ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Effect of drought stress on germination and antioxidants enzymes in Ferula gummosa Boiss
Marzieh Pakdaman*, Hasan Ebrahimzadeh, Nima Yazdanbakhsh, Vahid Niknam
Department of Biology, and Center of Excellence in Phylogeny of Living Organisms in Iran, School of Biology College of Science University of Tehran, Iran
Abstract
Ferula gummosa is a monocarpic plant which is native to Iran. Drought was imposed by applying 0%, 5%, 10% and 15% concentrations of polyethylene glycol (PEG) 6000. Rate and percentage of seed germination decreased by drought stress. In the treatment of 15% was not observed seed germination. The mass of seedlings decreased under drought. Protein content was elevated at 5% PEG. The catalase activity increased under drought stress, while other antioxidant enzymes (peroxidase, polyphenol oxidase, superoxide dismutase and ascorbate peroxidase) presented progressive decrease in their activity at all PEG concentrations. These results indicate that the seed germination of Ferula is sensitive to drought.
Keywords
Drought stress, Ferula gummosa, Medicinal plant, Seed germination, Plants
Introduction
Global warming has caused climate change and the spread of drought. Increasing temperature leads to serious problems, especially in arid and semi-arid areas [1]. Drought stress causes the production of reactive oxygen species (ROS), the first biochemical response of the plant under stress, leading to secondary defensive reactions [2]. Drought stress influences physiological processes such as plant water content, carbon dioxide uptake, cell surface oxidation, cell membrane stability, and enzyme activity [3]. Drought reduces the absorption of water and minerals, photosynthesis, and stomatal conductance [4]. One of the major biochemical changes that occur due to soil moisture reduction in plants is the change in the amount of plant protein production. ROS accumulation occurs in plant tissues under drought stress. Plants have evolved protective mechanisms for ROS detoxification [5]. The enzymatic and non-enzymatic defense systems reduce the destructive effects of the ROS. The enzymatic defense system consists of superoxide dismutase (SOD), ascorbate peroxidase (AXP) and catalase [6]. The selection of plants that can tolerate drought is very important in dry environments. However, drought control is difficult in natural conditions. Therefore, drought stress effect on seed germination is a useful criterion for plant tolerance to drought [7]. Therefore, the study of seed germination and seedling growth is very important in the production and quality of agricultural products. Water shortages in the soil delay and reduce germination, decrease the quality and grain yield [8]. Ferula gummosa is a traditional herb which has aromatic compounds and grows extensively on the mountainous slopes of Asia, Iran, Turkmenistan and India. In general, increasing irrigation improves seed size and yield, and dehydration affects the speed, severity and ability of seed germination .The seeds germinate in difficult conditions and have a long sleep, and the seed does not grow until optimized for germination. This feature is useful on the one hand for the plant and its generation's survival. Because there is enough time for plant dispersal. But on the other hand, due to increased production and cultivation of medicinal plants, having long sleep in seeds becomes a problem [9]. The main objective of this study was to evaluate the influence of drought stress on seed germination, seedling growth and antioxidative enzyme activities in Ferula.
Materials and Methodologies
Plant material and culture conditions
This research was carried out in the Laboratory of Plant Physiology at the University of Tehran in October 2015. The seeds were collected from the Urpalang region in Semnan with a length of 37 ° 53 ' and a width of 35 ° 5' at 2800 meters. Seeds surface were sterilized in 20% (v/v) sodium hypochlorite solution for 15 min, followed by three washes with sterile distilled water. The 25 seeds sterilized were placed on a filter paper in 9-cm Petri dishes containing 7 mL of distilled water or 5%, 10%, and 15% of polyethylene glycol 6000 (PEG). The Petri dishes kept according to a completely randomized design in a growth chamber at a temperature of 4 ± 1 ͦ C for 90 days in dark conditions. Each treatment was carried out in triplicate and seedlings were collected and kept at −70°C and used for all the experiments.
Measurement of the percentage of germination and growth parameters
Observing the germination situation of Ferula seeds every day. Germination percentage (GP) and Germination Rate (GP) of seeds were determined after 90 days after culture. The fresh and dry weight of seeds was measured under drought treatment.
(1) 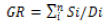
GR= Germination Rate
Si= Number of germinated seeds
Di= Number of days elapsed
(2) 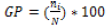
GP= Germination percentage
Ni= Number of germinated seeds in day
N= Total number of seeds
Determination content protein and antioxidant enzymes activity
Seedling material (0.5 g) was homogenized at 4°C with 2 M Tris–HCl (pH 6.8) to estimate different enzyme activities. The homogenate was then centrifuged at 13,249× g for 20 min at 4°C and the obtained supernatant was kept at −70°C and later used for enzyme assays. Protein content was measured by the Bradford method [10].
CAT activity was assayed by measuring the initial rate of disappearance of H2O2. The reaction mixture contained 50 mM phosphate buffer (pH 7.0), H2O2 (3%), and 10 μ L enzyme extract. The decrease in absorption was followed for 180 s and CAT activity was expressed as units per mg of protein [11].
Peroxidase (POX) activity was measured by the reaction mixture contained 2 mL of 0.2 M acetate buffer (pH 4.8), 0.2 mL H2O2 (3%), 0.1 mL 40 mM benzidine, and 0.1 mL enzyme extract. The increase in absorbance was recorded at 530 nm [12].
SOD activity was estimated by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT). In a reaction mixture contained 50 mM potassium phosphate buffer (pH 7.5), 13 mM methionine, 75 μ M NBT, 75 μ M riboflavin, 0.1 mM EDTA, and 100 μ L of enzyme extract. The reaction mixture was irradiated for 16 min and absorbance was read at 560 nm against the non-irradiated blank [13].
Polyphenol oxidase (PPO) activity was assayed by the reaction mixture contained 2.5 mL of 200 mM potassium phosphate buffer (pH 6.8), 0.2 mL of 20 mM pyrogallol, and 20 μ L enzyme extract. The temperature of the reaction mixture was 40°C [14].
APX activity was measured by the reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM H2O2, and 10 μ L of enzyme extract in a total volume of 1 mL. The concentration of oxidized ascorbate was determined by the decrease in absorbance at 290 nm. The concentration of oxidized ascorbate was calculated by using the extinction coefficient (ε = 2.8 mM−1 cm−1). One unit of APX was defined as 1 μ M oxidized ascorbate per min per mg protein [15].
Statistical analysis
experiment was carried out in a completely randomized design. All experiments were performed with three independent replications. Means comparison was performed using SPSS 22 software and analysis of variance by multivariate analysis of variance (MANOVA) and Duncan's test to determine the significance of differences at the level of p ≤ 0.05 %. Principal component analysis (PCA) and hierarchical cluster analysis were used for evaluating the correlation between each pair of variables and performed using XLSTAT (2016) and online CIMminner software and graphs are plotted with Graph Pad Prism 7 software.
Result
Growth parameters
According to the results seeds were not germinated at 15% PEG. Germination decreased with increasing intensity of stress, so germination was completely inhibited at high concentration of stress. At 5% and 10% PEG concentration, dry weight, fresh weight, percentage germination and rate germination decreased (Figure 1).
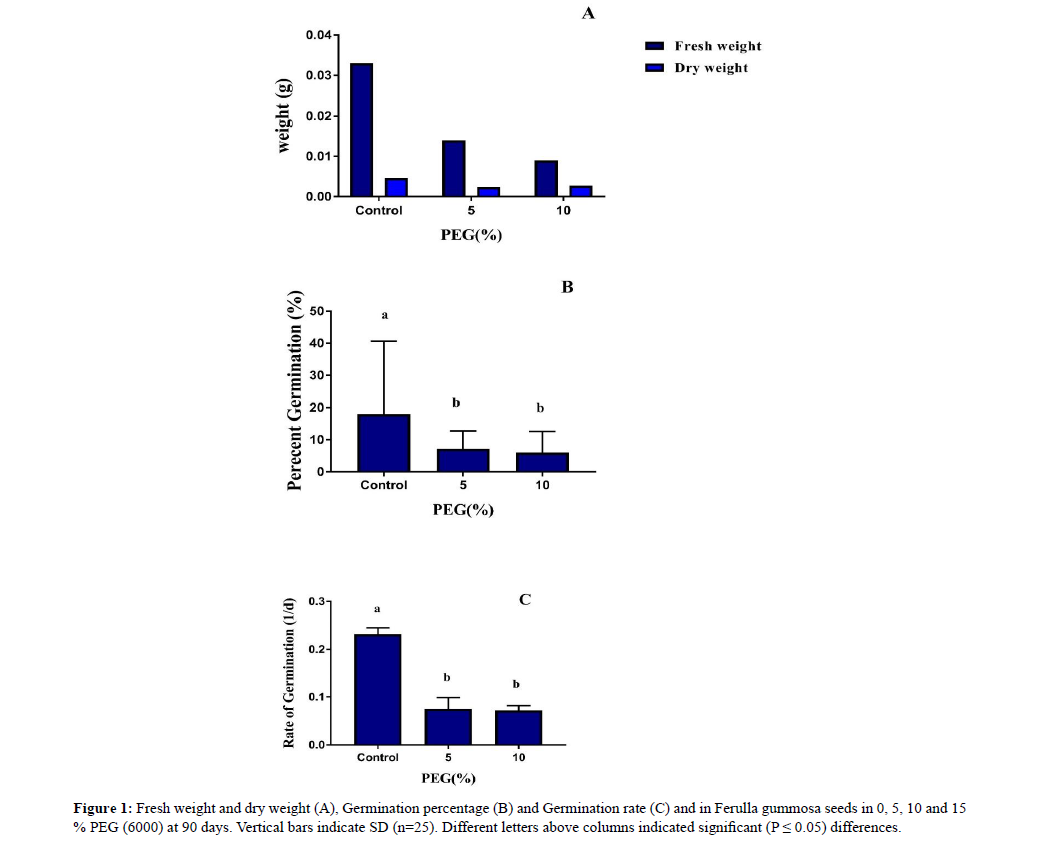
Figure 1: Fresh weight and dry weight (A), Germination percentage (B) and Germination rate (C) and in Ferulla gummosa seeds in 0, 5, 10 and 15 % PEG (6000) at 90 days. Vertical bars indicate SD (n=25). Different letters above columns indicated significant (P ≤ 0.05) differences.
Antioxidant enzymes activity
CAT activity increased under drought stress, but the activity of PPO, POX, SOD and APX decreased under drought condition (Figure 2). The maximum of CAT activity observed at 5% PEG.
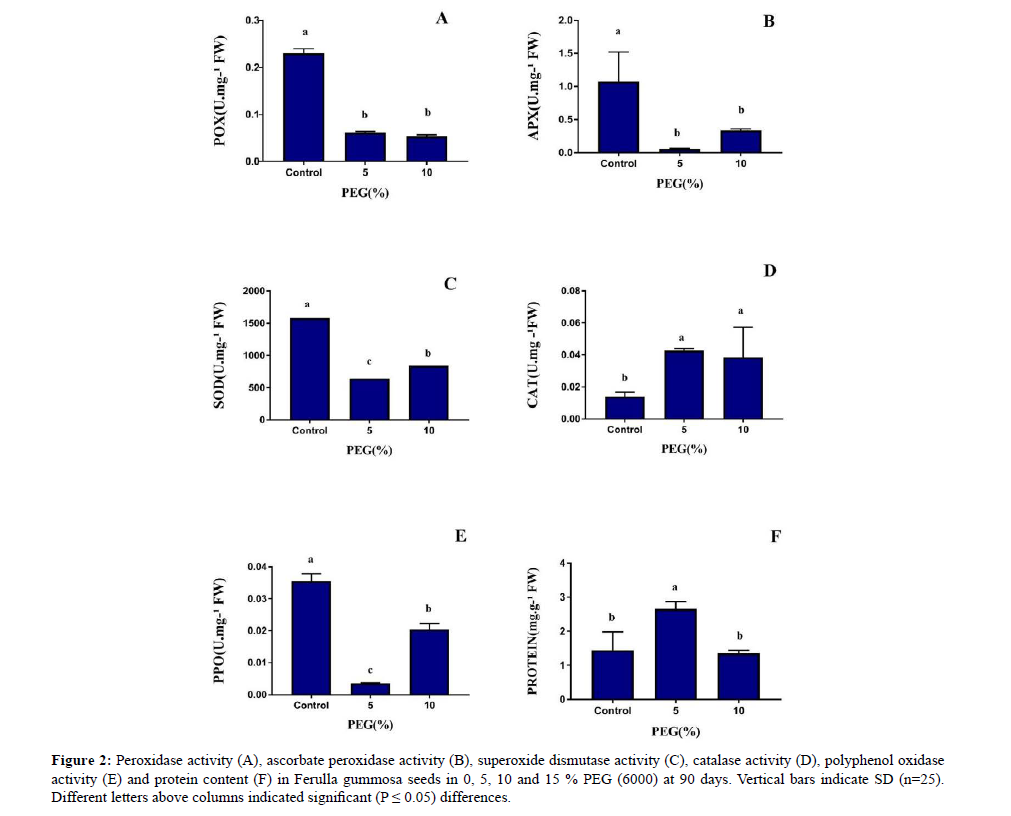
Figure 2: Peroxidase activity (A), ascorbate peroxidase activity (B), superoxide dismutase activity (C), catalase activity (D), polyphenol oxidase activity (E) and protein content (F) in Ferulla gummosa seeds in 0, 5, 10 and 15 % PEG (6000) at 90 days. Vertical bars indicate SD (n=25). Different letters above columns indicated significant (P ≤ 0.05) differences.
Protein content
Protein content initially increased under stress condition then decreased (Figure 3). In PEG concentration of 5% showed highest protein content.
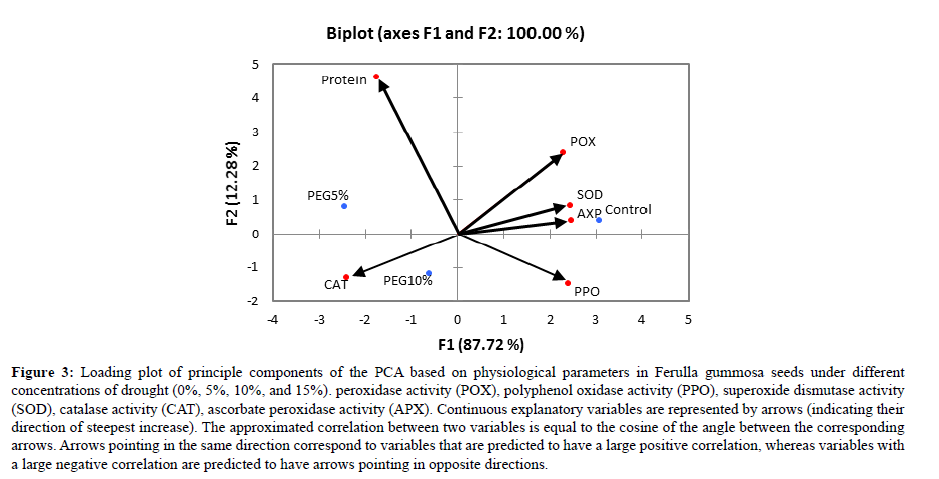
Figure 3: Loading plot of principle components of the PCA based on physiological parameters in Ferulla gummosa seeds under different concentrations of drought (0%, 5%, 10%, and 15%). peroxidase activity (POX), polyphenol oxidase activity (PPO), superoxide dismutase activity (SOD), catalase activity (CAT), ascorbate peroxidase activity (APX). Continuous explanatory variables are represented by arrows (indicating their direction of steepest increase). The approximated correlation between two variables is equal to the cosine of the angle between the corresponding arrows. Arrows pointing in the same direction correspond to variables that are predicted to have a large positive correlation, whereas variables with a large negative correlation are predicted to have arrows pointing in opposite directions.
PCA analyses
According to correlation analyses based on Pearson’s coefficient in Ferulla seedling, SOD showed a positive correlation with POX and APX as well as it showed a negative correlation with CAT.
Discussion
Water stress is one of the largest environmental factors limiting the productivity of plants. Plants have developed physiological and biochemical mechanisms for adaptation and response to environmental stresses [16]. Seed germination is a pivotal stage for plant establishment, which plant is more sensitive to drought stress at this stage. Our results indicated that the delay in germination, decrease in dry weight, germination rate, and germination percentage under drought stress that were consistent with the published reports in other studies on lentil and corn plants. It caused a decrease in growth and Cellular development also makes the synthesis of wall carbohydrates and its longitudinal growth sensitive to drought [17,18].
Abiotic stresses induce accumulation of ROS in cells, which can cause oxidative damage. The system of antioxidant enzymes, including CAT, POX, PPO, etc. Play an important role in the removal of ROS. The results in this study showed a decrease in the activity of antioxidant enzymes, and only CAT activity increased, which is consistent with the results of corn plants [19]. The activity of antioxidant enzymes in plants are indicators for assessing drought resistance [20]. O2- in chloroplasts, mitochondria, cytoplasm, and Peroxisome are changed by SOD to H2O2. POX also plays a key role in suppressing produced H2O2 by SOD. CAT is the main enzyme for H2O2 removal of mitochondria and thus helps to improve the destructive effects of stress. Maintaining a high level of antioxidant activity in the plant will increase its tolerance to stress-induced damage. The ability of antioxidants to eliminate ROS and reduce their destructive effects may correlate with plant resistance to drought stress [21]. Protein content decreased during stress. In stress conditions, the plant makes new proteins to cope with stress. But when the intensity of the stress increases, the peptides are activated and the protein decomposition predominates. Proteins react with free radicals and cause changes in amino acids. Drought stress increases the protein hydrolytic enzymes and causes the accumulation of free amino acids such as proline [22,23]. Dryness also causes changes in the expression of genes. This change in gene expression causes the synthesis of new proteins or alters proteins from the active to an inactive form. Plants that live in arid areas change their genes due to water shortages and reduce protein production [24].
Conclusion
In conclusion, the present study showed that seeds are very sensitive to water stress conditions. Growth parameters, protein content, and activity of the enzymes showed that the seedlings require high humidity and low temperature in the germination stage and have little resistance to drought stress. The natural habitat is snow-covered mountains, so the plant's low resistance to dehydration is not expected. On the other hand, it is very difficult that this plant is cultivated in vitro so cultivate Ferula gummosa under in vitro conditions is important due to its usage in economics, industry, and pharmacology.
References
- Zhang SH, Xu XF, Sun YM, et al. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J Integr Agric. 2018,17(2):336-347.
- Rezayian M, Niknam V, Ebrahimzadeh H. Effects of drought stress on the seedling growth, development, and metabolic activity in different cultivars of canola. Soil Sci Plant Nutr. 2018,64(3):360-369.
- Hellal F, El-Shabrawi H, El-Hady MA, et al. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J Genet Eng Biotechnol. 2017,17(2):203-212.
- Farooq M, Ullah A, Lee D-J, et al. Desi chickpea genotypes tolerate drought stress better than kabuli types by modulating germination metabolism, trehalose accumulation, and carbon assimilation. Plant Physiol Biochem. 2018,126:47-54.
- Wang X, Cai X, Xu C, et al. Drought-Responsive Mechanisms in Plant Leaves Revealed by Proteomics. Int J Mol Sci. 2016, 17(10): 1706.
- Catalá A, Díaz M. Editorial: Impact of lipid Peroxidation on the physiology and Pathophysiology of cell Membranes. Frontiers in Physiology. 2016,7.
- Li H, Li X, Zhang D, et al. Effects of drought stress on the seed germination and early seedling growth of the endemic desert plant Eremosparton songoricum (Fabaceae). EXCLI J. 2013,12:89–101.
- Mut Z, Akay H. Effect of seed size and drought stress on germination and seedling growth of naked oat (Avena sativa L.). Bulg J Agric Sci. 2010,16(4):459-67.
- Rezaeyan S, Rafati AM, Ahmadi G S, et al. The Study of Ultrasonic Waves on dormancy-breaking in Ferula assa-foetida, Ferula gummusa and Myrtus communis. Int J Adv Bio Biomed Res. 2014,2(4):129-33.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976,72(2):248-54.
- Aebi H. [13] Catalase in vitro. Method Enzymol. 1984,105:121-126.
- Abeles FB, Biles CL. Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol. 1991,95(1):269-273.
- Giannopolitis CN, Ries SK. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977,59(2):315-318.
- Raymond J, Rakariyatham N, Azanza J. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochem. 1993, 34(4):927-931.
- Nakano Y, Asada K. Purification of Ascorbate Peroxidase in Spinach Chloroplasts; Its Inactivation in Ascorbate-Depleted Medium and Reactivation by Monodehydroascorbate Radical. Plant Cell Physiol. 198,28(1):131-140.
- Liu H, Wang X, Wang D, et al. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind Crop Prod. 2011,33(1):84-8.
- Keshavarzi MHB. The Effect of Drought Stress on Germination and Early Growth of Sesamum indicum Seedling’ s Varieties under Laboratory Conditions. Int J Agri Manag Dev. 2012,2(4).
- Muscolo A, Sidari M, Anastasi U, et al. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J Plant Interact. 2014,9(1):354-363.
- Li-Ping B, Fang-Gong S, Ti-Da G, et al. Effect of Soil Drought Stress on Leaf Water Status, Membrane Permeability and Enzymatic Antioxidant System of Maize. Pedosphere. 2006,16(3):326-32.
- Abedi T, Pakniyat H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed. 2010,46(1):27-34.
- Anjum SA, Xie X-y, Wang L-c, et al. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011,6(9):2026-32.
- Ashrafi Parchin R, Shaban M. Study on protein Changes in wheat under drought stress. Int J Adv Bio Biomed Res. 2014,2(2):317-20.
- Mohammadkhani N, Heidari R. Effects of drought stress on soluble proteins in two maize varieties. Turk J Biol. 2008,32(1):23-30.
- Selote DS, Khannaâ?Chopra R. Droughtâ?induced spikelet sterility is associated with an inefficient antioxidant defence in rice panicles. Physiol Plant. 2004,121(3):462-71.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
