved in the reaction (20 mL plasma) and the brain homogenization 20 mL taken from (100 mg brain tissue / 3 ml of buffer phosphates is pH 8.1. The difference in cholinesterase activities within plasma and brain represents the fundamental and natural variations in cholinesterase activities within different animals [48]. The coefficients of variation of the modified Michael method are 17.78% and 19.37% for plasma and brain, respectively (see Table 2). Meanwhile, for Ellman method, the coefficients of variation are 21.72% and 41.21% for plasma and brain, respectively (see Table 2). In general, coefficient of variation represents the intralaboratory measurement accuracy [49]. The correlation between the plasma and brain samples in the modified Michael method is R=0.92 (Figure 1a). For the Ellman method, the correlation coefficient between the plasma and brain samples is R=0.958 (Figure 1b). From Figure 2a, the correlation coefficient between the modified Michael and Ellman methods applied in the plasma samples is R=0.902. For the brain samples, the correlation coefficient is R=0.951 (see Figure 2b).
Conclusions
Cholinesterase activity can be measured in blood plasma and brain easily by using the modified Michael method and the Ellman method. Both methods are efficient in measuring the cholinesterase activity. The efficiencies of both methods have been determined from the coefficients of variation in plasma and brain. The results are acceptable, indicating the efficiency and accuracy of both methods. By using the modified Michael method, the correlation between plasma and brain samples is R=0.92. For the Ellman method, the correlation coefficient between plasma and brain samples is R=0.958. Also, the correlation coefficient between the modified Michael method and the Ellman method applied in the plasma samples is R=0.902. In the brain samples, the correlation coefficient is R=0.951, which is very high. Therefore, both methods are accurate and efficient in measuring the cholinesterase activity.
Acknowledgments
The authors are grateful to the College of Veterinary Medicine, University of Kirkuk for providing the laboratory, cages and animals. In addition, they would like to thank Professor Dr. Najat Ali, Dr. Kamal Salih, and Dr. Adell Hayder for their scientific advices and facilities.
References
- Koelle GB (2013) Cholinesterases and anticholinesterase agents. Springer Science & Business Media, p: 15.
- Gerebtzoff MA (2013) Cholinesterases: a histochemical contribution to the solution of some functional problems. Elsevier, p: 3.
- Tse YC, Sharp CR, Evans T (2013) Mechanical ventilation in a dog with acetylcholinesterase inhibitor toxicosis. Journal of Veterinary Emergency and Critical Care 23: 442-446.
- Caloni F, Cortinovis C, Rivolta M, Davanzo F (2016) Suspected poisoning of domestic animals by pesticides. Science of The Total Environment 539: 331-336.
- Oz M, Petroianu G, Lorke DE (2016) α7-nicotinic acetylcholine receptors: new therapeutic avenues in Alzheimer’s disease. Nicotinic Acetylcholine Receptor Technologies, pp: 149-169.
- Bacalhau P, Marques C, Martins MR, Caldeira AT, Burke A (2014) The role of acetylcholinesterase in Alzheimer’s disease. Enzymatic Inhibition Studies 2: 4-10.
- Oda E (2015) Associations between serum cholinesterase and incident hyper LDL cholesterolemia, hypertriglyceridemia and hypo-HDL cholesterolemia as well as changes in lipid levels in a health screening population. Atherosclerosis 241: 1-5.
- Tvarijonaviciute A, Tecles F, Ceron JJ (2010) Relationship between serum butyrylcholinesterase and obesity in dogs: a preliminary report. The Veterinary Journal 186: 197-200.
- Casida JE, Quistad GB (2005) Quistad, Serine hydrolase targets of organophosphorus toxicants. Chemico-Biological Interactions 157: 277-283.
- Wilson BW, Henderson JD, Ramirez Al, Malley MA (2002) Standardization of clinical cholinesterase measurements. International Journal of Toxicology 21: 385-388.
- Lu Y, Pang YP, Park Y, Gao X, Yao J, et al. (2012) Genome organization, phylogenies, expression patterns, and three-dimensional protein models of two acetylcholinesterase genes from the red flour beetle. PLoS ONE 7: 32288.
- Holmes RS, Cox LA, VandeBerg JL (2009) A new class of mammalian carboxylesterase CES6. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 4: 209-217.
- Fujii T, Takada TY, Kawashima K (2008) Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. Journal of Pharmacological Sciences 106: 186-192.
- Nunes B (2011) The use of cholinesterases in ecotoxicology. In: Reviews of Environmental Contamination and Toxicology 212: 29-59.
- Wang HP, Liang YJ, Sun YJ, Hou WY, Chen JX, et al. (2014) Subchronic neurotoxicity of chlorpyrifos, carbaryl, and their combination in rats. Environmental Toxicology 29: 1193-1200.
- Gunduz A, Kalkan A, Turedi S, Durmus I, Turkmen S, et al. (2012) Pseudocholinesterase levels are not decreased in grayanotoxin (mad honey) poisoning in most patients. The Journal of Emergency Medicine 43: 1008-1013.
- Pezzementi L, Chatonnet A (2010) Evolution of cholinesterases in the animal kingdom. Chemico-biological Interactions 187: 27-33.
- Romano D, Bonomi F, de Mattos MC, de Sousa Fonseca T, de Oliveira MF, et al. (2015) Esterases as stereoselective biocatalysts. Biotechnology Advances 33: 547-565.
- Tsim K, Soreq H (2013) Acetylcholinesterase: old questions and new developments. Acetylcholinesterase: Old Questions and New Developments, p: 4.
- Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7: 88-95.
- Doctor B, da Silva LC (1987) Microtiter assay for acetylcholinesterase. Analytical Biochemistry 166: 399-403.
- Fairbrother A, Marden BT, Bennett JK, Hooper MJ (1991) Methods used in determination of cholinesterase activity. Chemicals in Agriculture 2: 35-72.
- Šinko G, Čalić M, Bosak A, Kovarik Z (2007) Limitation of the Ellman method: Cholinesterase activity measurement in the presence of oximes. Analytical Biochemistry 370: 223-227.
- Askar KA, Kudi AC, Moody AJ (2011) Comparative analysis of cholinesterase activities in food animals using modified Ellman and Michel assays. Canadian Journal of Veterinary Research 75: 261-270.
- Adak A, Eswarappa R, Nagarajan S, Mukhopadhayay SK (2015) Validation of Cholinesterase (Acetyl and Butyryl) Activity Estimation in the Blood and Brain of Wistar Rats. JSRST 4: 255-259.
- Wilson BW (1996) Factors in standardizing automated cholinesterase assays. Journal of Toxicology and Environmental Health Part A 48: 187-196.
- Michel HO (1949) An electrometric method for the determination of red blood cell and plasma cholinesterase activity. Journal of Laboratory and Clinical Medicine 34: 1564-1568.
- Ellin RI, Vicario PP (1975) ΔpH Method for Measuring Blood Cholinesterase: A Study on the Effect of Temperature. Archives of Environmental Health: An International Journal 30: 263-265.
- Mohammad F, Omer VS (1982) Modifications of Michel's electrometric method for rapid measurement of blood cholinesterase activity in animals: a minireview. Veterinary and Human Toxicology 24: 119.
- Wills J, Dubois KP (1972) The measurement and significance of changes in the cholinesterase activities of erythrocytes and plasma in man and animals. CRC Critical Reviews in Toxicology 1: 153-202.
- Mohammad F, Faris G, Al-Kassim N (1997) A modified electrometric method for measurement of erythrocyte acetylcholinesterase activity in sheep. Veterinary and Human Toxicology 39: 337-339.
- Ecobichon D, Comeau A (1973) Pseudocholinesterases of mammalian plasma: physicochemical properties and organophosphate inhibition in eleven species. Toxicology and Applied Pharmacology 24: 92-100.
- Silvestri R (1977) New techniques to measure blood cholinesterase activity in domesticated animals. American Journal of Veterinary Research 38: 659-662.
- Mohammad F, Al-Baggou BK, Alias AS, Faris GA (2006) Application of an electrometric method for measurement of in vitro inhibition of blood cholinesterases from sheep, goats and cattle by dichlorvos and carbaryl. Veterinarni Medicina Praha 51: 45.
- Faris G, Mohammad F (1996) Cholinesterase inhibition by dichlorvos and diphenhydramine in mice. Iraqi Journal of Veterinary Sciences 9: 7-13.
- Al-Hashimi M, Mohammad F (2000) Toxicity and interaction of diminazene in rabbits. Iraqi Journal of Veterinary Sciences 13: 13-21.
- Naik RS, Liu W, Saxena A (2013) Development and validation of a simple assay for the determination of cholinesterase activity in whole blood of laboratory animals. Journal of Applied Toxicology 33: 290-300.
- Cunha TJ, Cheeke PR (2012) Rabbit feeding and nutrition. Elsevier 21: 80-86.
- Abass KS (2004) Validation of an electrometric method for cholinesterase measurement in the plasma and tissues of the chicken. Proceedings of the 11th Scientific Congress, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt, pp: 241-259.
- Horowitz IH, Yanco EG, Landau S, Nadler Valency R, Anglister N, et al. (2016) Whole blood cholinesterase activity in 20 species of wild birds. Journal of Avian Medicine and Surgery 30: 122-126.
- Dingova D, Leroy J, Check A, Garaj V, Krejci E, et al. (2014) Optimal detection of cholinesterase activity in biological samples: Modifications to the standard Ellman s assay. Analytical Biochemistry 462: 67-75.
- Abass KS (2014) A method for fast assessment of OP/CB exposure in the Japanese quail (Coturnix coturnix japonica) using combined esterases enzyme activity as biomarkers. Enzyme Research, pp: 1-15.
- Aurbek N, Thiermann H, Szinicz L, Eyer P, Worek F (2006) Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase. Toxicology 224: 91-99.
- Al-Jobory MMH, Mohammad FK (2004) A pH method for measuring blood cholinesterase activity in goats. in Abstract Book of the 12th Congress of Mediterranean Federation for Health and Production of Ruminants, Istanbul, Turkey. Citeseer 75: 16-19.
- Lenglet C, Rousson M, Deriche R, Faugeras O (2006) Statistics on the manifold of multivariate normal distributions: Theory and application to diffusion tensor MRI processing. Journal of Mathematical Imaging and Vision 25: 423-444.
- Choi JY, Yu J, Yang DB, Ra K, Kim KT, et al. (2011) Acetylthiocholine (ATC) cleaving cholinesterase (ChE) activity as a potential biomarker of pesticide exposure in the Manila clam, Ruditapes philippinarum, of Korea. Marine Environmental Research 71: 162-168.
- Mohammad FK, Bhattacharyya H, Fazili M, Nasreen S, Jeelani SG, et al. (2007) Review of a practical electrometric method for determination of blood and tissue cholinesterase activities in animals. Feedback 2: 16.
- Farage-Elawar M (1991) Development of esterase activities in the chicken before and after hatching. Neurotoxicology and Teratology 13: 147-152.
- Taverniers I, de Loose M, Van Bockstaele E (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends in Analytical Chemistry 23: 535-552.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences