ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Correlation Between Black Soybean (Glycine Max L.) Genotypes with Harvesting Time on Seeds Acceleration Ageing and Electric Conductivity in Two Seasons
Omer A. Mohamed*, Mohammed A. Mohammed Ali, Kamal Eldin E. M.A.Taha
Department of Aronomy, Faculty of Agriculture, Omdurman Islamic University, P.O.BOX 382, Sudan
Abstract
This study aimed to correlate between nine black soybean genotypes and three harvesting time on seeds acceleration ageing and electric conductivity in two seasons in UNPAD university top farm with split plot design. The parameters were measure seeds acceleration ageing, seeds germination and electric conductivity. The results showed in wet and dry seasons significant different between genotypes. After acceleration aging where high germination in wet season was KA3 94.00% and low was KA2 37.77%, in dry season where high germination was KH4 87.77 % and low was KA2 54, 88%.for three harvesting times the results revealed significant different in wet and dry season where high germination H2 80,37% and low was H3 68,22% in wet season and in dry season were high germination H1 76.59% and low was H356.74%. In wet and dry season electric conductivity at harvesting time and after storage 90 days the result showed that significant different between genotypes. In three harvesting times in wet and dry season at harvesting time and after storage 90 days storage the results revealed no significant different.
Keywords
Black soybean; Genotypes; Harvesting times; Acceleration ageing; Electric conductivity
Introduction
The genetic diversity is a key component of any agricultural yield system. The material from the diverse geographical origin of the crop species can help to ensure conservation of co-adapted gene complexes [1]. The application of genetic variation can also be manipulated either for selecting superior genotypes or to be utilized as parents for the development of future cultivars through hybridization. Genetic improvements could be accelerated if physiological attributes were used as selection criteria [2]. Physiological maturity of seed is considered to occur when the seed has accumulated its maximum dry weight accumulation of seed dry weight and complete transition from green to yellow color. The percent moisture (wet-weight basis) of soybean seed at the maximum dry weight is variable, ranging from 50-62%. There have been multiple attempts to describe PM of soybeans and the developmental stage when it occurs. Determine PM by correlating it with qualitative characteristics was reached at the reproductive development stage R7, described as “pods yellowing” and “50% of leaves yellow. When one mature pod on the main stem was an acceptable indicator of PM and found that seed moisture at PM ranged from 54-62% in soybeans. In the past, a common method for determination of PM of a single seed was to describe the seed as completely yellow, which was not useful for the determination of PM of the whole plant. The study usefulness of several visual indicators of PM determination, and concluded that results from their previous research proved to be a useful indicator in the determination of PM for a single plant or field population of soybeans. Change of color in the soybean hilum was found characterize PM, similar to the presence of an abscission layer (black layer) in corn. The loss of green color from pods may be a useful tool for prompt determination of PM. Seed shrinkage may also be a useful indicator PM in soybeans because seed shrinkage occurred immediately following the loss of green color in seeds [3]. Under subtropical and tropical environments soybean seed (Glycine max (L.) Merrill) are harvested early to avoid deterioration from weathering. Careful afterharvest drying is required and is an important step in maintaining the physiological quality of the seed. Soybean seed should be harvested when the moisture content is in a range of 16-20%. Traditional drying utilizes a high-temperature air stream passed through the seed mass without dehumidification. The drying time is long because the system is inefficient and the high temperature increases the risk of thermal damage to the seed. New technology identified as heat pipe technology (HPT) is available and has the unique feature of removing the moisture from the air stream before it is passed through the seed mass at the same environmental temperature [4].
Two studies were conducted to evaluate the performance of HPT for dry soybean seed. In the first study the seeds were dried from 17.5 to 11.1% in 2 hours and 29 minutes and in the second study the seeds were dried from 22.6 to 11.9% in 16 hours and 32 minutes. This drying process caused no reduction in seed quality as measured by the standard germination, tetrazolium-viability, accelerated aging and seedling vigor classification tests. The only parameter that indicated a slight seed quality reduction was tetrazolium vigor in the second study. It was concluded that the HPT system is a promising technology for drying soybean seed when efficiency and maintenance of physiological quality are desired [5].
On the other hand, seed conservation potential during storage is directly related to environmental conditions (mainly temperature and relative humidity) as pointed out by other researchers. Consequently, it is important that soybean seeds are stored at moderate temperatures and relative humidity below to 70% field-damaged soybean seeds stored at high moisture levels deteriorated even faster. Their oil presented high free fatty acid content, unpleasant flavor and high refining losses. The higher the unsaturation degree of the fatty acids, the faster their concentrations were reduced. A high correlation between free fatty acid content and iron content was found in injured soybean seeds leading to poor quality refined oils. Oil acidity increase during storage was proportional to initial free fatty acid content [6].
The closer to the ideal seed moisture content, the less triglyceride hydrolysis occurred in soybean during storage. Seeds stored at 13% moisture level prevented peroxide formation during 50 days. Based on these facts, this work was conducted aiming mainly to study the relationship between the time of harvesting and soybean storability, seeking to characterize the interaction between physiological and chemical manifestations of the deterioration process. In the natural environment and when stored at ambient room conditions, seeds respond to constantly changing relative humidity and temperatures. Maintaining seeds under controlled conditions lowers metabolic activity, thereby reducing the aging process and increasing the longevity of the seed lot. For most seeds, a cool and dry environment is preferred and for orthodox seeds the cooler and drier the greater the longevity that can be achieved. Which one percent reduction in moisture content doubles the life of the seed and 10 degrees F reduction in temperature doubles the life of the seed [7].
Seed deteriorates through normal physiological reactions and changes that occur within the seed over time. These changes result in the accumulation of deleterious by-products that increase the seed’s vulnerability to external challenges and decrease the ability of the seed to survive. According to optimum protocols for seed storage must take into account the chemical composition of the seed, the physiological status of the seed, and the physical status of water within the seed [8,9]
In addition, it was concluded that the critical moisture content for storage for each seed lot would increase with decreasing storage temperature. The preservation of seed viability and quality in storage is an important trait both for food usage and for seed use. Generally, viability and quality of seeds gradually deteriorate after harvest, but the deterioration in long-term storage depends on the environment, biochemical, biological, and genetic factors. Changes characterized during the aging process in seeds include alterations in membrane protein composition, disruption of the nuclear envelope, protein degradation oxidative stress, and decreases in mRNA translation (Kumar and DNA replication capabilities. Reduced levels of antioxidant enzymes such as superoxide dismutase, catalase, and ascorbate peroxidase can also lead to oxidative damage [10,11]
The longevity of a seed lot is the length of time the seeds remain viable after reaching physiological maturity [12]. For seed storage purposes, longevity is used synonymously with storability. To preserve the initial seed quality, seeds must be properly stored between the time of harvest and the planting of a subsequent crop. The total seed storage period as comprising segments of bulk storage, which is the period from harvest through packaging including conditioning. This study try to now the correlation between nine black soybean genotypes and three harvesting time on seeds acceleration ageing and electric conductivity in two seasons [13].
Materials and Methods
Time and Place of The Experiments
The experiments were conducted at the station of experiment Padjadjaran University Ciparanje Jatinangor, Sumedang Regency, West Java Province. Location attitude of 720 m above sea level, with an average rainfall of 175.3 mm per month and daily temperature 23oC. The experiment during December 2015 until march 2016. There were cultivate nine of soybean genotypes 1. KH 4.2. KBI, CK 5.4. CK 6.5. KA 2.6. KA 3.7. KA 6.8. CIKURAY. 9. DETAM 1. This experiment designing in split plot design These genotypes were main plots and subplots three-time of harvesting H1 harvesting after 50 % physiological maturity when the pods beginning to change color from green to yellow, H2 harvesting after 50 % full change in color and H3 harvesting after 50 % the full maturity and the pods lost its moisture contents, with three replications, each plot consisting of 5 rows each of 300 cm in length and width 200 cm with a row to row distance 40 cm with 15 cm of plant space and the space between plots 100 cm. The crop was grown under Field conditions. Land preparing manuals to make plots. Before sewing add pesticide powder in the holes this sowing is done by hand. The irrigation depended on rain. Weeds control by manuals. Randomly selection plants for samples the samples hold to the green house for drying. Then storage in the storage room under 25oC for 90 days. The aim of this experiment was try to correlate between nine soybean genotypes correlation with harvesting time in seeds acceleration ageing and electric conductivity in two seasons [14].
Accelerated Ageing Test
The accelerated aging test was performed on 50 of seeds placed on a wire mesh screen and suspended over 40 ml of distilled water inside plastic boxes (15.0 × 11.0 × 6 cm), held at 41°C and near 100% air relative humidity for 72 h. After the aging period, seeds were tested for standard germination, and the number of normal seedlings, five-day-old seedlings was evaluated. The germination was calculated as:

After final germination day, the lengths of germinated seedlings were measured (ISTA, 2008), at harvesting time and after 6 months in two seasons.
Electrical Conductivity Test (EC)
Three replications (50-seed) were weighed and immersed in 75 ml of deionizing water within glass cups and kept in a germinator at 25°C, during 24 h. After this period, EC was determined in the imbibition’s solution with a conductivity meter. Analysis of variance was carried out using ANOVA and for mean comparisons LSD.at harvesting time and after 6 months in two seasons.
Statistical Analysis
In these experiments will using Analysis of variance was carried out using ANOVA with SPSS and for mean comparison DUNKN.LSD.
Results and Discussion
Acceleration Aging in Two Seasons
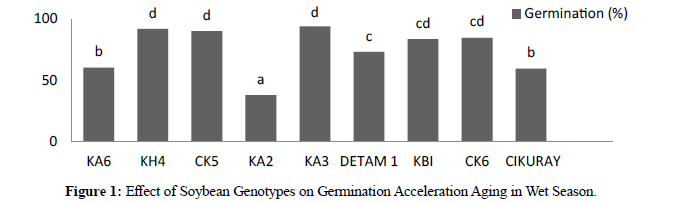
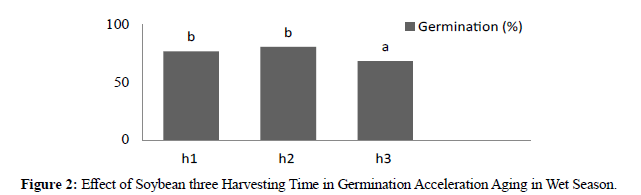
In wet season the result showed significant different between genotypes. After acceleration aging by alcohol where high germination was KA3 94.00% and low was KA2 37.77%. In three harvesting times revealed significant different where H1 76.44%, H2 80,37% and 68,22%. In wet season acceleration aging by alcohol test for vigor the result showed significant different between genotypes where high vigor was KA3 9,12 and low KA2 3.77. For three harvesting times revealed significant different between genotypes where H1 7,61, H2 8,01 and H3 6,73
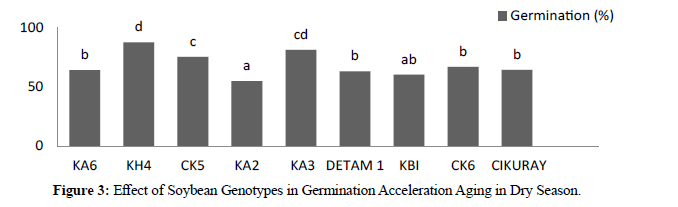
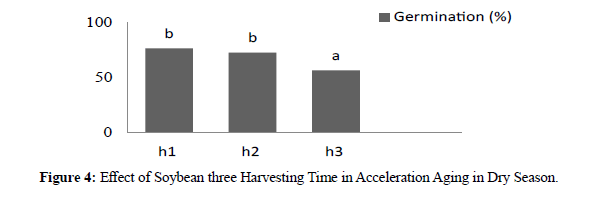
In dry season the result showed significant different between genotypes. After acceleration aging by alcohol where high germination was KH4 87.77 % and low was KA2 54.88%. In three harvesting times revealed significant different where H1 76,59%, H2 72,74% and 56,74%. In dry season acceleration aging by alcohol the result showed significant different between genotypes where high vigor was KA3 7,91 and low was KA2 5,43. For three harvesting times revealed significant different where H1 7,44, H2 7,08, and H3 5,53. Acceleration ageing in two season compered between germination and vigor in Acceleration ageing in wet season was more better than in dry season accept there genotypes showed more better in dry season (KA6, KA2, and cikuray) and seed longevity during stronger for long time due deterioration of this seeds in dry season emergence to environment during seeds harvesting. (Tables 1).
| Treatments | Wet Season (December 2015-March 2016) | Dry Season (April 2016-July 2016) |
|---|---|---|
| Germination (Aceleration Aging) | Germination (Aceleration Aging) | |
| Genotype | ||
| KA6 | 60,44 b | 64,22 b |
| KH4 | 91,77 d | 87,77 d |
| CK5 | 90,22 d | 75,33 c |
| KA2 | 37,77 a | 54,88 a |
| KA3 | 94,00 d | 81,33 cd |
| DETAM 1 | 73,11 c | 63,11 b |
| KBI | 83,55 cd | 60,22 ab |
| CK6 | 84,66 cd | 66,88 b |
| CIKURAY | 59,55 b | 64,44 b |
| CV | 16,8% | 29,3% |
| Harvesting Time | ||
| h1 | 76,44b | 76,59 b |
| h2 | 80,37b | 72,74 b |
| h3 | 68,22a | 56,74 a |
| CV | 16,6% | 20% |
Note: same letter means did not have significant differet effect. Different letter means had significant effect.
Table 1: Acceleration Aging in Two Seasons.
Electric Conductivity in Two Seasons
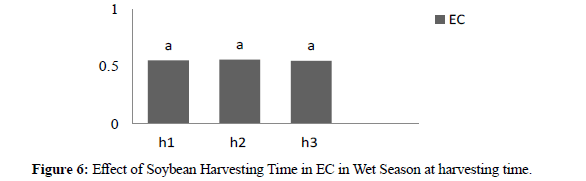
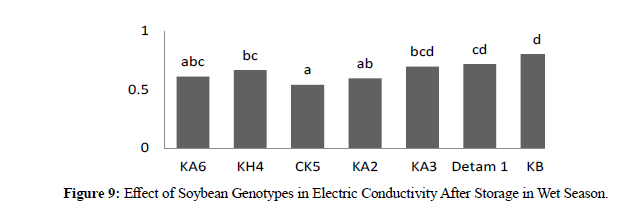
In wet season electric conductivity at harvesting time the result showed that significant different between genotypes where high electric conductivity was CIKURAY 0,631 and low was CK6 0,477. In three harvesting times revealed no significant where H1 0,554, H2 0,559 and H3 0,550. In wet season electric conductivity after storage (90) days the result showed significant different between genotypes where high electric conductivity was KBI 0,803, and low was CK6 0,506. For three harvesting times the result showed no significant different where H1 0,658, H2 0,670 and H3 0,651. (Table 2)
| Treatments | Wet Season (December 2015-March 2016) | Dry Season (April 2016-July 2016) | ||
|---|---|---|---|---|
| EC B. Storage | ECA.Storage 90 days | EC B. Storage | EC A. storage 90 days | |
| Genotype | ||||
| KA6 | 0,508 ab | 0,613 abc | 0,504 a | 0,620 bc |
| KH4 | 0,611 cd | 0,668 bc | 0,605 b | 0,666 cd |
| CK5 | 0,481 a | 0,541 a | 0,463 a | 0,557 ab |
| KA2 | 0,570 bcd | 0,598 ab | 0,504 a | 0,545 a |
| KA3 | 0,554 bc | 0,698 bcd | 0,615 b | 0,681 cd |
| DETAM 1 | 0,544 abc | 0,718 cd | 0,633 b | 0,723 de |
| KBI | 0,612 cd | 0,803 d | 0,706 c | 0,747 e |
| CK6 | 0,477 a | 0,506 a | 0,495 a | 0,557 ab |
| CIKURAY | 0,631 a | 0,790 d | 0,633 b | 0,707 de |
| CV | 11,7% | 13,5% | 7,7% | 8,4% |
| Harvesting Time | ||||
| H1 | 0,554 a | 0,658 a | 0,578 a | 0,652 a |
| H2 | 0,559 a | 0,670 a | 0,569 a | 0,640 a |
| H3 | 0,550 a | 0,651 a | 0,574 a | 0,643 a |
| CV | 13,1% | 15,8% | 12,31% | 9,7% |
Note: same letter means did not have significant differet effect. Different letter means had significant effect
Table 2: Electric Conductivity in Two season.
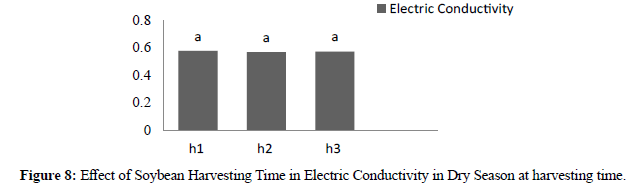
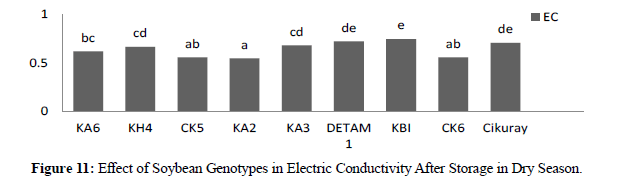
In dry season at harvesting time electric conductivity showed significant different between genotypes where high was KBI 0,706 and low was CK5 0,495. For three harvesting times the result showed no significant different where H1 0,578, H2 0,569 and H3 0,574. In dry season electric conductivity after storage (90) days the result showed significant different between genotypes where high was KBI 0,747 and low was CK5 0,557 and CK6 0,557. In three harvesting times there were no significant different where H1 0,652, H2 0,640 and H3 0,643. Compared with two seasons in electric conductivity between genotypes at harvesting time the result showed low leaching and after storage showed high leaching this due to storage and affected to seed membrane the result similar (Table 3).
| DH | WBD | WAD | P% | A% | C% | O% | |
|---|---|---|---|---|---|---|---|
| EC | 0.67* | -0.42 NS | 0.28 NS | 0.34 NS | 0.72* | -0.46 NS | 0.03 NS |
NS=Means no significant. * means significant. ** means highly significant
Table 3: Correlation between Chemical composition and Electric Conductivity (Genotypes) in Wet Season.
Correlation between electric conductivity and DH, A%, P%, WAD and O% showed positive correlation. Electric conductivity test showed negative correlation with WBD and carbohydrate (Table 4).
| DH | WBD | WAD | P% | A% | C% | O% | |
|---|---|---|---|---|---|---|---|
| E | -0.77* | 0.40 NS | -0.99** | -0.66* | -0.06 NS | 0.90** | -0.74* |
NS=Means no significant. * means significant. ** means highly significant
Table 4: Correlation between Chemical composition and Electric Conductivity (Harvesting Time) in Wet Season.
Correlation between electric conductivity and C% showed highly positive correlation and positive with WBD. Electric conductivity test showed negative correlation with WAD, P%, A%, O% and DH (Table 5).
| DH |
|---|
| WAD | P% | A% | C% | O% | |||
|---|---|---|---|---|---|---|---|
| EC | 0.870** | -0.232 NS | -0.392 NS | 0.262 NS | 0.882** | 0.098 NS | -0.801 |
NS=Means no significant. * means significant. ** means highly significant.
Table 5: Correlation between Chemical Composition and Electric Conductivity (Genotypes) in Dry season.
Correlation between electric conductivity and DH, A%, P%, WAD and C% showed positive correlation. Electric conductivity test showed negative correlation with WBD, WAD and O% (Table 6).
| DH | WBD | WAD | P% | A% | C% | O% | |
|---|---|---|---|---|---|---|---|
| EC | -0.373 NS | 0.610* | 0.330 NS | 0.917** | -0.642* | -.0.100 NS | 0.735* |
N =Means no significant. * means significant. ** means highly significant
Table 6: Correlation between Chemical Composition and Electric Conductivity (Harvesting Time) in Dry Season.
Correlation between electric conductivity and C%, DH and A% showed negative correlation and positive with WBD, WAD and O% (Figures 1-12).
Figure 1:Effect of Soybean Genotypes on Germination Acceleration Aging in Wet Season.
Figure 2:Effect of Soybean three Harvesting Time in Germination Acceleration Aging in Wet Season.
Figure 3:Effect of Soybean Genotypes in Germination Acceleration Aging in Dry Season.
Figure 4:Effect of Soybean three Harvesting Time in Acceleration Aging in Dry Season.
References
- Frankel OH, Brown ADH, Burdon JJ. The Conservation of Plant Biodiversity. Cambridge University Press, Cambridge. 1995, pp:299.
- Saleem K, Abdul L, Sahibzada Qayyum A, Farhad A, Mehvish F. Genetic Variability Analysis in Some Advanced Lines of Soybean (Glycine max L.). Asian Journal of Agricultural Sciences. 2011, 3(2): 138-141.
- Ennen, Ross David. Earlier harvest and drying of soybean seed within intact pods maintains seed quality. 2011.
- Hurburgh, Charles R. Soybean Drying and Storage. Agriculture and Environment Extension Publications. 2008, Book 134.
- Krzyzanowski FC, West SH, Jose De Barros Franca. Drying Soybean Seed Using Air Ambient Temperature At Low Relative Humidity. 2006, 28(2):77-83.
- Shaban, Morad. Review on physiological aspects of seed deterioration. International Journal of Agriculture and Crop Sciences. 2013.
- Copeland LC, Mc Donald MB. Principles of seed science and technology. 2001.
- Gupta A, Aneja KR. Seed deterioration in soybean varieties during storage-physiological attributes. Seed Res. 2004, 32:26-32.
- Justice L, Brass N. Principles and Practices of seed storage. 1978
- Vertucci CW, Leopold AC. Oxidative processes of soybean and pea seeds. Plant Physiol. 1990, 84:1038-1043.
- De Castro RD, Zheng X, Bergervoet JHW, De Vos CHR, Bino RJ. Ã?-tubulin accumulation and DNA replication in imbibing tomato seeds. Plant Physiol. 1995, 109:499-504.
- Bailly C, Benamar A, Corbineau F, Come D. Free radical scavenging as affected by accelerated aging and subsequent priming in sunflower seeds. Physiol Plant. 1998, 104:646-652.
- Delouche JC, Baskin CC. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci Tech. 1973, 1:427-452.
- ISTA (International Seed Testing Association).International Rules for Seed Testing. Zurich: International Seed Testing Association. Seed Sci Tech. 2008.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences