ISSN : 2573-4466
Insights in Enzyme Research
Lipoprotein Lipase: A General Review
Moacir Couto de Andrade Júnior1,2*
1Post-Graduation Department, Nilton Lins University, Manaus, Amazonas, Brazil
2Department of Food Technology, Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Amazonas, Brazil
- *Corresponding Author:
- MC Andrade Jr
Post-Graduation Department
Nilton Lins University, Manaus, Amazonas, Brazil
Tel: +55 (92) 3633-8028
E-mail: moacircoutjr@gmail.com
Received date: March 07, 2018; Accepted date: April 10, 2018; Published date: April 17, 2018
Citation: Andrade Jr MC (2018) Lipoprotein Lipase: A General Review. Insights Enzyme Res Vol.2 No.1:13. doi:10.21767/2573-4466.100013
Copyright: © 2018 Andrade Jr MC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Carbohydrates (e.g., glucose) and lipids (e.g., free fatty acids or FFAs) are the most important sources of energy for most organisms, including humans. Lipoprotein lipase (LPL) is an extracellular enzyme (EC 3.1.1.34) that is essential in lipoprotein metabolism. LPL is a glycoprotein that is synthesized and secreted in several tissues (e.g., adipose tissue, skeletal muscle, cardiac muscle, and macrophages). At the luminal surface of the vascular endothelium (site of the enzyme action), LPL hydrolyzes triglyceride-rich lipoproteins (e.g., chylomicrons, very lowdensity lipoproteins), providing FFAs and glycerol for tissue use. Therefore, LPL plays a key metabolic role in providing substrates for lipogenesis and lipid storage, and in supplying immediate energy for different tissues. Knowledge about this enzyme has greatly increased over the past decade. A detailed understanding of the fascinating, although complex, apparatus by which LPL exerts its catalytic activity in the turbulent bloodstream is just one of the examples. Additionally, interest in LPL activity has been reinforced by its pathophysiological relevance in chronic degenerative diseases such as dyslipidemia, obesity, type 2 diabetes mellitus, and Alzheimer's disease, and in other contexts of disordered lipid metabolism such as severe hypertriglyceridemia and the (potentially) associated acute pancreatitis as well as in non-alcoholic fatty liver disease. This work aimed at critically reviewing the current knowledge of historical, terminological, biochemical, pathophysiological, and therapeutic aspects of human LPL activity.
Keywords
Diabetes mellitus; Lipid-lowering drugs; Lipogenesis; Lipoprotein lipase (LPL); Obesity; Polyphenols; Starvation
Lipoprotein Lipase: Historical Hallmarks, Enzymatic Activity, Characterization and Present Relevance in Human Pathophysiology and Therapeutics
Macheboeuf, in 1929, first described chemical procedures for the isolation of a plasma protein fraction that was very rich in lipids but readily soluble in water, such as a lipoprotein [1]. In 1943, Hahn reported, the clearing of severe alimentary lipemia in dogs after transfusion of heparin-containing blood [1-3]. Korn, in 1955, isolated an enzyme from normal rat heart and considered it to be a clearing factor because it effectively hydrolyzed chylomicron triacylglycerol or triglyceride (TG), and he named it lipoprotein lipase (LPL) [3-5]. The first cases of LPL deficiency were described in 1960 by Havel and Gordon [6,7]. LPL deficiency is a rare inherited disease that is characterized by severe hypertriglyceridemia, chylomicronemia, and the risk of recurrent pancreatitis, among other potential complications [8]. Another important step towards understanding LPL activity came with the discovery, in 1970, of apoprotein C2, an obligatory cofactor of the enzyme [5,9]. An apoprotein (APO) is the protein moiety of a conjugated protein, or a protein complex (this term is synonym for apolipoprotein, which was originally coined by John Oncley in 1963) [10,11]. In 1970, human LPL was also purified [12].
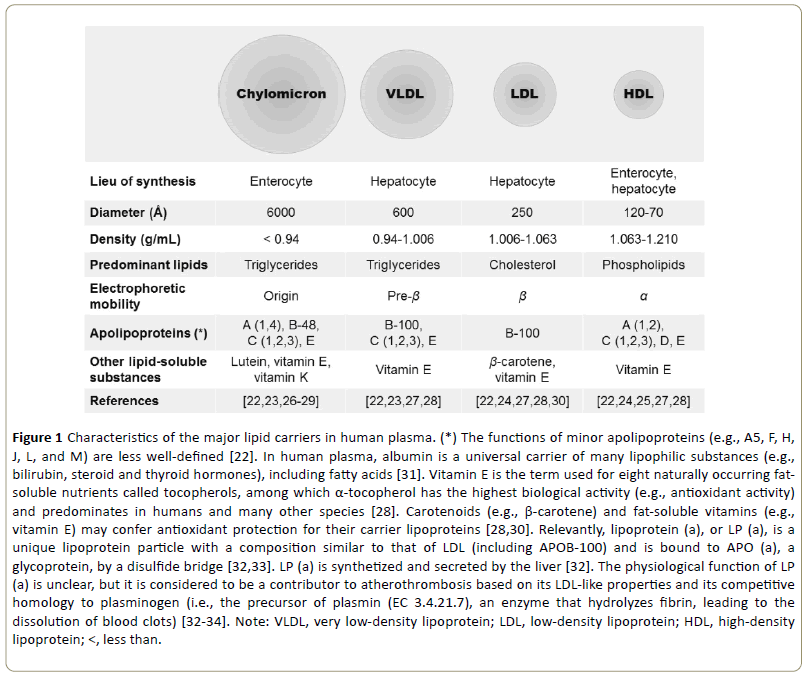
According to the Enzyme Commission (EC) number, LPL is a hydrolase (EC 3) that acts on ester bonds (EC 3.1), and it is characterized as a carboxylic ester hydrolase (EC 3.1.1) of its own (EC 3.1.1.34). Besides chylomicron TG (preferential substrate), LPL (EC 3.1.1.34) also hydrolyses other triglyceriderich lipoproteins (TRLs) in plasma such as very low-density lipoproteins (VLDLs), providing free fatty acids (FFAs) and glycerol for tissue use; experimentally, this was demonstrated by inhibition of the enzyme with antisera that leads to the accumulation of TG in the plasma [13-16]. LPL affects the maturation of several classes of lipoprotein particles [17]. Besides releasing energy-rich lipids such as fatty acids (9 kcal/g) for uptake by tissues, the lipolytic processing also produces atherogenic remnant lipoproteins (e.g., low-density lipoproteins or LDLs) and provides lipid conjugates (i.e., apolipoproteins, phospholipids) for the biogenesis of highdensity lipoproteins (HDLs) [14,18,19]. In 1978, Breckenridge et al. reported the first case of apolipoprotein C2 deficiency [20]. In 1990, Austin coined the term atherogenic lipoprotein profile, which describes the syndrome of small, dense LDL, elevated VLDL, and low HDL (Figure 1) [21].
Figure 1: Characteristics of the major lipid carriers in human plasma. (*) The functions of minor apolipoproteins (e.g., A5, F, H, J, L, and M) are less well-defined [22]. In human plasma, albumin is a universal carrier of many lipophilic substances (e.g., bilirubin, steroid and thyroid hormones), including fatty acids [31]. Vitamin E is the term used for eight naturally occurring fatsoluble nutrients called tocopherols, among which α-tocopherol has the highest biological activity (e.g., antioxidant activity) and predominates in humans and many other species [28]. Carotenoids (e.g., β-carotene) and fat-soluble vitamins (e.g., vitamin E) may confer antioxidant protection for their carrier lipoproteins [28,30]. Relevantly, lipoprotein (a), or LP (a), is a unique lipoprotein particle with a composition similar to that of LDL (including APOB-100) and is bound to APO (a), a glycoprotein, by a disulfide bridge [32,33]. LP (a) is synthetized and secreted by the liver [32]. The physiological function of LP (a) is unclear, but it is considered to be a contributor to atherothrombosis based on its LDL-like properties and its competitive homology to plasminogen (i.e., the precursor of plasmin (EC 3.4.21.7), an enzyme that hydrolyzes fibrin, leading to the dissolution of blood clots) [32-34]. Note: VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; <, less than.
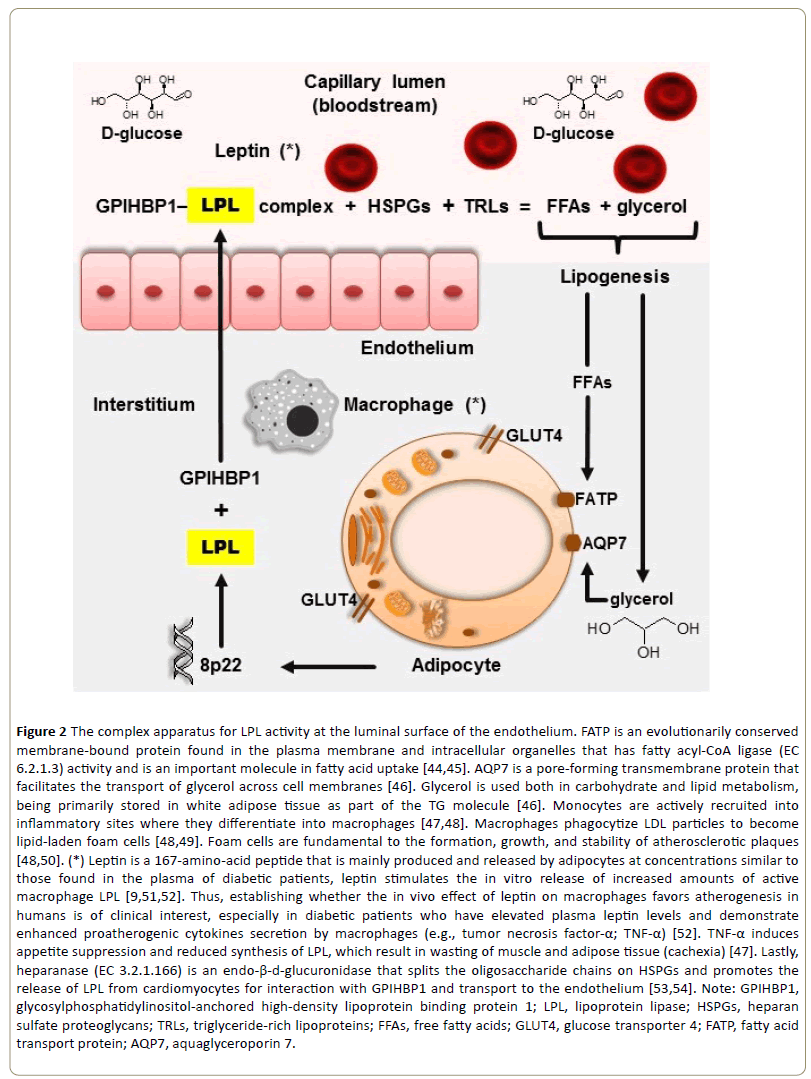
Human LPL was sequenced in 1987 from a complementary deoxyribonucleic acid (cDNA) clone coding for a mature protein of 448 amino acids with a calculated molecular weight of 50,394 [17,35]. Analysis of the sequence indicated that human LPL, hepatic lipase, and pancreatic lipase are members of a gene family [17,36]. The human gene that encodes LPL is located on the short arm of chromosome 8, residing in the p22 region of the same chromosome and containing nine introns and ten exons (or coding regions) [36-38]. LPL is a glycoprotein synthesized and secreted into the interstitial space in several tissues (e.g., adipose tissue, skeletal muscle, cardiac muscle, and macrophages) [36,38-40]. The tissue-specific regulation of LPL is discussed ahead. The enzyme is then bound by glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1), which transports LPL to the capillary lumen, which is the site of the enzyme’s action [39,40]. LPL contains heparin-binding domains that interact with heparan sulfate proteoglycans (HSPGs) and contains lipidbinding sequences that bind TRLs, which bridges capillary HSPGs and circulating TRLs (in a stable multimolecular structure) along the capillary endothelium through the margination process [39]. Unlike heparin, which is only found in mast cells, heparan sulfate is ubiquitously expressed on the cell surface and in the extracellular matrix of all animal cells [41]. However, like heparin, heparan sulphate is a linear polysaccharide consisting of alternating uronic acid and α-(1– 4)-D-glucosamine residues, with the difference of exhibiting a reduced degree of sulphation; nevertheless, heparan sulphate’s high density of negative charges attracts positively charged LPL molecules and holds them by electrostatic and sequence-specific interactions with highly sulfated domains [42,43]. As further discussed in this review, the endothelial location of LPL is strategically important in lipid metabolism (Figure 2).
Figure 2: The complex apparatus for LPL activity at the luminal surface of the endothelium. FATP is an evolutionarily conserved membrane-bound protein found in the plasma membrane and intracellular organelles that has fatty acyl-CoA ligase (EC 6.2.1.3) activity and is an important molecule in fatty acid uptake [44,45]. AQP7 is a pore-forming transmembrane protein that facilitates the transport of glycerol across cell membranes [46]. Glycerol is used both in carbohydrate and lipid metabolism, being primarily stored in white adipose tissue as part of the TG molecule [46]. Monocytes are actively recruited into inflammatory sites where they differentiate into macrophages [47,48]. Macrophages phagocytize LDL particles to become lipid-laden foam cells [48,49]. Foam cells are fundamental to the formation, growth, and stability of atherosclerotic plaques [48,50]. (*) Leptin is a 167-amino-acid peptide that is mainly produced and released by adipocytes at concentrations similar to those found in the plasma of diabetic patients, leptin stimulates the in vitro release of increased amounts of active macrophage LPL [9,51,52]. Thus, establishing whether the in vivo effect of leptin on macrophages favors atherogenesis in humans is of clinical interest, especially in diabetic patients who have elevated plasma leptin levels and demonstrate enhanced proatherogenic cytokines secretion by macrophages (e.g., tumor necrosis factor-α; TNF-α) [52]. TNF-α induces appetite suppression and reduced synthesis of LPL, which result in wasting of muscle and adipose tissue (cachexia) [47]. Lastly, heparanase (EC 3.2.1.166) is an endo-β-d-glucuronidase that splits the oligosaccharide chains on HSPGs and promotes the release of LPL from cardiomyocytes for interaction with GPIHBP1 and transport to the endothelium [53,54]. Note: GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1; LPL, lipoprotein lipase; HSPGs, heparan sulfate proteoglycans; TRLs, triglyceride-rich lipoproteins; FFAs, free fatty acids; GLUT4, glucose transporter 4; FATP, fatty acid transport protein; AQP7, aquaglyceroporin 7.
The incidence (i.e., new cases) of cardiovascular diseases has increased significantly over the past decade, seriously affecting human health and quality of life [55]. This is largely a result of the detrimental impact of risk factors such as dyslipidemia, obesity, and type 2 diabetes mellitus. LPL is an integral part of lipoprotein metabolism and its clinical importance derives chiefly from the role of this metabolic sector in atherogenesis (typically described as the formation of atheromatous lesions in the arterial intima) [9,10]. However, endothelial dysfunction is the primum movens (i.e., the starting point) in the pathogenesis of atherosclerosis because it appears long before clinical symptoms arise, and it is eligible to be a surrogate endpoint for the risk of cardiovascular disease [56]. Additionally, interest in LPL has been reinforced over the past decade by its great pathophysiological relevance in the abovementioned diseases and in other contexts of disordered lipid metabolism such as severe hypertriglyceridemia and the potentially associated acute pancreatitis, as well as in non-alcoholic fatty liver disease (NAFLD) [9]. There has been a renewed interest in the possible role of TG as a marker for the risk of cardiovascular disease [57]. Currently, numerous patients with such chronic degenerative diseases may benefit from effective long-term pharmacological treatments. However, only those drugs that modulate LPL activity (e.g., fibrates, nicotinic acid) were revised here. New non-pharmacologic therapeutic options have been developed, especially in the field of phytomedicine (e.g., phytochemicals such as polyphenols). These and other pertinent aspects (e.g., physiological regulation of LPL activity) have been developed and discussed in this review article.
Metabolic Overview of The Major Lipids in Human Beings and Other Important Aspects of Lipoprotein Lipase Activity
Phospholipids, cholesterol, and triglycerides (TGs) are the major lipids circulating in human blood [58,59]. Phospholipids are fundamental biological building blocks that maintain the proper functioning of plasma and other cellular membranes and are crucial for the survivability of cells and the existence of multicellular life [59]. Phospholipids may be synthesized de novo via the Kennedy pathway (which was named after Eugene Patrick Kennedy (1919–2011) who discovered it more than 50 years ago), and salvaged and recycled in a pathway requiring the mitochondria-associated endoplasmic reticulum membrane [60-62]. As mentioned in Figure 1, the lipid content of HDL is predominantly composed of phospholipids, accounting for 35–50% of the HDL lipids, with phosphatidylcholine as the major species [25].
Cholesterol has no energy value, but it serves as a building block for many important compounds (e.g., steroid hormones, vitamin D, bile acids) and is a component of the outer membranes of all body cells [63]. The amphipathic (or amphiphilic) surface of lipoproteins is composed of unesterified cholesterol, phospholipids, and apolipoproteins while the hydrophobic core of these plasma lipid transport vehicles are composed of TGs and cholesterol esters [28]. Of pathophysiological importance, circulating cholesterol is a major component of atherosclerotic plaques [64]. The liver synthesizes more than 80% of the body’s cholesterol and less than 20% of it comes from food sources (e.g., egg yolk, meat, seafood, butter) [65-67]. The 3-hydroxy-3-methylglutaryl coenzyme A reductase (EC 3.1.3.47; HMG-CoA reductase), is the key enzyme in the mevalonate pathway for cholesterol biosynthesis [68]. This is important because all statins inhibit HMG-CoA reductase by binding to the active site of the enzyme [69]. HMG-CoA reductase inhibitors (or statins) are a group of medications currently used to treat hypercholesterolemia and other dyslipidemias [70,71].
TGs are chemically characterized as esters of three fatty acids and one glycerol molecule [72]. Although in humans most fatty acids come from the diet rather than from de novo synthesis, preferential oxidation of carbohydrates rather than lipids would leave fatty acids available for TG synthesis [73]. Thus, lipogenesis is a broad term that may be defined as the fatty acid synthesis, including the de novo synthesis, and the subsequent conversion of fatty acids to TGs in the liver and adipose tissue, as partially illustrated in Figure 2 [74]. It should be emphasized that the term de novo lipogenesis is reserved for the biochemical process of converting non-lipid precursors (e.g., glucose, fructose, leucine, and isoleucine) into fatty acids for storage as energy [75-78]. Additionally, lipogenesis occurs preferentially in adipose tissue, but it also happens in the liver [79]. Conversely, lipolysis is defined as the hydrolytic cleavage of ester bonds in TGs, resulting in generation of FFAs and glycerol [80]. As discussed previously (Figure 2), LPL requires a complex apparatus to perform TRL hydrolysis in the turbulent bloodstream. In this respect, LPL may be appropriately defined as an extracellular enzyme because its catalytic activity takes place outside the secreting cells [81]. APOA5 is associated with TRLs and enhances TG hydrolysis and remnant lipoprotein clearance [82,83]. However, APOC3 inhibits LPL activity [54,83]. Luminal (or endothelial) LPL is referred to as the functional LPL pool, as it represents the portion of tissue LPL that is actively involved in plasma TG hydrolysis [83]. After LPL hydrolyzes TRLs, sortilin, a member of the vacuolar protein sorting 10 (or VPS10) family, facilitates the uptake of secreted LPL and transfers it to endosomes in parenchymal cells, and LPL ends up in lysosomes for degradation [50,84].
Glycerol is a sugar alcohol that exists naturally in foods and living tissues, and it is constantly being produced by the breakdown of lipids in the gastrointestinal tract and absorbed by the mucosa [85]. When oxidized as an energy substrate, glycerol is converted to carbon dioxide and water, with the concomitant release of 4.32 kcal/g of usable energy [85]. Glyceroneogenesis is defined as de novo synthesis of glycerol-3-phosphate from pyruvate, lactate, and certain amino acids [86]. It is correctly considered an abbreviated version of gluconeogenesis (i.e., glucose synthesis from nonglycosidic substrates) [87]. Glycerol metabolism is closely associated with that of carbohydrates [81,85,87].
Physiological Factors Regulating Lipoprotein Lipase Activity
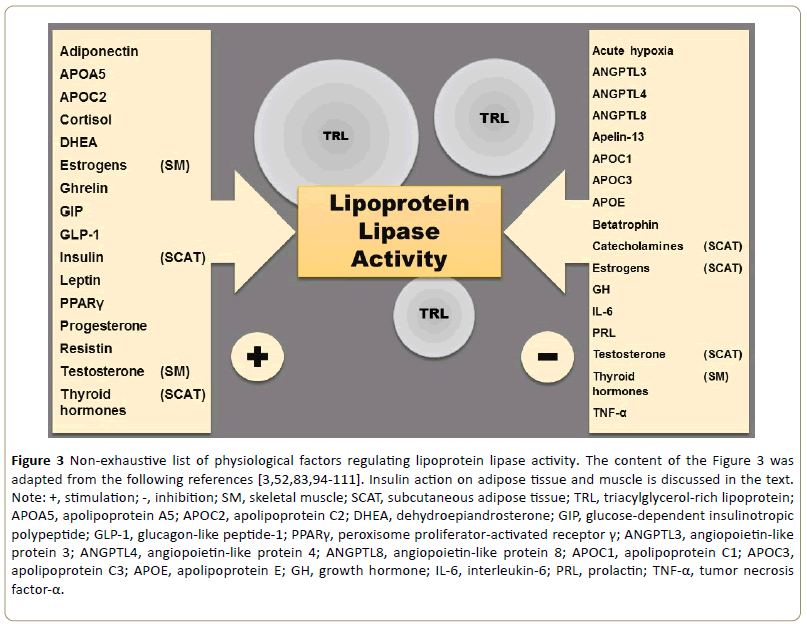
Regulation of LPL activity has so many different features that the adjective multidimensional would be perhaps more appropriate to qualify it [88]. LPL activity is finely regulated by a multitude of factors at the transcriptional, translational, and posttranslational levels [83,89-91]. These are the basic concepts in the central dogma of genetics that was proposed by Francis Crick in 1957 [91,92]. Genes encoded by deoxyribonucleic acid (DNA) are transcribed in the cellular nucleus to produce messenger ribonucleic acid (mRNA), which in turn is translated at the endoplasmic reticulum into functional proteins such as LPL [91,92]. Regulation of DNA transcription is responsible for the upregulation of LPL gene expression and activity during cardiomyogenesis and adipogenesis [83]. Chaperons are proteins with the function of assisting the folding and assembly of other proteins [93]. LPL is synthesized as an inactive monomer in parenchymal cells and, with the support of specific endoplasmic reticulum chaperones (i.e., lipase maturation factor 1 and Sel-1 suppressor of lin-12- like protein), a noncovalent, active LPL dimer is formed, although both active and inactive forms of LPL are secreted [54]. Most of the physiological variation in LPL activity (e.g., during exercise and fasting) appears to be driven via posttranslational mechanisms by extracellular proteins [83]. The Figure 3 is a schematic summary of some physiological factors that regulate LPL activity.
Figure 3: Non-exhaustive list of physiological factors regulating lipoprotein lipase activity. The content of the Figure 3 was adapted from the following references [3,52,83,94-111]. Insulin action on adipose tissue and muscle is discussed in the text.
Note: +, stimulation; -, inhibition; SM, skeletal muscle; SCAT, subcutaneous adipose tissue; TRL, triacylglycerol-rich lipoprotein; APOA5, apolipoprotein A5; APOC2, apolipoprotein C2; DHEA, dehydroepiandrosterone; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; PPARγ, peroxisome proliferator-activated receptor γ; ANGPTL3, angiopoietin-like protein 3; ANGPTL4, angiopoietin-like protein 4; ANGPTL8, angiopoietin-like protein 8; APOC1, apolipoprotein C1; APOC3, apolipoprotein C3; APOE, apolipoprotein E; GH, growth hormone; IL-6, interleukin-6; PRL, prolactin; TNF-α, tumor necrosis factor-α.
Besides the multiple factors listed below, LPL activity is also regulated by daily circumstances, such as exercise, fed and fasting states, and starvation and cold, in a very intricate manner, with many pertinent aspects that are yet to be elucidated.
Exercise may be concisely defined as a series of specific movements for the purpose of training or developing the body through systematic practice, or as a bodily exertion for the promotion of physical health [112]. LPL has been found to be increased in the skeletal muscle and adipose tissue as well as in the plasma of people engaged in exercise compared to those not engaged in exercise [83,113]. Moreover, exercise induces an acute increase in postheparin LPL that in turn leads to enhanced TG clearance and decreases plasma clearance of HDL constituents [113]. It is known that during exercise, energy turnover increases and adrenergic mechanisms play an important role in this regulation [114]. Plasma catecholamines effectively inhibit LPL via the α1-adrenoceptors (Figure 3) [109]. Nonetheless, exercise induces LPL and GLUT4 protein in the muscle independent of adrenergic-receptor signaling [115]. Both adipose tissue and intramuscular fat can be stimulated by catecholamines, and both LPL and hormonesensitive lipase (HSL; EC 3.1.1.79) play important roles in this regulation [114]. HSL is the predominant regulator of lipolysis from adipocytes, releasing FFAs from stored TGs [116]. In this dynamic metabolic process, LPL replenishes while HSL depletes the adipocyte fat store.
In the fed state, postprandial metabolism is essentially characterized by high insulin levels that are responsible for antilipolytic action (e.g., by inhibiting HSL) and antigluconeogenic action (by suppressing this metabolic pathway) as well as for lipogenic action (e.g., by stimulating LPL) [87,117]. Human insulin is a 51-amino acid peptide hormone that is produced by pancreatic β-cells in addition to be a major regulator of LPL activity (Figure 3) [110,118]. Briefly, insulin inhibits gluconeogenesis in the liver and the kidney because of the tissue-specific expression of hormonesensitive metabolic enzymes involved in this process [119]. Thus, insulin may inhibit, for example, glucose-6-phosphatase (EC 3.1.3.9), among other gluconeogenic enzymes [120-122]. However, because higher insulin concentrations are required to suppress gluconeogenesis than to inhibit glycogenolysis (i.e., the breakdown of glycogen), or increase glycogen synthesis, gluconeogenically derived glucose-6-phosphate can be diverted into hepatic glycogen even during mild hyperinsulinemia [87,123]. Thus, the fed state is an insulinsufficient state in which insulin affects the internal machinery of cells in the liver, adipose tissue, and muscles to promote energy production and storage [124]. Postprandially, LPL activity is elevated in adipose tissue compared with heart and muscle, resulting in the channeling of circulating TG fatty acids into lipid depots [125].
Conversely, the fasting state is characterized by low plasma insulin and high insulin counterregulatory hormones (e.g., glucagon, catecholamines) that determine the catabolic changes in fuel selection and the metabolite fluxes [87,126]. Reference [87] includes an overview of the hormonal and metabolic alterations during food deprivation. With such a hormonal profile, LPL activity is decreased in certain tissues (i.e., white adipose tissue) [83,127,128]. However, during fasting, relatively high heart and muscle LPL activities redirect TG fatty acids appropriately into these tissues and away from adipose stores [79,125]. Additionally, a study demonstrated that a ten-hour period of fasting caused a 25% decrease in LPL activity in adipose tissue whereas LPL activity in muscle remained unchanged, while a 30-hour period of fasting caused an incremental 50% decrease in LPL activity in adipose tissue and a 100% increase LPL activity in muscle; this likely reflects an increase in activity and mass of LPL in skeletal muscle [129]. Thus, a role for the tissue-specific regulation of LPL activity seems to be plausible, especially when LPL activity channels fatty acids to adipose tissue for storage in the fed state and to muscle tissues as energy fuel during times of food deprivation such as fasting [129]. Finally, intermittent fasting has been much discussed in the current literature for its potential health benefits [130-134]. In fact, LPL has been reported to accumulate in senile plaques of Alzheimer's disease (AD) brains, and as a molecular chaperone to bind to amyloid-β peptide (the major component of the plaques) [130]. In one study, intermittent fasting (i.e., alternate-day fasting) alleviated the increase of LPL expression in the brain of a mouse model of AD possibly by mediation of an increase in ketone body levels (i.e., β-hydroxybutyrate) subsequent to the induced ketosis [130].
Fasting and starvation are not synonymous terms, but the expression “prolonged fasting” is currently used as a synonym for starvation [87]. The term starvation is used to describe a state of extreme hunger resulting from a prolonged lack of essential nutrients [87]. Starvation is, in principle, longer, potentially harmful, and may lead to a lethal outcome [87]. The response to starvation is also integrated at all levels of organization and is directed toward the survival of the species [87]. For example, in the presence of low insulin levels during starvation, LPL in the muscle is more active than in the adipose tissue, and fatty acids from triglyceride-rich VLDLs are shunted in addition to the readily available FFAs into skeletal muscle cells to produce energy by oxidation [135]. Thus, in extreme circumstances such as starvation, the enzymatic activity of LPL seems to be adjusted to the actual energy metabolism needs of the organism.
Lastly, it is important to mention the role of brown adipose tissue (BAT) in the rat in increasing LPL activity by β3- adrenergic stimulation during cold [83]. The development of BAT with its characteristic protein, uncoupling protein-1, likely had a role in determining the evolutionary success of mammals, because its thermogenesis enhances neonatal survival and allows active life even in cold surroundings [136]. However, in humans, BAT is retained into adulthood, and it also retains the capacity to have a significant role in energy balance [137]. BAT is currently a primary target organ in obesity prevention strategies [137].
Alterations of Lipoprotein Lipase Activity in Metabolic Disorders
Enzymes are very sensitive biomolecules that require optimum conditions for their maximal operation. Experimentally, researchers seek to define this ideal operational profile of the enzymes by testing various influential factors, such as different substrates, temperatures, pH, buffers, activators, inhibitors, and durations. Thus, it is possible to define enzymatic stability, i.e., the ability to retain the catalytic activity of the biomolecule [138]. Knowing the enzymatic stability makes it possible to adapt the enzyme use to the most diverse biotechnological activities, including those aimed at human health. However, it is not possible to directly extrapolate in vitro results to in vivo results, but there is a great variability of enzymatic activity (lato sensu) among, for example, microorganism strains and animal species, including humans [139-142].
As discussed above, LPL activity is regulated by multiple physiological factors and daily circumstances such as exercise and fasting. Additionally, numerous diseases may affect human metabolism and LPL activity. The most prevalent metabolic disorders are obesity, diabetes mellitus, dyslipidemia, metabolic syndrome, and osteoporosis [143-145]. However, metabolic syndrome is composed of a constellation of interrelated cardiovascular risk factors of metabolic origin and includes visceral (or android) obesity, glucose intolerance, insulin resistance, dyslipidemia, and hypertension [146-148]. Consequently, metabolic syndrome is a heterogeneous entity rather than an unequivocal entity and it has profuse synonymy (e.g., plurimetabolic syndrome, syndrome X, Reaven syndrome, atherothrombogenic syndrome) [148,149]. Two common LPL gene variants (447 Ter and 291 Ser) were associated with metabolic syndrome through their effect on high TG and low HDL cholesterol [149].
Obesity may be broadly defined as an excess of body fat mass [150]. Adipogenesis is a process of adipocyte differentiation and lipid accumulation, and the expression of LPL mRNA has often been considered to be an early sign of adipocyte differentiation [151,152]. During adipogenesis, transcription of the LPL gene is stimulated by adipogenic transcription factor PPARγ, fatty acids, and other PPARγ agonists in differentiated adipocytes [83]. Additionally, insulin has a major effect on LPL activity in adipose tissue during adipocyte differentiation by increasing LPL gene transcription [89]. In mature adipocytes, insulin not only increases the level of LPL mRNA but also regulates LPL activity through both posttranscriptional and posttranslational mechanisms [89]. A major portion of available fatty acids for adipocyte uptake is derived from LPL-mediated hydrolysis of circulating lipoprotein particles; but in humans, de novo lipogenesis occurs mainly in the liver and to a lesser extent in the adipocyte [153-155]. Obesity studies in rodents and humans have revealed increased adipose tissue LPL activity [90]. Obese subjects also have elevated adipose tissue LPL activity per fat cell when compared with lean control subjects [156]. Remarkably, weight loss increases adipose tissue LPL activity probably in an attempt to maintain lipid stores [90]. Finally, obesity is a significant risk factor for type 2 diabetes mellitus [157]. It is estimated that approximately 80% of type 2 diabetic patients are obese, explaining the tight association of adiposity with insulin resistance and justifying the term diabesity [158,159].
Type 2 diabetes mellitus is the most common endocrine disorder in the world [160]. It is a chronic, progressive disease, characterized by multiple defects in glucose metabolism, the core of which is insulin resistance, which is the impaired ability to respond to insulin especially in muscle, liver, and adipocytes, and by a gradual β-cell failure [161,162]. Type 1 diabetes mellitus is, however, much less prevalent, and is characterized by profound insulin deficiency caused by pancreatic β-cell destruction [163,164]. A novel subtype of type 1 diabetes mellitus, known as fulminant type 1 diabetes, is responsible for approximately 20% of all ketosis-onset type 1 diabetes cases in the Japanese population [164]. As discussed above, LPL activity in both adipose tissue and skeletal muscle depends on insulin and varies in diabetes mellitus according to ambient insulin level and insulin sensitivity [165]. Briefly, in untreated type 1 diabetes mellitus, LPL activity in both adipose tissue and muscle tissue is low, but it increases with insulin therapy [165]. In chronically insulin-treated patients with good control, LPL activity in postheparin plasma is increased [165]. In untreated type 2 diabetic patients, the average LPL activity in adipose tissue and postheparin plasma is normal (or below normal) and therapy with oral agents or insulin results in good glycemic control, followed by an increase in LPL activity in both adipose tissue and postheparin plasma [165]. Of utmost interest are the alterations in lipoproteins following changes in LPL activity in diabetic patients. These changes include high VLDLs and low HDLs in insulin deficiency with low LPL activity, normal or low VLDLs and high HDLs in chronically insulintreated patients with high LPL activity, and high TGs and low HDLs in untreated type 2 diabetic patients [165]. Thus, the most obvious lipid defect in uncontrolled diabetes mellitus is the elevated TG level and a corollary is the reduced HDL level [166]. These metabolic disorders must be clinically followed up and continuously treated.
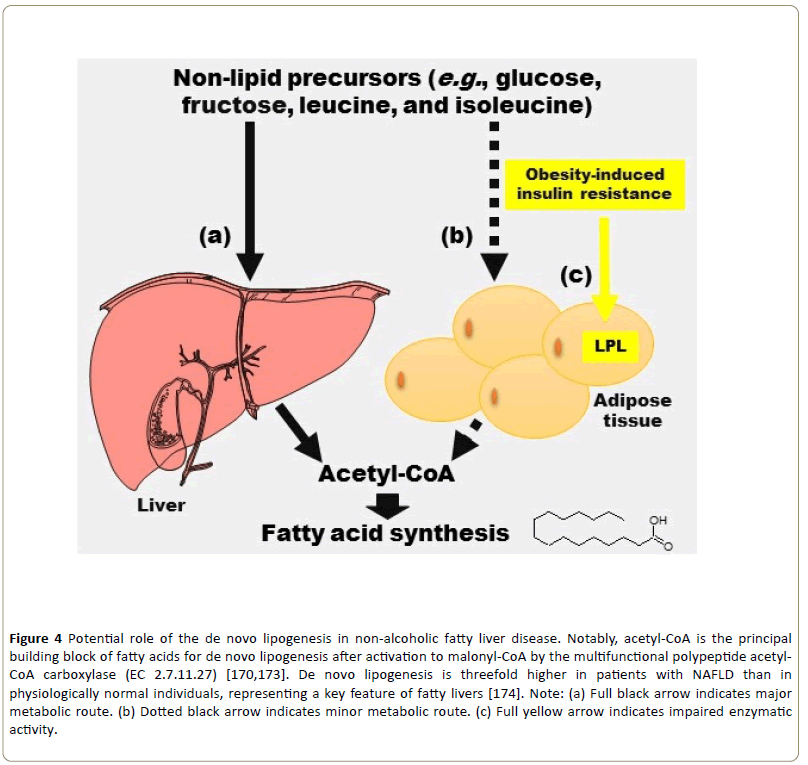
Lastly, fatty liver may occur in up to 80% of diabetic patients and is more commonly associated with type 2 diabetes mellitus (in which the degree of hepatic lipid accumulation is related to the severity of the associated obesity) [167]. Thus, non-alcoholic fatty liver disease (NAFLD) is closely associated with several metabolic syndrome features and has even been recognized as the hepatic expression of metabolic syndrome [168,169]. In this pathophysiological context, de novo lipogenesis is thought to contribute to the origin of NAFLD, which is often associated with insulin resistance [78,170]. However, the high activity of LPL in the white adipose tissue of extremely obese individuals is impaired by insulin resistance [171,172]. Hence, the pathophysiological role of LPL activity in NAFLD remains elusive (Figure 4).
Figure 4: Potential role of the de novo lipogenesis in non-alcoholic fatty liver disease. Notably, acetyl-CoA is the principal building block of fatty acids for de novo lipogenesis after activation to malonyl-CoA by the multifunctional polypeptide acetyl-CoA carboxylase (EC 2.7.11.27) [170,173]. De novo lipogenesis is threefold higher in patients with NAFLD than in physiologically normal individuals, representing a key feature of fatty livers [174]. Note: (a) Full black arrow indicates major metabolic route. (b) Dotted black arrow indicates minor metabolic route. (c) Full yellow arrow indicates impaired enzymatic activity.
Drugs and Phytochemicals Affecting Lipoprotein Lipase Activity
Many compounds exert part of their beneficial effects on the disordered metabolism of lipoproteins through the modulation of LPL activity. Fibrates are one of the oldest lipidlowering drugs, beginning in the late 1950s with the firstgeneration agent (clofibrate), subsequently, gemfibrozil, the second-generation fibrate that has been used worldwide, while the third-generation agents comprise fenofibrate, bezafibrate, and etofibrate [175]. Fibrates are derivatives of fabric acid whose mechanism of action relies on the activation of the nuclear receptor peroxisome proliferator-activated receptors alpha (PPARα), which leads to several changes in metabolism, including a reduction in the production of APOC3 (the already mentioned physiological inhibitor LPL) and an increase in the expression of LPL, both alterations leading to a significant rise in LPL activity and a reduction in TG level in bloodstream [9,175-177]. Nicotinic acid and its derivatives (pyridylcarbinol, xanthinol nicotinate, and acipimox) activate LPL, thereby mainly lowering TG levels [175,178,179]. However, at the start of nicotinic acid therapy, a prostaglandinmediated vasodilation may occur with flushing, hypotension, that can be prevented by low doses of acetylsalicylic acid [178]. Some statins, such as pitavastatin, simvastatin, and atorvastatin, also increase the expression and the activity of LPL [180-183].
Phytochemicals may be defined as substances found in plants that exhibit a potential for modulating human metabolism in a manner that is beneficial for the prevention of chronic and degenerative diseases [184]. The growing interest in phytochemicals is in part due to the high prevalence of metabolic disorders and an urgent need for new therapeutic avenues [19]. Among phytochemicals, polyphenols have drawn attention for their many health virtues, particularly their antioxidant activity [81]. In addition, many plants rich in these phytochemicals have been currently tested for their effects on LPL activity [185-187]. This research area holds promise for improving patients’ quality of life.
Concluding Remarks
LPL plays a crucial physiological role not only in lipoprotein metabolism but also in fuel metabolism. LPL is strategically located at the dynamic blood-tissue interface (vascular endothelium) from where it can more easily redirect the use of energy-rich substrates, such as FFAs, according to the metabolic demands of the organism. LPL activity seems to adapt to even more extreme circumstances of energy shortage, such as prolonged fasting (or starvation), by favoring the energy supply to tissues such as the muscles. As discussed in this review, this complex tissue-specific regulation of LPL activity is physiologically desirable. Pathophysiologically, however, there are still gaps in the comprehension of the role played by LPL in metabolic disorders such as obesity, diabetes mellitus, and NAFLD, among others. Furthermore, the metabolic syndrome continues to expand (conceptually), making the understanding of the role of LPL activity in this heterogeneous illness even more difficult. Finally, complex diseases, such as metabolic disorders, require multifarious therapeutic approaches. Nonetheless, some LPL activators (e.g., fibrates) have been proven effective in the treatment of hypertriglyceridemia.
Acknowledgement
The author is deeply grateful to his loving mother, Graciema Britto de Andrade, for her permanent, untiring, and enthusiastic support (in memoriam).
Conflict of Interest
The author declares no conflict of interest.
References
- Mann GV (1958) A short history of lipoproteins. In: Homburger F, Bernfeld P (eds.) The lipoproteins. Methods and Clinical Significance. Basel, Karger, Switzerland, pp: 7-13.
- Hahn P (1943) Abolishment of alimentary lipemia following injection of heparin. Science 98: 19-20.
- Hamosh M, Hamosh P (2011) Lipoproteins and lipoprotein lipase. In: Comprehensive Physiology, pp: 387-418.
- Korn E (1955) Clearing factor, a heparin-activated lipoprotein lipase I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem 215: 1-14.
- Fredrickson DS (1993) Phenotyping. On reaching base camp (1950-1975). Circulation 87: III-1-III-15.
- Hoeg JM, Osborne Jr JC, Gregg RE, Brewer Jr HB (1983) Initial diagnosis of lipoprotein lipase deficiency in a 75-year-old man. Am J Med 75: 889-892.
- Havel RJ, Gordon RS (1960) Idiopathic hyperlipemia: metabolic studies in an affected family. J Clin Invest 39: 1777-1790.
- Gaudet D, Méthot J, Kastelein J (2012) Gene therapy for lipoprotein lipase deficiency. Curr Opin Lipidol 23: 310-320.
- Malloy M, Kane J (2011) Disorders of lipoprotein metabolism. In: Gardner D, Shoback D (eds.) Greenspan's basic and clinical endocrinology. McGraw-Hill, New York, USA, pp: 675-698.
- Dorland WA (1901) The American illustrated medical dictionary: A new and completed dictionary of the terms used in medicine, surgery, dentistry, pharmacy, chemistry, and the kindred branches with their pronunciation, derivation, and definition. Saunders, USA.
- Patsch W, Gotto Jr A (1996) Apolipoproteins: Pathophysiology and clinical implications. ?Methods Enzymol 263: 3-32.
- Fielding CJ (1970) Human lipoprotein lipase I. Purification and substrate specificity. Biochim Biophys Acta 206: 109-117.
- Björnson E, Adiels M, Taskinen MR, Borén J (2017) Kinetics of plasma triglycerides in abdominal obesity. Curr Opin Lipidol 28: 11-18.
- Momin A, Bankar M, Bhoite G (2016) Study of common genetic variant S447X in lipoprotein lipase and its association with lipids and lipoproteins in type 2 diabetic patients. Indian J Clin Biochem 31: 286-293.
- Braun J, Severson D (1992) Tissue-specific regulation of lipoprotein lipase. Can Med Assoc J 147: 1192.
- Bensadoun A (1991) Lipoprotein lipase. Annu Rev Nutr 11: 217-237.
- Wion KL, Kirchgessner TG, Lusis AJ, Schotz MC, Lawn RM (1987) Human lipoprotein lipase complementary DNA sequence. Science 235: 1638-1641.
- Davies B, Beigneux A, Fong L, Young S (2012) New wrinkles in lipoprotein lipase biology. Curr Opin Lipidol 23: 35-42.
- Andrade Jr MC, Andrade J, Costa SS, Leite EAS (2017) Nutrients of cubiu fruits (Solanum sessiliflorum Dunal, Solanaceae) as a function of tissues and ripening stages. J Food Nutr Res 5: 674-683.
- Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M (1978) Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med 298: 1265-1273.
- Tulenko T, Sumner A (2002) The physiology of lipoproteins. J Nucl Cardiol 9: 638-649.
- Jonas A, Phillips MC (2008) Lipoprotein structure. In: Vance DE, Vance JE (eds.) Biochemistry of lipids, lipoproteins and membranes. Elsevier BV, Amsterdam, Netherland, pp: 485-506.
- Karam I, Yang YJ, Li JY (2017) Hyperlipidemia background and progress. SM Atheroscler J 1: 1-8.
- Ryeo-Eun G, Kyung AH, Ye-Seul K, Seung-Hee K, Ki-Hoan N, et al. (2015) Effects of palm and sunflower oils on serum cholesterol and fatty liver in rats. J Med Food 18: 363-369.
- Choi HY, Hafiane A, Schwertani A, Genest J (2017) High-density lipoproteins: biology, epidemiology, and clinical management. Can J Cardiol 33: 325-333.
- Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, et al. (2014) Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr 100: 168-175.
- Aaseth E, Fagerland MW, Aas AM, Hewitt S, Risstad H, et al. (2015) Vitamin concentrations 5 years after gastric bypass. Eur J Clin Nutr 69: 1-7.
- Bjørneboe A, Bjorneboe GE, Drevon CA (1990) Absorption, transport and distribution of vitamin E. J Nutr 120: 233-242.
- Kazeem MI, Ogunwande IA (2012) Role of fixed oil and fats in human physiology and pathophysiology. RPMP 33: 85-103.
- Faisel H, Pittrof R (2000) Vitamin A and causes of maternal mortality: association and biological plausibility. Public Health Nutr 3: 321-327.
- Daneshian M, Guenther A, Wendel A, Hartung T, Von Aulock S (2006) In vitro pyrogen test for toxic or immunomodulatory drugs. J Immunol Methods 313: 169-175.
- Rigamonti F, Carbone F, Montecucco F, Bonaventura A, Liberale L, et al. (2018) Serum lipoprotein (a) predicts acute coronary syndromes in patients with severe carotid stenosis. Eur J Clin Invest, 48.
- Kotani K, Banach M (2017) Lipoprotein (a) and inhibitors of proprotein convertase subtilisin/kexin type. J Thorac Dis 9: E78-E82.
- Juo PS (2001) Concise dictionary of biomedicine and molecular biology. CRC Press, Boca Raton, Florida.
- Antonian E (1988) Recent advances in the purification, characterization and structure determination of lipases. Lipids 23: 1101-1106.
- Kobayashi J, Mabuchi H (2015) Lipoprotein lipase and atherosclerosis. Ann Clin Biochem 52: 632-637.
- Sparkes RS, Zollman S, Klisak I, Kirchgessner TG, Komaromy MC, et al. (1987) Human genes involved in lipolysis of plasma lipoproteins: Mapping of loci for lipoprotein lipase to 8p22 and hepatic lipase to 15q21. Genomics 1: 138-144.
- Wang G, Wang X, Zhang Q, Ma Z (2007) Response to pioglitazone treatment is associated with the lipoprotein lipase S447X variant in subjects with type 2 diabetes mellitus. Int J Clin Pract 61: 552-557.
- Goulbourne CN, Gin P, Tatar A, Nobumori C, Hoenger A, et al. (2014) The GPIHBP1–LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab 19: 849-860.
- Gadek KE, Wang H, Hall MN, Sungello M, Libby A, et al. (2018) Striated muscle gene therapy for the treatment of lipoprotein lipase deficiency. PLoS ONE, 13: e0190963.
- Weiss RJ, Esko JD, Tor Y (2017) Targeting heparin and heparan sulfate protein interactions. Org Biomol Chem 15: 5656-5668.
- Khan S, Gor J, Mulloy B, Perkins SJ (2010) Semi-rigid solution structures of heparin by constrained X-ray scattering modelling: new insight into heparin–protein complexes. J Mol Biol 395: 504-521.
- Nelson DL, Cox MM (2013) Lehninger principles of biochemistry. WH Freeman and Company, New York, USA.
- Nishiyama K, Fujita T, Fujimoto Y, Nakajima H, Takeuchi T, et al. (2018) Fatty acid transport protein 1 enhances the macrophage inflammatory response by coupling with ceramide and c-Jun N-terminal kinase signaling. Int Immunopharmacol 55: 205-215.
- Golebiowski M, Sosnowska A, Puzyn T, Bogus MI, Wieloch W, et al. (2014) Application of two-way hierarchical cluster analysis for the identification of similarities between the individual lipid fractions of Lucilia sericata. Chem Biodivers 11: 733-748.
- Iena FM, Lebeck J (2018) Implications of aquaglyceroporin 7 in energy metabolism. Int J Mol Sci 19: E154.
- Abbas AK, Lichtman AH, Pillai S (2007) Cellular and molecular immunology. WB Saunders Company, Philadelphia, Pennsylvania, USA.
- Remmerie A, Scot CL (2018) Macrophages and lipid metabolism. Cell Immunol.
- Plank BG, Doling MJ, Knight PA (2012) Coronary artery disease. In: Bisognano JD, Beck GR, Connell RW (eds.) Manual of outpatient cardiology. Springer, London, UK, pp: 179-216.
- He PP, Jiang T, OuYang XP, Liang YQ, Zou JQ, et al. (2018) Lipoprotein lipase: biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clin Chim Acta 480: 126-137.
- Barchetta I, Ciccarelli G, Cimini FA, Ceccarelli V, Ortho-Melander M, et al. (2018) Association between systemic leptin and neurotensin concentration in adult individuals with and without type 2 diabetes mellitus. J Endocrinol Invest.
- Maingrette F, Renier G (2003) Leptin increases lipoprotein lipase secretion by macrophages: involvement of oxidative stress and protein kinase C. Diabetes 52: 2121-2128.
- Ricciuti B, Foglietta J, Chiari R, Sahebkar A, Banach M, et al. (2018) Emerging enzymatic targets controlling angiogenesis in cancer: Preclinical evidence and potential clinical applications. Med Oncol 35: 4.
- Olivecrona G (2016) Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol 27: 233-241.
- Jin ZX, Xiong Q, Jia F, Sun CL, Zhu HT, et al. (2015) Investigation of RNA interference suppression of matrix metalloproteinase-9 in mouse model of atherosclerosis. Int J Clin Exp Med 8: 5272-5278.
- Bruyndonckx L, Hoymans VY, Lemmens K, Ramet J, Vrints CJ (2016) Childhood obesity–related endothelial dysfunction: an update on pathophysiological mechanisms and diagnostic advancements. Pediatr Res 79: 831-837.
- Goldberg IJ (2018) Fat in the blood, fat in the artery, fat in the heart: triglyceride in physiology and disease. Arterioscler Thromb Vasc Biol 38: 1-7.
- Cavusoglu E, Chhabra S, Jiang XC, Hojjati M, Chopra V, et al. (2007) Relation of baseline plasma phospholipid levels to cardiovascular outcomes at two years in men with acute coronary syndrome referred for coronary angiography. Am J Cardiol 100: 1739-1743.
- Koivuniemi A (2017) The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Letters 591: 2700-2713.
- Area-Gomez E, Schon E (2017) On the pathogenesis of Alzheimer’s disease: The MAM hypothesis. FASEB J 31: 864-867.
- Wickner W (2011) Eugene Patrick Kennedy, 1919-2011. PNAS 108: 19122-19123.
- Gibellini F, Smith T (2010) The Kennedy pathway-de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62: 414-428.
- Nellipudi K, Ramasubramania R, Sreenivasulu M, Lalitha C, Salma S (2015) Anti-hyperlipidemic activity of chloroform fraction of Camellia sinensis leaf. WJPR 4: 530-540.
- Huang Y, Tan J, Cui L, Zhou Z, Zhang Z, et al. (2018) Graphene and Au NPs co-mediated enzymatic silver deposition for the ultrasensitive electrochemical detection of cholesterol. ?Biosens Bioelectron 102: 560-567.
- Mirkin G (1983) Foods and nutrition for exercise. In: Bove AA, Lowenthal DT (eds). Exercise medicine: physiological principles and clinical applications. Elsevier, London, UK, pp: 89-109.
- Xu G, Liu D, Zhao G, Chen S, Wang J, et al. (2016) Effect of eleven antioxidants in inhibiting thermal oxidation of cholesterol. J Am Oil Chem Soc 93: 215-225.
- Lamarche B (2017) Saturated fat: friend or foe? In: Rippe J (ed). Nutrition in lifestyle medicine, nutrition and health. Springer, Cham, Switzerland, pp: 387-394.
- Ding Y, Peng Y, Deng L, Fan J, Huang B (2017) Gamma-tocotrienol reverses multidrug resistance of breast cancer cells with a mechanism distinct from that of atorvastatin. J Steroid Biochem Mol Biol 167: 67-77.
- Calabro P, Yeh ETH (2004) Multitasking of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor: beyond cardiovascular diseases. Curr Atheroscler Rep 6: 36-41.
- Marie S, Cisternino S, Buvat I, Declèves X, Tournier N (2017) Imaging probes and modalities for the study of solute carrier O (SLCO)-transport function in vivo. J Pharm Sci 106: 2335-2344.
- Tadros RO, Vouyouka AG, Chung C, Malik RK, Krishnan P, et al. (2013) The effect of statin use on embolic potential during carotid angioplasty and stenting. Ann Vasc Surg 27: 96-103.
- Plank M, Wachtmeister G, Thuneke K, Remmele E, Emberger P (2017) Effect of fatty acid composition on ignition behavior of straight vegetable oils measured in a constant volume combustion chamber apparatus. Fuel 20: 293-301.
- Tornheim K, Ruderman NB (2011) Intermediary metabolism of carbohydrate, protein, and fat. In: Ahima R (ed.) Metabolic basis of obesity. Springer Science, New York, USA, pp: 25-51.
- Wada T, Gao J, Xie W (2009) PXR and CAR in energy metabolism. Trends Endocrinol Metab 20: 273-279.
- Dias S, Paredes S, Ribeiro L (2018) Drugs involved in dyslipidemia and obesity treatment: focus on adipose tissue. Int J Endocrinol 2018.
- Iwakoshi-Ukena E, Shikano K, Kondo K, Taniuchi S, Furumitsu M, et al. (2017) Neurosecretory protein GL stimulates food intake, de novo lipogenesis, and onset of obesity. eLife 6: e28527.
- Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, et al. (2016) Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol 12: 15-21.
- Solinas G, Borén J, Dulloo AG (2015) De novo lipogenesis in metabolic homeostasis: more friend than foe? Mol Metab 4: 367-377.
- Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: An endocrine organ. Arch Med Sci 9: 191-200.
- Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, et al. (2014) Measurement of lipolysis. Methods Enzymol 538: 171-193.
- Andrade Jr MC, Andrade JS (2015) Fermented foods in general and ethnic fermented foods in particular. Lambert Academic Publishing, Saarbrücken, German.
- Forte TM, Ryan RO (2015) Apolipoprotein A5: extracellular and intracellular roles in triglyceride metabolism. Curr Drug Targets 16: 1274-1280.
- Kersten S (2014) Physiological regulation of lipoprotein lipase. Biochim Biophys Acta 1841: 919-933.
- Al-Akhrass H, Naves T, Vincent F, Magnaudeix A, Durand K, et al. (2017) Sortilin limits EGFR signaling by promoting its internalization in lung cancer. Nat Commun 8: 1182.
- Tao RC, Kelley RE, Yoshimura NN, Benjamin F (1983) Glycerol: its metabolism and use as an intravenous energy source. J Parenter Enteral Nutr 7: 479-488.
- Okamura T, Shimizu H, Nagao T, Ueda R, Ishii S (2007) ATF-2 regulates fat metabolism in Drosophila. ?Mol Biol Cel 18: 1519-1529.
- Andrade Jr MC (2017) Metabolism during fasting and starvation: understanding the basics to glimpse new boundaries. J Nutr Diet 1: e02.
- Hegele RA (2016) Multidimensional regulation of lipoprotein lipase: impact on biochemical and cardiovascular phenotypes. ?J Lipid Res 57: 1601-1607.
- Wang H, Eckel RH (2009) Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297: E271-E288.
- Mead JR, Irvine SA, Ramji DP (2002) Lipoprotein lipase: Structure, function, regulation, and role in disease. J Mol Med 80: 753-769.
- Ioannou D, Tempest HG (2018) Does genome organization matter in spermatozoa? A refined hypothesis to awaken the silent vessel. Syst Biol Reprod Med.
- Cobb M (2017) 60 years ago, Francis Crick changed the logic of biology. PLoS Biol 15: e2003243.
- Biro JC (2005) Nucleic acid chaperons: a theory of an RNA-assisted protein folding. Theor Biol Med Model 2: 35.
- Yeoh BS, Vijay-Kumarm M (2018) Altered microbiota and their metabolism in host metabolic diseases. In: Sun J, Dudeja PK (eds). Mechanisms underlying host-microbiome interactions in pathophysiology of human diseases. Physiology in health and disease. Springer, Boston, USA, pp: 129-165.
- Su X, Peng DQ (2018) New insights into ANGPLT3 in controlling lipoprotein metabolism and risk of cardiovascular diseases. Lipids Health Dis 17: 12.
- Gholami S, Gheibi N, Falak R, Chegini KG (2018) Cloning, expression, purification and CD analysis of recombinant human betatrophin. Rep Biochem Mol Biol 6: 158-163.
- Nauck MA, Meier JJ (2018) Incretin hormones: their role in health and disease. Diabetes Obes Metab 20: 5-21.
- Zhang X, Ye Q, Gong D, Lv Y, Cheng H, et al. (2017) Apelin-13 inhibits lipoprotein lipase expression via the APJ/PKCa/miR-361-5p signaling pathway in THP-1 macrophage-derived foam cells. Acta Biochim Biophys Sin 49: 530-540.
- Lath R, Jibhkate A, Shendye R (2017) Study of lipid profile and high sensitivity C reactive protein in women with polycystic ovary syndrome. Int Arch BioMed Clin Res 3: 80-83.
- Schwetz V, Librizzi R, Trummer C, Theiler G, Stiegler C, et al. (2017) Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metab Brain Dis 32: 155-161.
- Meirelles RMR (2017) Functional hypogonadism: Diabetes mellitus, obesity, metabolic syndrome, and testosterone. In: Hohl A (ed). Testosterone. Springer, Cham, Switzerland, pp: 147-159.
- Lee JA, Cho YR, Hong SS, Ahn EK (2017) Anti-obesity activity of saringosterol Isolated from Sargassum muticum (yendo) fensholt extract in 3T3-L1 cells. Phytother Res 31: 1694-1701.
- Mahat B, Chassé E, Mauger JF, Imbeault P (2016) Effects of acute hypoxia on human adipose tissue lipoprotein lipase activity and lipolysis. J Transl Med 14: 212.
- Ebner N, Springer J, Kalantar-Zadeh K, Lainscak M, Doehner W, et al. (2013) Mechanism and novel therapeutic approaches to wasting in chronic disease. Maturitas 75: 199-206.
- Saikia H, Lama A (2011) OTC–Availability of emergency contraceptive levonorgestrel: a review. J Pharm Res 4: 67-71.
- Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthén L, et al. (2008) Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr 87: 1743-1749.
- Saleh J, Sniderman AD, Cianflone K (1999) Regulation of plasma fatty acid metabolism. Clin Chim Acta 286: 163-180.
- Ottosson M, Vikman-Adolfsson K, Enerbäck S, Elander A, Björntorp P, et al. (1995) Growth hormone inhibits lipoprotein lipase activity in human adipose tissue. JCEM 80: 936-941.
- Ruffolo Jr RR, Nichols AJ, Hieble JP (1991) Metabolic regulation by a1- and a2-adrenoceptors. Life Sci 49: 171-183.
- Semenkovich CF, Wims M, Noe L, Etienne J, Chan L (1989) Insulin regulation of lipoprotein lipase activity in 3T3-Ll adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem 264: 9030-9038.
- Weinberg RB (1987) Lipoprotein metabolism: hormonal regulation. Hosp Pract 22: 223-243.
- Campello M, Nordin M, Weiser S (1996) Physical exercise and low back pain. Scand J Med Sci Sports 6: 63-72.
- Ikekpeazu JE, Oranwa JC, Ogbu IS, Onyekwelu KC, Esom EA, et al. (2017) Lipid profile of people engaged in regular exercise. Ann Med Health Sci Res 7: 36-39.
- Kjœr M, Lange K (2000) Adrenergic regulation of energy metabolism. In: Warren MP, Constantini NW (eds.) Sports endocrinology. Humana Press, New Jersey, United States, pp: 181-188.
- Greiwe JS, Holloszy JO, Semenkovich CF (2000) Exercise induces lipoprotein lipase and GLUT-4 protein in muscle independent of adrenergic-receptor signaling. J Appl Physiol 89: 176-181.
- Zolotov S, Xing C, Mahamid R, Shalata A, Sheikh-Ahmad M, et al. (2017) Homozygous LIPE mutation in siblings with multiple symmetric lipomatosis, partial lipodystrophy, and myopathy. Am J Med Genet A 173: 190-194.
- Mortensen LS, Holmer-Jensen J, Hartvigsen ML, Jensen VK, Astrup A, et al. (2012) Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur J Clin Nutr 66: 799-805.
- Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, et al. (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. ?Nat Rev Neurol.
- White MF (2012) Mechanisms of insulin action. In: Skyler JS (ed). Atlas of Diabetes (4th edn). Springer, USA, pp: 19-38.
- De Castro GS, Calder PC (2018) Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin Nutr 37: 37-55.
- Otero YF, Stafford JM, McGuinness OP (2014) Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux. J Biol Chem 289: 20462-20469.
- Linke A (1962) Enzyme induction in starvation. Acta Endocrinol 40: S170.
- Edgerton DS, Ramnanan CJ, Grueter CA, Johnson KMS, Lautz M et al. (2009) Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 58: 2766-2775.
- Lam DWH, Feng Y, Fleckman AM (2015) Acute hyperglycemic syndromes: diabetic ketoacidosis and the hyperosmolar state. In: Poretsky L (ed). Principles of diabetes mellitus. Springer, Cham, Switzerland, pp: 1-17.
- Doolittle MH, Ben-Zeev O, Elovson J, Martin D, Kirchgessner TG (1990) The response of lipoprotein lipase to feeding and fasting. Evidence for posttranslational regulation. J Biol Chem 265: 4570-4577.
- Daniel H, Sailer M (2012) Metabolomics applications in human nutrition. In: Suhre K (ed). Genetics meets metabolomics: From experiment to systems biology. Springer, New York, USA, pp: 125-137.
- Hesse D, Radloff K, Jaschke A, Lagerpusch M, Chung B, et al. (2014) Hepatic trans-Golgi action coordinated by the GTPase ARFRP1 is crucial for lipoprotein lipidation and assembly. ?J Lipid Res 55: 41-52.
- Fried SK, Hill JO, Nickel M, DiGirolamo M (1983) Novel regulation of lipoprotein lipase activity in rat brown adipose tissue: Effects of fasting and caloric restriction during refeeding. J Nutr 113: 1870-1874.
- Ruge T, Svensson M, Eriksson JW, Olivecrona G (2005) Tissue-specific regulation of lipoprotein lipase in humans: effects of fasting. Eur J Clin Invest 35: 194-200.
- Zhang J, Li X, Ren Y, Zhao Y, Xing A, et al. (2018) Intermittent fasting alleviates the increase of lipoprotein lipase expression in brain of a mouse model of Alzheimer's disease: possibly mediated by ß-hydroxybutyrate. Front Cell Neurosci 12: 1.
- Templeman I, Thompson D, Gonzalez J, Walhin JP, Reeves S, et al. (2018) Intermittent fasting, energy balance and associated health outcomes in adults: Study protocol for a randomised controlled trial. Trials 19: 186.
- Shin BK, Kang S, Kim DS, Park S (2018) Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer's disease-induced estrogen deficient rats. Exp Biol Med 243: 334-343.
- Kivelä R, Alitalo K (2017) White adipose tissue coloring by intermittent fasting. Cell Res 27: 1300-1301.
- Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, et al. (2015) Intermittent fasting and human metabolic health. J Acad Nutr Diet 115: 1203-1212.
- Neumann-Haefelin C, Beha A, Kuhlmann J, Belz U, Gerl M, et al. (2004) Muscle-type specific intramyocellular and hepatic lipid metabolism during starvation in Wistar rats. Diabetes 53: 528-534.
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277-359.
- Symonds ME (2013) Brown adipose tissue growth and development. Scientifica.
- Weijers SR, Van't Riet K (1992) Enzyme stability in downstream processing part 1: enzyme inactivation, stability and stabilization. Biotechnol Adv 10: 237-249.
- Navarro M, Aparicio C, Charles-Harris M, Ginebra MP, Engel E, et al. (2006) Development of a biodegradable composite scaffold for bone tissue engineering: physicochemical, topographical, mechanical, degradation, and biological properties. Adv Polym Sci 200: 209-231.
- Lee HS, Gilliland SE, Carter S (2001) Amylolytic cultures of Lactobacillus acidophilus: potential probiotics to improve dietary starch utilization. J Food Sci 66: 338-344.
- Kato K, Yamamoto M, Peerapon K, Fukada H, Biswas A, et al. (2014) Effects of dietary taurine levels on epidermal thickness and scale loss in red sea bream, Pagrus major. ?Aquacult Res 45: 1818-1824.
- Shen C, Zhang B, Liu Z, Tang Y, Zhang Y, et al. (2017) Effects of ABCB1, ABCC2, UGT2B7 and HNF4a genetic polymorphisms on oxcarbazepine concentrations and therapeutic efficacy in patients with epilepsy. Seizure-Eur J Epilep 51: 102-106.
- Rohini A, Agrawal N, Kumar H, Kumar V (2018) Emerging role of branched chain amino acids in metabolic disorders: a mechanistic review. Pharma Nutrition 6: 47-54.
- Tabatabaei-Malazy O, Larijani B, Abdollahi M (2015) Targeting metabolic disorders by natural products. J Diabetes Metab Disord 14: 57.
- Dehghan M, Pourahmad-Jaktaji R, Farzaneh Z (2016) Calcitonin receptor AluI (rs1801197) and Taq1 calcitonin genes polymorphism in 45-and over 45-year-old women and their association with bone density. Acta Inform Med 24: 239-243.
- Kina-Tanada M, Sakanashi M, Tanimoto A, Kaname T, Matsuzaki T, et al. (2017) Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia 60: 1138-1151.
- Muller CJF, Malherbe CJ, Chellan N, Yagasaki K, Miura Y, et al. (2018) Potential of rooibos, its major C-glucosyl flavonoids, and Z-2-(ß-D-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit Rev Food Sci Nutr 58: 227-246.
- Leslie BR (2005) Metabolic syndrome: historical perspectives. Am J Med Sci 330: 264-268.
- Vishram JKK, Hansen TW, Torp-Pedersen C, Madsbad S, Jørgensen T, et al. (2016) Metabolic syndrome and related disorders. Metab Syndr Relat Disord 14: 442-448.
- Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, et al. (2017) Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev 38: 267-296.
- Nuermaimaiti N, Liu J, Liang X, Jiao Y, Zhang D (2018) Effect of lncRNA HOXA11-AS1 on adipocyte differentiation in human adipose-derived stem cells. Biochem Biophys Res Commun 495: 1878-1884.
- Niemelä S, Miettinen S, Sarkanen JR, Ashammakhi N (2008) Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. In: Ashammakhi N, Reis R, Chiellini F (eds). Topics in Tissue Engineering.
- Gonzales AM, Orlando RA (2007) Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab 4.
- Ziegler O, Böhme P, Valet P (2017) De la dysfonction du tissu adipeux blanc aux phénotypes anatomocliniques de l'obésité. Obésité 12: 16-41.
- Bartelt A, Weigelt C, Cherradi ML, Niemeier A, Tödter K (2013) Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochim Biophys Acta 1831: 934-942.
- Schwartz RS, Brunzell JD (1981) Increase of adipose tissue lipoprotein lipase activity with weight loss. J Clin Invest 67: 1425-1430.
- Watanabe RM (2018) Physiologic interpretation of GWAS signals for type 2 diabetes. In: DiStefano JK (ed.) Disease gene identification. Methods in molecular biology. New York: Humana Press, pp: 323-351.
- Jayanthy G, Devi VR, Ilango K, Subram SP (2017) Rosmarinic acid mediates mitochondrial biogenesis in insulin resistant skeletal muscle through activation of AMPK. J Cell Biochem 118: 1839-1848.
- Andrade Jr MC, Andrade JS (2014) Amazonian fruits: an overview of nutrients, calories and use in metabolic disorders. FNS 5: 1692-1703.
- Sakuma K, Aizawa M, Wakabayashi H, Yamaguchi A (2017) The autophagy-dependent signaling in skeletal muscle. In: Sakuma K, (ed.) The plasticity of skeletal muscle. Springer, Singapore, pp: 93-111.
- Howard-Thompson A, Khan M, Jones M, George CM (2018) Type 2 diabetes mellitus: Outpatient insulin management. Am Fam Physician 97: 29-37.
- Martin CR (1995) Dictionary of endocrinology and related biomedical sciences. Oxford University Press, New York, USA.
- Vionnet AC, Jornayvaz FR (2015) Classification du diabète: vers une hétérogénéité croissante. Rev Med Suisse 11: 1234-1237.
- Takahashi N, Tsujimoto T, Chujo D, Kajio H (2018) High risk of renal dysfunction in patients with fulminant type 1 diabetes. J Diabetes Investig 9: 146-151.
- Taskinen MR (1987) Lipoprotein lipase in diabetes. Diabetes Metab Res Rev 3: 551-570.
- Tomkin GH, Owens D (2017) Diabetes and dyslipidemia: characterizing lipoprotein metabolism. Diabetes Metab Syndr Obes 10: 333-343.
- Burt AD, MacSween RNM, Peters TJ, Simpson KJ (1992) Non-alcoholic fatty liver: causes and complications. In: McIntyre N, Benhamou JP, Bircher J, Rizzetto M, Rodes J (eds.) Oxford Textbook of Clinical Hepatology. Oxford Univesity Press, Oxford, UK, pp: 865-871.
- Targher G, Bertolini L, Padovani R, Poli F, Scala L, et al. (2006) Non-alcoholic fatty liver disease is associated with carotid artery wall thickness in diet-controlled type 2 diabetic patients. J Endocrinol Invest 29: 55-60.
- Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, et al. (2009) Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis 19: 646-653.
- Sanders FWB, Griffin JL (2016) De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev 91: 452-468.
- Reccia I, Kumar J, Akladios C, Virdis F, Pai M, et al. (2017) Non-alcoholic fatty liver disease: a sign of systemic disease. Metab Clin Exp 72: 94-108.
- Park SH, Kim BI, Yun JW, Kim JW, Park DI, et al. (2004) Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol 19: 694-698.
- Hellerstein MK, Schwar JM, Neese RA (1996) Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nurr 16: 523-557.
- Tilg H, Moschen AR, Roden M (2017) NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 14: 32-42.
- Stein EA (1994) Drug and alternative therapies for hyperlipidemia. Atherosclerosis 108: S105-S116.
- Okopien B, Buldak L, Boldys A (2017) Fibrates in the management of atherogenic dyslipidemia. Expert Rev Cardiovasc Ther 15: 913-921.
- Dhanalakshmi R, Balamurugan K, Manavalan R (2014) Hypolipidemic and antiatherosclerotic activity of some medicinal plants: A review. WJPPS 3: 328-340.
- Lüllmann H, Hein L, Mohr K, Bieger D (2005) Color Atlas of Pharmacology. Thieme, Stuttgart, Germany.
- Carlson LA (2005) Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 258: 94-114.
- Vergès B, Florentin E, Baillot-Rudoni S, Monier S, Petit JM, et al. (2008) Effects of 20 mg rosuvastatin on VLDL1-, VLDL2-, IDL- and LDL-ApoB kinetics in type 2 diabetes. Diabetologia 51: 1382-1390.
- Lamon-Fava S, Diffenderfer MR, Barrett PHR, Buchsbaum A, Matthan NR, et al. (2007) Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res 48: 1746-1753.
- Schneider JG, Eynatten M, Dugi KA (2005). Atorvastatin increases lipoprotein lipase expression in vitro and activity in vivo. J Atheroscler Thromb 12: 332-333.
- Verd JC, Peris C, Alegret M, Díaz C, Hernández G, et al. (1999) Different effect of simvastatin and atorvastatin on key enzymes involved in VLDL synthesis and catabolism in high fat/cholesterol fed rabbits. Br J Pharmacol 127: 1479-1485.
- Baky MH, Kamal AM, Elgindi MR, Haggag EG (2016) A review on phenolic compounds from family Sapotaceae. J Pharmacogn Phytochem 5: 280-287.
- Arbex PM, De Castro Moreira ME, Toledo RCL, Cardoso LDM, Pinheiro-Sant'ana HM, et al. (2018) Extruded sorghum flour (Sorghum bicolor L.) modulate adiposity and inflammation in high fat diet-induced obese rats. J Funct Foods 42: 346-355.
- Liu L, Yasen M, Tang D, Ye J, Aisa HA, et al. (2018) Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed Pharmacother 100: 29-35.
- Baek J, Lee J, Kim K, Kim T, Kim D, et al. (2013) Inhibitory effects of Capsicum annuum L. water extracts on lipoprotein lipase activity in 3T3-L1 cells. Nutr Res Pract 7: 96-102.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences