Can Transforming Growth Factor Beta Affect Breast Cancer by Targeting MicroRNA 195?
Ghada Mahmoud Abd El –Aziz2, Mahmoud Mohamed Kamel3, Marwa Alkaffas1*, Ebtehal Gamal Abdelhady2 and Laila Ahmed Rashed1
1Faculty of Medicine, Department of Medical Biochemistry, Unit of Biochemistry and Molecular Biology,Cairo University, Cairo, Egypt
2Faculty of Medicine, Department of Medical Biochemistry and Molecular Biology, Beni-Suef University, Cairo, Egypt
3Clinical Pathology Departement, National Cancer Institute, Cairo University, Cairo, Egypt
- *Corresponding Author:
- Marwa Alkaffas
Faculty of Medicine
Department of Medical Biochemistry
Unit of Biochemistry and Molecular Biology
Cairo University, Cairo, Egypt
Tel: 01148899400
E-mail: madho_taal@yahoo.com
Received date: June 01, 2018; Accepted date: June 11, 2018; Published date: June 17, 2018
Citation: El – Aziz GMA, Kamel MM, Alkaffas M, Abdelhady EG, Rashed LA (2018) Can Transforming Growth Factor Beta Affect Breast Cancer by Targeting MicroRNA 195? J Mol Cell Biochem. Vol.2 No.1:4
Abstract
Background: Breast cancer is commonest non-skin cancer and is the second cause of cancer death in women. miRNA-195 is a tumor suppressor that is downregulated in many cancers. TGF-β is an inflammatory cytokine that stimulates or inhibits miRNA maturation.
Aim: Detect level of miRNA-195 in metastatic & non-metastatic breast cancer patients and study its role in tumorigenesis of breast cancer via possible association with the inflammatory cytokine, TGF-β.
Methods: Expression of miRNA-195 and level of TGF-β were detected in 60 breast cancer female patients and 20 controls using quantitative real-time PCR and ELISA respectively.
Results: MiRNA-195 expression was significantly lower while TGF-β level was significantly higher in metastatic cancer patients compared to non-metastatic patients and healthy controls. Negative correlation was found between miRNA-195 expression and TGF-β in breast cancer patients.
Conclusion: MiRNA-195 may play role as tumor suppressor of breast cancer, particularly in metastatic group, may be through TGF-β signaling.
Keywords
Breast cancer; MiRNA-195; TGF-β
Introduction
Breast cancer (BC) is the leading cause of cancer death among females. Breast cancer is a heterogeneous tumor; it has a wide array malignant characteristics and clinical features. Not only the diversity in molecular abnormalities, but also the aberrant posttranscriptional modulation of gene expression by microRNAs (miRNAs) contributes to the imbalance between oncogenes and tumor suppressors in BC [1].
MicroRNAs are a novel class of small non-coding single-stranded RNA molecules involved in various biological processes; posttranscriptional gene regulation which affects mRNA stability and protein translation, apoptosis, development, proliferation and differentiation. So, miRNAs play fundamental role in tumorigenesis [2]. They included in the regulation of the inflammatory microenvironment in cancer [3].
Inflammatory mediators constitute a major part of the tumor microenvironment. They also have important roles in regulation of tumor initiation, proliferation and metastasis. Transforming growth factor-β (TGF-β) is an inflammatory cytokine that contributes to both tumor suppression and promotion; but, its underlying mechanism is still unclear. Recent evidence suggests an association between TGF-β signaling and miRNAs, which provides new insight into the interactions occurring in the inflammatory microenvironment of the tumors [4].
Previous studies suggest that TGF-β pathway can stimulate or inhibit miRNA maturation [4]. One unique feature of TGF-β is its paradoxical role; it acts as a tumor suppressor in the low-malignant stages of tumorigenesis, whereas it promotes proliferation of the high-malignant cancer cells. The mechanisms by which TGF-β affects miRNA expression are either transcriptional or posttranscriptional [5]. The most prominent examples of miRNAs that are down-regulated by TGF-β signaling are miRNA-200, miRNA-584, miRNA-34a and miRNA-450b-5p. Whereas others up-regulated by TGF-β signaling are miRNA-21, miRNA-181, miRNA-494, and miRNA-10 [4]. MicroRNA-195 (miRNA-195) is a tumor suppressor as it is down-regulated in many cancers as hepatocellular, bladder, gastric, tongue, ovarian, peritoneal, adrenocortical, and colorectal. However, the results of the studies that examined the role of miRNA-195 in breast cancer progression and carcinogenesis is still inconsistent [3].
Materials and Methods
Study subjects
The study included 60 Egyptian women with breast cancer at different stages, their age ranged from (23-76 years). They were recruited from KasrAlainy hospital. Patients were diagnosed by clinical examination and confirmed by mammography and surgical biopsies 20. Healthy controls recruited during routine checkup, who were proven to be healthy with no family history of breast cancer.
Informed consent was obtained from the participants in this study after ethical committee approval from Medical Biochemistry Department, Faculty of Medicine Cairo University. All cases were subjected to estimation of expression level of miRNA 195 and TGF-β in serum.
The studied subjects were divided into two groups as follows
Group I: (n=20) healthy females as a control group with no family history of breast cancer, or any other diseases.
Group II: (n=34) non-metastatic breast cancer patients with or without nodal affection.
Group III: (n=26) metastatic breast cancer patients with nodal affection and distant metastasis (bone, liver and lung).
Blood sampling
Blood samples were collected from all subjects, 60 breast cancer patients and 20 healthy females as normal controls. Serum was separated from blood sample and stored at–80°C to be used for detecting level of miRNA-195 using qRT PCR and levelof TGF-β by ELISA.
Detection of miRNA195 gene expression by quantitative real-time PCR: RNA extraction
Total RNA was extracted from 100μL of serum using (Qiagen, Valencia, CA, USA) according tothe manufacturer’s instructions. The concentration and purity of RNA were determined using NanoDrop® ND-1000.
cDNA synthesis and quantitative reverse transcriptase polymerase chain reaction (RTPCR)
RNA (5μg) was used per 20 μL reaction to generate cDNA using Gene-specific primers from the TaqMan microRNA Assays and reagents from the TaqMan microRNA ReverseTranscription kit (Applied Biosystems, Foster City, CA, USA), real-time PCR was carried out using an Invitrogen kit. Primers for miR-195 supplied from Applied Biosystems (TaqManmiRNA Assays ID 000452) in Step One Plus (Applied Biosystem,USA) device. MicroRNA expressions were normalized to small RNA U6, with the similar efficiency of microRNAs. Data of quantitative RT-PCRwere demonstrated by nominal CT value (normalized toU6), and fold changes were calculated by ΔΔCt. The ΔΔCt (ΔΔCt=ΔC samples− ΔCtcontrol) to qualify the expression rate of miR-195.
Detection of TGF-β using ELISA
TGF-β was detected by Quantikine® ELISA kit supplied by (R&D systems, USA, Catalog Number DB100B) according to manufacturer instruction.
Statistical analysis
Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 24. Data was summarized using mean, standard deviation, median deviation in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data using the non-parametric Kruskal-Wallis and Mann-Whitney tests [5]. Correlations between quantitative variables were done using Spearman correlation coefficient [6]. P-values less than 0.05 were considered as statistically significant.
Results
Clinical characteristics of the patients
Clinical characteristics between metastatic and non-metastatic groups were compared showing no statistically significant difference about menopausal status (p=0.255) and family history (p=0.223), while staging was significantly different between both groups (p<0.001), as shown in Table 1.
| Comparison | Metastasis | No metastasis | P value | |||
|---|---|---|---|---|---|---|
| Count | % | Count | % | |||
| Menopause | Pre | 21 | 80.8% | 23 | 67.6% | 0.255 |
| Post | 5 | 19.2% | 11 | 32.4% | ||
| Family history | Yes | 9 | 34.6% | 7 | 20.6% | 0.223 |
| No | 17 | 65.4% | 27 | 79.4% | ||
| Staging | II | 0 | 0.0% | 17 | 50.0% | <0.001 |
| II then IV | 1 | 3.8% | 0 | 0.0% | ||
| III | 0 | 0.0% | 17 | 50.0% | ||
| III then IV | 9 | 34.6% | 0 | 0.0% | ||
| IV | 16 | 61.5% | 0 | 0.0% | ||
Table 1: Comparison between metastatic and non-metastatic groups regarding the clinical data.
Pathological characteristics of the patients
Comparing pathological data of metastatic and non-metastatic groups showed no statistically significant difference in ER (p=0.234), PR (p=0.956), site (p=0.155) and histological type (p=0.114). Whereas, HER-2 status (p=0.016) and molecular subtypes (p<0.001) were statistically significant, as shown in Table 2.
| Comparison | Metastatic | Non-metastatic | P value | |||
|---|---|---|---|---|---|---|
| Count | % | Count | % | |||
| HER 2 | Positive | 14 | 53.8% | 8 | 23.5% | 0.016 |
| Negative | 12 | 46.2% | 26 | 76.5% | ||
| PR | Positive | 17 | 65.4% | 22 | 64.7% | 0.956 |
| Negative | 9 | 34.6% | 12 | 35.3% | ||
| ER | Positive | 18 | 69.2% | 28 | 82.4% | 0.234 |
| Negative | 8 | 30.8% | 6 | 17.6% | ||
| Histological type & grade | IDC & ILC | 1 | 3.8% | 4 | 11.8% | 0.114 |
| IDC II | 19 | 73.1% | 28 | 82.4% | ||
| IDC III | 4 | 15.4% | 1 | 2.9% | ||
| ILC | 2 | 7.7% | 0 | .0% | ||
| Medullary | 0 | 0.0% | 1 | 2.9% | ||
| Site | Bilateral | 3 | 11.5% | 0 | 0.0% | 0.155 |
| Left | 12 | 46.2% | 17 | 50.0% | ||
| Right | 11 | 42.3% | 17 | 50.0% | ||
| Molecular subtypes | Luminal A | 1 | 3.8% | 4 | 11.8% | <0.001 |
| Luminal B | 9 | 34.6% | 17 | 50.0% | ||
| HER-2 enriched | 14 | 53.8% | 8 | 23.5% | ||
| Triple negative | 2 | 7.7% | 5 | 14.7% | ||
Table 2: Comparison between both groups regarding pathological data.
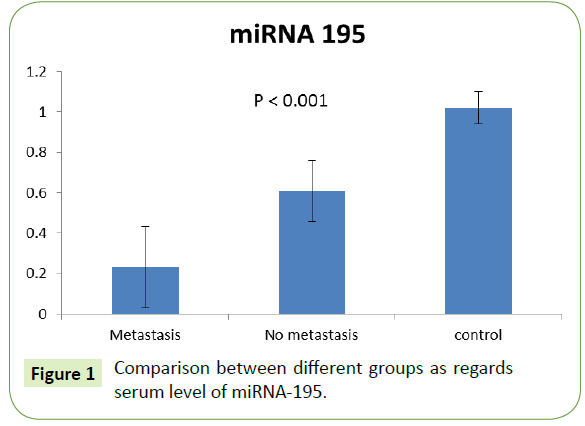
Serum level of MiRNA-195 among groups
The expression of miRNA-195 was significantly lower in metastatic group (0.23 ± 0.20) rather than in the non-metastatic one (0.61 ± 0.15). Both showed significant decrease compared to control group with p value (<0.001), as shown in Figure 1.
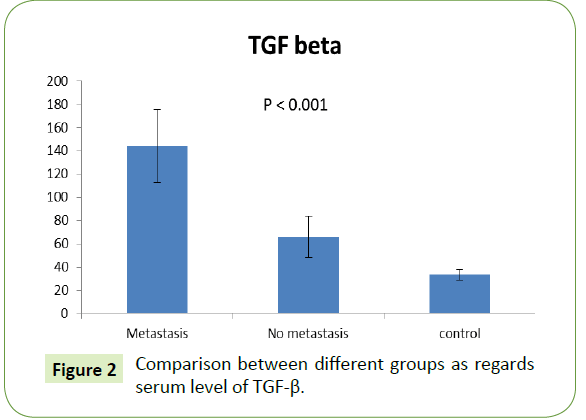
Serum level of TGF-β among different groups
The serum level of TGF-β was significantly higher in metastatic group (65.90 ± 17.93) rather than in the non-metastatic one (0.61 ± 0.15). Both showed significant increase compared to control group with p value (<0.001), as shown in Figure 2.
Comparison between miRNA and TGF-β regarding stage at diagnosis, single or multiple organs metastatic disease and Patients diagnosed as metastatic disease from the start & those developed metastasis after period of time
As shown in Table 3, For subgroup analysis among non-metastatic group according to stage at diagnosis: there was no statistically significant difference (p=0.849) in miRNA-195 expression between stage II group patients (0.61+0.16) and stage III group (0.60+0.15). Also, there was no significant difference (p=0.184) between patients with Stage II (69.76 ± 17.99) and stage III (62.03 ± 17.53) regarding TGF- β.
| Comparison | miRNA 195 Mean ± Standard deviation |
P value | TGF-β (pg/ml) Mean ± Standard deviation |
P value |
|---|---|---|---|---|
| Stage in non-metastatic group II |
0.61 ± 0.16 | 0.849 | 69.76 ± 17.99 | 0.184 |
| Stage in non-metastatic group III | 0.60 ± 0.15 | 62.03 ± 17.53 | ||
| Single organs metastatic disease | 0.27 ± 0.24 | 0.391 | 146.40 ± 31.96 | 0.586 |
| Multiple organs metastatic disease | 0.17 ± 0.07 | 140.57 ± 31.35 | ||
| Diagnosed as metastatic disease from the start | 0.25 ± 0.24 | 0.452 | 157.60 ± 30.67 | 0.004 |
| Developed metastasis after period of time | 0.21 ± 0.11 | 122.65 ± 17.50 |
Table 3: Comparison between miRNA and TGF-β regarding stage at diagnosis, single or multiple organs metastatic disease and Patients diagnosed as metastatic disease from the start and those developed metastasis after period of time.
As well as in metastatic group, there was no significant difference (p=0.391) in serum miRNA-195 expression between patients with single organ of metastasis (0.27 ± 0.24) and patients with multiple organs of metastasis (0.17 ± 0.07), As well asserum TGF-β level, there was no significant difference (p=0.586) between patients with single organ of metastasis (146.40 ± 31.96) and in patients with multiple organ of metastasis (140.57 ± 31.35).
Also, no significant difference (p=0.452) in miRNA-195 expression between patients who were diagnosed as non-metastatic & developed metastasis after period of time (0.21+0.11) and those diagnosed as metastatic from the start (0.25+0.24). Serum TGF-β showed significantly lower (p=0.004) in patients who were diagnosed as non-metastatic & developed metastasis after period of time (122.65 ± 17.50) rather than those diagnosed as metastatic from the start (157.60 ± 30.67).
Association between miRNA-195, TGF-β and hormonal status (ER & PR)
There was no significant difference between serum miRNA-195 level and hormonal status for both ER and PR as well asthe relation between serum TGF-β level & hormonal status for both ER & PR as shown in Table 4.
| Association | miRNA 195 Mean ± Standard Deviation |
P value | TGF-β (pg/ml) Mean ± Standard Deviation |
P value |
|---|---|---|---|---|
| ER positive | 0.45 ± 0.23 | 0.570 | 94.33 ± 40.84 | 0.202 |
| ER negative | 0.43 ± 0.34 | 117.81 ± 58.33 | ||
| PR positive | 0.44 ± 0.24 | 0.969 | 92.88 ± 41.71 | 0.156 |
| PR positive | 0.46 ± 0.29 | 112.68 ± 51.84 |
Table 4: Association between miRNA-195 and TGF-β regarding (ER&PR) status.
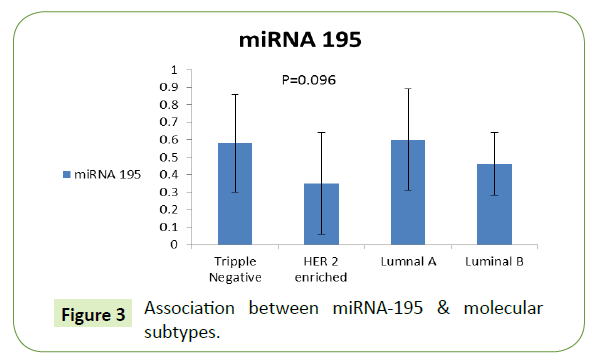
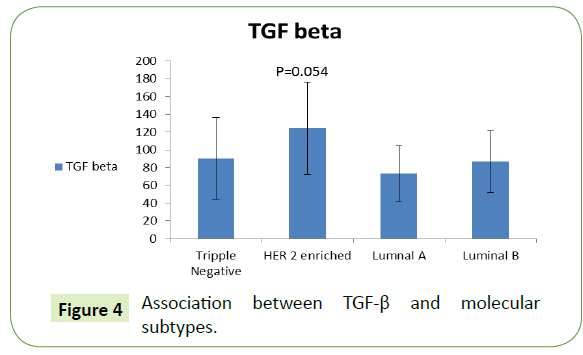
Association between miRNA-195, TGF-β & molecular subtypes
The level of miRNA-195 was higher for luminal A patients (0.60 ± 0.29), followed by TNBC subtype then luminal B then HER-2 enriched subtype with no significant difference as shown in Figure 3.
There was higher level of TGF-β for HER-2 enriched patients (124.45 ± 51.99), followed by TNBC then luminal B then luminal A no significant difference (Figure 4).
Discussion and conclusion
Breast cancer is the commonest non-skin cancer and it is the second cause of cancer death in women worldwide [7]. Early detection of breast cancer is the key to successful treatment and patient survival [8]. Screening techniques are of limited use in screening due to their reduced sensitivity and specificity [8]. So, searching for effective non-invasive biomarkers is vital for the screening of breast cancer.
MicroRNA (miRNA) is a non-coding short RNA; about 22 nucleotides. miRNAs are estimated to regulate at least the third of human genes. They contribute by either negative or positive regulation [9]. Previous studies reported that the aberrant miRNAs expression had been implicated in the development and progression of breast cancer in addition to their involvement in the tumor-associated signal transduction pathways [8]. Transforming growth factor-β (TGF-β) is an inflammatory cytokine that contributes to both tumor suppression and promotion; but, its underlying mechanisms still unclear. Recent evidence suggests an association between TGF-β signaling and miRNAs, which provides new insight into the interactions occurring in the inflammatory microenvironment of the tumors [2].
MicroRNA-195 (miR-195) is a tumor suppressor as it is downregulated in many cancers as hepatocellular, bladder, gastric, tongue, ovarian, peritoneal, adrenocortical, and colorectal. However, the results of the studies that examined the role of miR-195 in breast cancer progression and carcinogenesis is still inconsistent [10].
The aim of this work was to detect the level of microRNA-195 in metastatic and non-metastatic breast cancer patients and to study its role in tumorigenesis of breast cancer through its possible association with the inflammatory cytokine, TGF-β.
As regards clinicopathological data, [11] determined that worse prognosis of breast cancer was associated with overexpression of HER-2; as it was associated with premenopausal status, invasive ductal carcinoma and estrogen receptor positive tumors.
Regarding miRNA-195 level among metastatic group, [12] who reported that miRNA-195 expression was significantly decreased in 40 breast cancer specimens when compared with that in the adjacent normal breast tissues using quantitative polymerase chain reaction (qPCR), they also induced exogenous over-expression of miRNA-195 that leads to reduced cell colony formation, suppressed cell migration and caused an accumulation of cells in the G1 phase of the cell cycle. All of their data suggest that miRNA-195 is a tumor suppressor that may inhibit carcinogenesis in human breast cancer.
Also, these results were in accordance to [10] who compared miRNA-195 in 210 primary breast cancer and 102 age-matched normal serum samples obtained from healthy females using real-time RT-PCR. miRNA-195 was down-regulated in breast cancer compared with control samples [13], who reported that miRNA-195 was found to be significantly downregulated in breast cancer tissues and cell lines.
For subgroup analysis among non-metastatic group according to stage at diagnosis; [13] reported no significant differences of miRNA-195 expression among different breast cancer stages.
Regarding the serum level of TGF-β among metastatic group, [14] who used the immunohistochemistry to examine TGF-β protein expression in 497 breast cancer tissues and 40 benign breast tumor tissues as controls, and reported that the positive staining of TGF-β was mainly observed on the cytoplasm of cells in the breast cancer tissues but rarely detected in the benign breast tissues. In addition, [14] compared the TGF-β positive group with those with TGF-β negative staining, finding that the TGF-β positive group had poor differentiation in histology.
The result of [15] also supported this current study; as they treated MCF-7 and MDA-MB-231 breast cancer cells with TGF-β1; showing that TGF-β1 significantly induced the occurrence of epithelial mesenchymal transition, migration, invasion and metastasis in both cell lines.
The results of the present study was also in accordance to [16] who used ELISA to detect the level of TGF-β in the 24-hours drained fluid from the wound of breast-conserving surgery in 23 patients with breast cancer patients; finding that its level was increased.
Also the association between miRNA-195 expression and hormonal status was studied for both Estrogen receptors (ER) and Progesterone receptors (PR) [13], showed no significant difference of miRNA-195 expression neither between ER positive versus ER negative tumors, nor between PR positive and PR negative tumors. In addition, results of [10], reported no significant relationships between miRNA-195 expression and other clinic pathological parameters as ER or PR status of breast cancer. [17] who analyzed the expression status of miRNA-195 in different breast cancer subtypes. Their data obtained that the expression of miRNA-195 was significantly down-regulated in HER2-positive breast epithelial cells compared to normal cells. Also, it was significantly downregulated in the HER2-positive subtype compared to luminal subtypes (luminal A & luminal B) of breast cancers. Hence, miRNA-195 expression was significantly down-regulated in aggressive subtypes of breast cancers. Also, [18] who reported that the expression of miRNA-195 was found to be significantly downregulated in samples of TNBC patients when compared to their age matched healthy samples, measured using quantitative real-time PCR.
As regards the association between serum TGF-β level and molecular subtypes, [19] who measured mRNA expression and protein levels of TGF-β1 and β2 in human breast cancer cells lines (TNBC cells and non-TNBC cells) using real-time polymerase chain reaction (RT-PCR) and ELISA respectively, and reported that both TGF-β1 and TGF-β2 protein expression was significantly increased in TNBC cells compared to other subtypes.
Conflicts of Interest
Author does not have any conflict of interest to declare.
References
- Bisso A, Faleschini M, Zampa F, Capaci V, De Santa J, et al. (2013) Oncogenic miR-181a/b affects the DNA damage response in aggressive breast cancer. Cell Cycle 12: 1679-87.
- Guo L, Zhang Y, Zhang L, Huang F, Li J, et al. (2016) MicroRNAs, TGF- β signaling, and the inflammatory microenvironment in cancer. Tumour Biology 37: 115-125.
- Zhou Q, Xiao X, Wang C, Zhang X, Li F, et al. (2014) Decreased microRNA-155 expression in ocular Behcet’s disease but not in Vogt Koyanagi Harada syndrome. Investigative ophthalmology and visual science 53: 5665-5674.
- Gong C, Qu S, Liu B,Liu B, Pan S, et al. (2015) MiR-106b expression determines the proliferation paradox of TGF-β in breast cancer cells. Oncogene 34: 84-93.
- Chan YH (2003) Biostatistics 102: Quantitative data-parametric and non-parametric tests. Singapore Med J 44: 391-396.
- Chan YH (2003) Biostatistics 104: Correlational Analysis. Singapore Med J 44: 614-619.
- Hospital AT, Road NHE, Hong S (2013) FDG PET-CT for diagnosis of distant metastases in breast cancer patients: A meta-analysis. Surgical Oncology 22: 139-43.
- Fu L, Li Z, Zhu J, Wang P, Fan G, et al. (2016) Serum expression levels of microRNA-382-3p, -598-3p, -1246 and -184 in breast cancer patients. Oncology letters 12: 269-274.
- Wang H (2016) Predicting microRNA biomarkers for cancer using phylogenetic tree and microarray analysis. International journal of molecular sciences 17: E773.
- Wang F, Zhao Y, Dou X (2014) Serum microRNA-195 is down-regulated in breast cancer: A potential marker for the diagnosis of breast cancer. Mol Biol Rep 41: 5913-5922.
- Badowska-Kozakiewicz M, Sobol M, Patera J, Kozłowski W (2013) Immunohistochemical evaluation of human epidermal growth factor receptor 2 and estrogen and progesterone receptors in invasive breast cancer in women. Archives of Medical Science 9: 466-471.
- Luo Q, Wei C, Li X, Li J, Chen L, et al. (2014) MicroRNA-195-5p is a potential diagnostic and therapeutic target for breast cancer. Oncology reports 31: 109-1102.
- Li D, Zhao Y, Liu C, Chen X, Qi Y, et al. (2011) Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clinical Cancer Research 17: 1722-1730.
- Xu C, Wang Z, Cui R, He H, Lin X, et al. (2015) Co-expression of parathyroid hormone related protein and TGF-Beta in breast cancer predicts poor survival outcome. BMC Cancer 15: 925.
- Ma M, He M, Jiang Q, Yan Y, Guan S, et al. (2016) MiR-487a promotes TGF-β1-induced EMT, the migration and invasion of breast cancer cells by directly targeting MAGI2. Int J Biol Sci 12: 397-408.
- Scherer D, Bauer J, Schmaus A, Neumaier C, Herskind C, et al. (2016) TGF-β1 is present at High Levels in wound fluid from breast cancer patients immediately post-surgery and is not increased by intraoperative radiation therapy (IORT). PloS One 11: e0162221.
- Tashkandi H, Shah N, Patel Y, Chen H (2015) Identification of new miRNA biomarkers associated with HER2-positive breast cancers. Oncoscience 2: 924-929.
- Thakur S, Grover RK, Gupta S, Yadav AK, Das BC (2016) Identification of specific miRNA signature in paired sera and tissue samples of indian women with triple negative breast cancer. PLoS ONE 11: 1-21.
- Kim S, Lee J, Jeon M, Nam SJ, Lee JE (2015) Elevated TGF-β1 and -β2 expression accelerates the epithelial to mesenchymal transition in triple-negative breast cancer cells. Cytokine 75: 151-158.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences