ISSN : 0976-8505
Der Chemica Sinica
Prospects Chromatography-Mass Spectrometry Analysis Flavonoids Plant Extracts for Identification and Quantification of the Individual Components
Oshchepkova Yu I*, Kholmuradov ÃÂÂœK, Mamadrakhimov ÃÂÂÂÃÂÂÂ, Salakhutdinova ÃÂÂœK, Sulranova EM and Salikhov Sh I
Institute of Bioorganic Chemistry, Academician A.S.Sadykov, Uzbek Academy of Sciences, Tashkent, Uzbekistan
Abstract
Obtained extracts from the seeds of celery Apium graveolens, fenugreek Trigonélla foénum-graécum, Vitis vinifera grapes and leaves clover Trifolium and using chromatography-mass spectrometry methods in extracts of several plants identified the main flavonoids
Keywords
Celery Apium graveolens, Trigonélla foénum-graécum, Vitis vinifera, Clover Trifolium, Chromatography-mass spectrometry analysis, Flavonoids, Aglycones.
Introduction
In recent years, there has been an increased interest in medicinal products derived from plant material. Increasing requirements to the quality of medicines caused the need to develop more advanced methods and techniques for standardizing phytopreparations.
This problem can be solved with the use of modern physico-chemical methods for analyzing the main classes of active substances that make up the finished dosage form, such as flavonoids, anthocyanins, saponins, organic acids. The problem of qualitative and quantitative determination of all components in complex phytopreparations is quite a complex task both from the point of view of the choice of the analytical method and its instrumental design.
Medicinal plants containing flavonoids, due to their large structural diversity and biological activity are one of the most promising sources of phytopreparations [1,2].
Experimental and clinical studies have revealed their antioxidant, cytoprotective, hepatoprotective, antihypoxic and many other effects [3,4]. There is no single method of quantitative analysis for all flavonoids. At present, physicochemical methods of analysis, such as spectrophotometry, absorption spectroscopy, are widely used to identify and quantify flavonoids in medicines, where quercetin flavonoid is used as a standard. The most sensitive and informative method is chromatography-mass spectrometry, based on a combination of two independent methodschromatography and mass spectrometry [5,6].
Materials and Methods
Preparation of extracts
Degreased seeds of celery Apium graveolens, fenugreek Trigonélla foénum-graécum, grapes Vitis vinifera and leaves of Trifolium clover were crushed, and the extracts were obtained in the ratio "raw extractant" (1:1) using 70% ethanol as an extractant. The technology for obtaining thick extracts was carried out in accordance with the requirements of the general pharmacopoeia article "Extracts" [7].
Chromatography
The analysis was carried out using an Agilent Technologies-1260 liquid chromatograph equipped with a 6420 Triple Quad LC/MS mass spectrometer (Agilent Technologies, USA).
Separation of the extracts occurred on a column with a reverse phase of Eclipse XDB (2.1 × 150 mm) under the following conditions: mobile phase: component A-water (0.05% solution of formic acid); Component B-acetonitrile and C-isopropanol in a gradient of 0 min 18% B+2% C, 15 min 72% B+8% C, 18 min 18% B+2% C. The elution rate is 0.25 ml/min. The composition of the mobile phase at the exit from the chromatographic column was recorded by mass spectrometry. Chromatograms were recorded with negative ionization. The parameters of the mass spectrometer were chosen as follows: the scanning range was 100-2200 m/z, the gas flow of the desiccant was 3 L/min, the gas temperature was 3000C, the gas pressure on the needle was 20 psi, the evaporator temperature was 3000C, and the capillary voltage was 4000 V. The analysis was carried out on the day of preparation of the extraction to prevent the oxidation of flavonoids.
Sample preparation
Preparation of solutions of GSO Rutin, Quercetin, Catechin, Gallocatechin, Luteolin: about 0.05 g (accurate sample) of the preparation, previously dried at 130-13500C for 3 hours, is quantitatively transferred to a 50 cm3 volumetric flask, dissolved in 40 ml of 70% alcohol when heated in a water bath. After dissolution, the contents of the flask are cooled to room temperature and adjusted to 70% with alcohol to a mark.
Results and Discussion
One of the most common and numerous classes of secondary plant metabolites is phenolic compounds. A large group of natural phenolic compounds are phenylpropanoids I-V, containing in the structure one or more C6-C3 fragments (anthocyanins, flavonoids and their derivatives - lignans, flavolignanes) [8,9]. These compounds have recently become the subject of close attention of researchers in the search for promising biologically active compounds and the creation on their basis of biologically active drugs [10].
In recent years in the world of phytotherapy, there has been a trend towards a wider use of multicomponent herbal medicines. The currently available techniques for analyzing the aforementioned phytopreparations mostly do not meet modern pharmacopoeial requirements, since they are not specific; do not allow identification and quantification of the individual components of the mixture.
In European and British pharmacopoeias, qualitative analysis involves the use of standard substances of quercetin, hyperoside and routine. However, approaches to determining the authenticity of seeds, fruits and preparations of plant raw materials require, in our opinion, adjustments and widening of the spectrum of standard flavonoids. Based on the results of our research, it is possible to develop methodological and methodological approaches to standardizing raw medicinal plants containing flavonoids, as well as proposing changes to existing regulatory documentation and developing new normative documentation for medicinal raw materials, medicinal substances and preparations [13,14].
To determine the individual and group composition of low molecular weight organic substances of natural origin, an approach is proposed based on the analysis of chromatographic profiles obtained under standardized conditions and spectral characteristics of the main components (retention times, UV and mass spectra, UV spectral ratios) [15].
We performed a chromatography-mass spectrometric analysis of flavonoids of plant extracts obtained from seeds of grapes, celery, fenugreek and clover leaves.
Based on the grape seed extract, Endotelon preparations are available-Venotonic, Angioprotective and Revenol antioxidant. Grape seed extract contains a significant amount of proanthocyanins, which provides its ability to strengthen capillaries and allows use in various circulatory disorders, including cerebral circulation disorders. Grape seed antioxidants protect the core of the cell-the storehouse of genetic material from the damaging effects of free radicals.
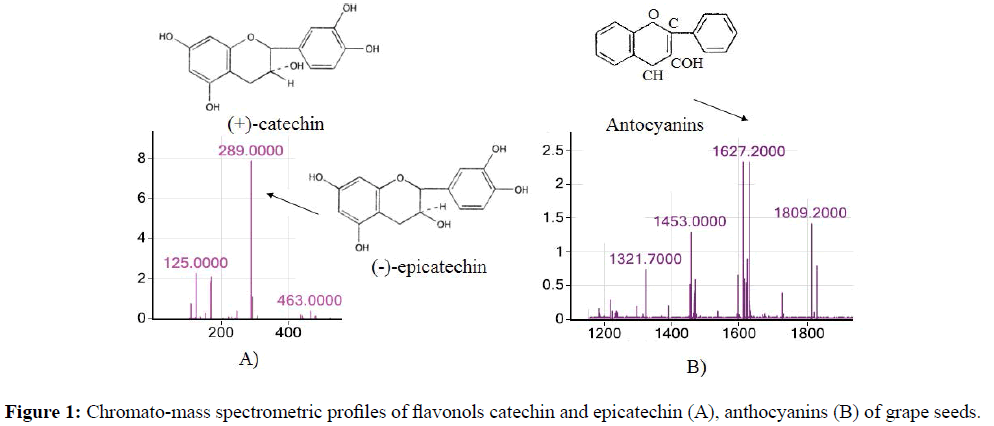
In the mass spectrum of the grape seed extract fraction, the most intense ion peak m/z ion=289.0, corresponding to the flavonols catechin and epicatechin (Figure 1A), are stereoisomers.
The presence of other less intense characteristic ion peaks m/z=1453.0, 1627.2 and 1809.2 indicates the presence of anthocyanins in minor amounts (Figure 1B).
On the basis of celery seeds, a complex agent of plant origin, Nephrokea, is produced, which promotes the elimination of toxins, which prevents the formation of kidney stones and has antibacterial and antioxidant properties.
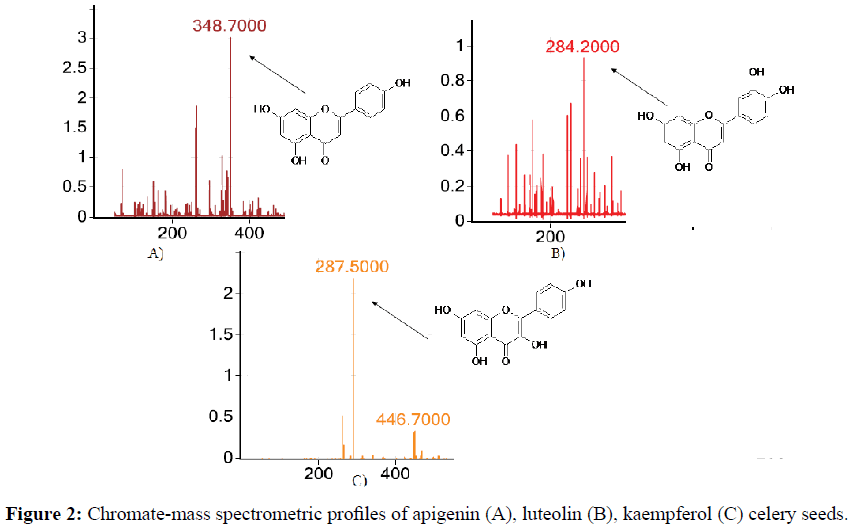
In the mass spectrum of the celery extract fraction, the most intense ion peak is m/z of the ion=348.7, corresponding to aglycon apigenin (Figure 2A). The presence of other less intense characteristic peaks of ions m/z=284.2 indicates the presence of luteolin in the minor quantities (Figure 2B) and the ion m/z ion=287.5, corresponding to kaempferol, and also the less pronounced ion peak m/z ion=446.7, Corresponding to the glycosidic structure in the form of a sodium form (Figure 2C). The data presented allowed us to characterize the substance as kaempferol-3-glycoside.
Based on red clover isoflavones, there is a non-hormonal drug Feminal for the treatment and prevention of climacteric disorders, effectively eliminating unwanted symptoms of menopause and supporting the health of the cardiovascular and bone systems. Clover isoflavones normalize fat metabolism, reduce blood cholesterol levels, and have strong antioxidant activity.
Red clover is the only naturally occurring source of 4 isoflavones, Biocanin A (Biochanin A), Formononetin (Formononetin), Daidzein, Genistein with the greatest concentration.
Isoflavones of red clover in their structure are closest to estrogens of the human body in comparison with other phytoestrogens. Due to this similarity, the use of red clover isoflavones compensates for the lack of own estrogens and thus can reduce menopausal manifestations.
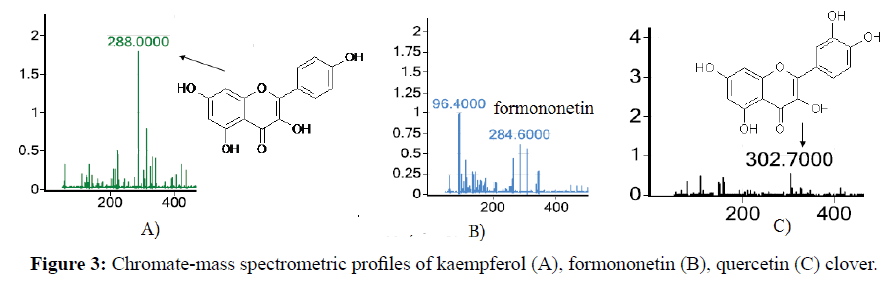
In the mass spectrum of the clover extract fraction, the most intense ion peak m/z ion=288.0, corresponding to kaempferol, is observed (Figure 3A). The presence of other less intense characteristic peaks of ions m/z=284.6 and m/z=303.293 indicates the presence of formononetine (Figure 3B) and quercetin (Figure 3C) in minor quantities, respectively.
Seeds of fenugreek are used to obtain the preparation "Pasenin", which possesses antisclerotic action. Flavonoids of fenugreek seeds act as natural antioxidants in the regulation of lipid peroxidation processes in cells, and polyphenols serve as a trap for free radicals.
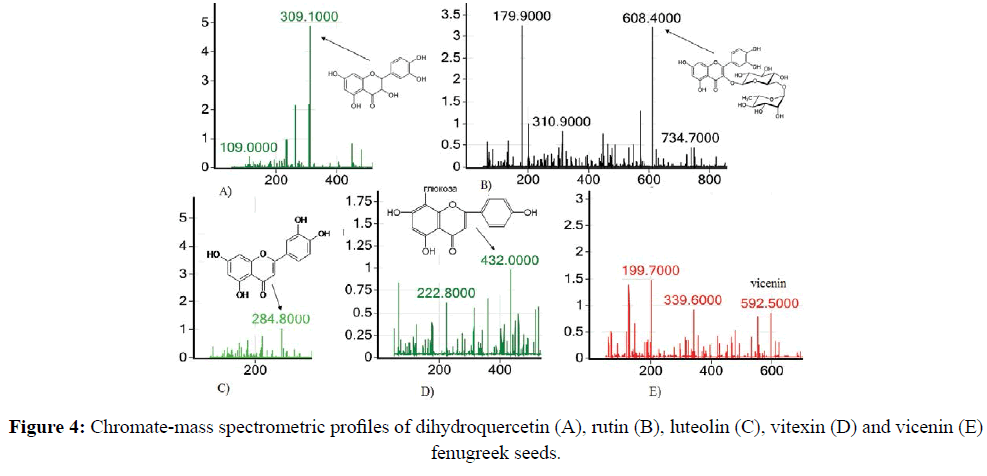
In the mass spectrum of the fenugreek extract fraction, the most intense peaks of ions m/z of the ion=309.1 and m/z of the ion=608.4, corresponding to flavonol dihydroquercetin (Figure 4A) and glycoside of the flavonoid quercetin routine (Figure 4B), respectively. The presence of other less intense characteristic peaks of ions m/z=284.8 indicates the presence of luteolin (Figure 4C) in the minor amounts of the aglycone (Figure 4B), the ion m/z ion=432.0, corresponding to the vitexin (Figure 4D) and the less pronounced ion ion m/z peak=592.5, corresponding to the vicenin (Figure 4E).
Conclusion
Thus, as a result of the study using mass spectrometry methods in extracts of a number of plants, the main flavonoids with therapeutic effects due to their chemical structure were identified. The presence of flavonoids and isoflavonoids in seeds of celery, grapes, fenugreek and leaves of clover meadow opens a wide perspective for the production of medicines for hypocholesterolemic and estrogenic effects. The obtained results can be used as a basis for objective methods of qualitative and quantitative analysis of this group of active substances, represented by flavones, flavonols and aglikons.
References
- Kurkin VA, Kurkina AV, Avdeeva EV (2013) Flavonoids as biologically active compounds of medicinal plants.Fundamental Research 11: 1897-1901.
- GrotewoldE (2006) The Science of flavonoids. Springer Science, New York p.273.
- Sentsov MF, Braslavsky VB, Kurkin VA, Zapesochnaya GG (1996) TheQuantitative estimation of silybin and of total flavolignansin Silybummarianum (L.) fruits. RastResources 32: 100-105.
- Ivashchenko NV, Samylina IA, Lapshikhin AA (2014) Investigation of the polyphenolic complex of russian olive (elaeagnusangustifolia) growing in Russia. Pharmacy7: 16-19.
- Seva DA, Beklemishev MK, Rodin IA, Ikhalainen AA, Antokhin AM, et al. (2015) Profile studies fitoekdisteroidnogo extract serratulacoronata (serratulacoronata) HPLCchromatography coupled with tandem mass spectrometry high resolution. Mass spectrometry12: 177-183.
- Chen G, Zhang H, Ye J (2000) Determination of rutin and quercetin in plants by capillary electrophoresis with electrochemical detection. AnalyticaChimicaActa423: 69-76.
- State Pharmacopoeia of the Union of Soviet Socialist Republics (1990), 11th Edition, Vol. 2. Medicina,Moscow [in Russian].
- Tomas-Barberan FA, Clifford MN (2000)Flavanones, chalcones and dihydrochalcones-Nature, ocurrence and dietary burden. J Sci Food Agr80: 1073-1080.
- Hollman PC, Arts CW (2000)Flavonols, flavons and flavanols-Nature, occurrence and dietary burden. J Sci Food Agr80: 1081-1093.
- Liangli Yu (2008) Wheat antioxidants. A John Wiley & Sons, Hoboken.
- Shaposhnikov AA, Khoroshevsky AY (2003) Isoflavonoids of plants of the family of legumes and their biological effect. Success of Modern Biol123: 76-81.
- Makarenko OA, Levitsky AP (2013) Physiological functions of flavonoids in plants. Physiology and biochemistry of cultivated plants 45: 100-112.
- Sandhar H, Kumar B, Prasher S (2011) A review of phytochemistry and pharmacology of flavonoids. Int Pharm Sci1: 25-41.
- Gudzenko AV (2011) Use of marker substances -modern approach to the standardization of multicomponent herbal medicines. Pharmaceutical Journal 5: 87-91.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences