ISSN : 2573-4466
Insights in Enzyme Research
A Preliminary Cost Effective Qualitative Assay for Diastase Analysis in Honey
Sudhanshu Saxena1 and Satyendra Gautam1,2,*
1Food Technology Division, Bhabha Atomic Research Centre, Mumbai, Maharashtra, India
2Homi Bhabha National Institute, Anushaktinagar, Mumbai, Maharashtra, India
- *Corresponding Author:
- Dr. S Gautam
Food Science and Safety Section
Food Technology Division
Bhabha Atomic Research Centre (BARC)
Homi Bhabha National Institute (HBNI)
Trombay, Mumbai-400 085, Maharashtra, India
Tel: +912225595379
Email: sgautam@barc.gov.in
Received Date: February 03, 2017 Accepted Date: March 04, 2017 Published Date: March 09, 2017
Citation: Saxena S, Gautam S. A Preliminary Cost Effective Qualitative Assay for Diastase Analysis in Honey. Insights Enzyme Res. 2017, 1:1. doi: 10.21767/2573-4466.100007
Abstract
Quality characteristics of honey can be evaluated in terms of some of its biochemical characteristics which are quite unique to honey and not easy to be adulterated. One such important quality index is its diastase enzyme activity. Primary source of diastase in honey is considered to be secretions from the salivary and hypopharyngeal glands of foraging bees. For ease of evaluation, a simple and cost effective “In-vitro Starch Hydrolysis Plate Assay (ISHPA)” is proposed here that has been compared with the existing standard Schade method (Diastase number) and displayed a good correlation (R2=0.93). The proposed method is quite simple, qualitative, cost effective and suitable for routine analysis of diastase activity in different honey samples.
Keywords
Schade method; Starch hydrolysis; HMF; ISHPA
Introduction
Honey is naturally occurring functional food having immense medicinal importance [1-4]. It is produced by bees (Apis mellifera) from biochemical transformation of carbohydrate rich exudates produced by plants. Honey is primarily a complex mixture mainly comprising of reducing sugars, water and other minor components including amino acids, proteins, enzymes, hormones, vitamins, essential oils, lipids, phenolic compounds, pigments, sterols, phospholipids, organic acids, and mineral salts [5]. The composition, color, aroma and flavor of honey depend mainly on the flowers, geographical regions, climate and honeybee species involved in its production. Besides it is also affected by weather conditions, processing, manipulation, packaging and storage time [5]. Diastase is an enzyme naturally present in honey and routinely used as an index for its quality evaluation [6]. Its function is to digest the starch molecule in a mixture of maltose (disaccharide) and maltotriose (trisaccharide). Diastase activity should be determined after processing and blending for all retail honey (USDA draft; Codex draft). As the diastase enzyme is sensitive to heat (thermolabile), therefore its reduced activity consequently indicates about the overheating of honey during thermal processing. Besides, during prolonged storage of honey also the diastase activity significantly reduces [7]. In general two different methods are routinely used to determine honey diastase. The traditional Schade method uses starch as a substrate and determines the diastase activity expressed in Schade units [8]. The Phadebas method works on the same principle however; it uses an artificial substrate [8]. There is a very good correlation between the diastase activity expressed in Schade units and the absorbance measured with the Phadebas test. In the current study, a simple preliminary qualitative assay system for measuring the diastase activity of honey is proposed. The test was performed with seventeen unprocessed honey samples collected from different geographical regions of India. Correlation analysis was also performed with standard diastase analysis methods that indicated that zone of clearance could serve as an effective qualitative index to enumerate the diastase number in honey. Thus the proposed methodology offers a cost effective, simple qualitative alternative to ascertain the diastase activity in honey in terms of zone of clearance.
Materials and Methods
Different floral raw (unprocessed) honey samples seventeen in number were procured from different geographical regions of India such as Indo Gangetic region (IGR); North Eastern region (NER); Southern Peninsular Region (SPR) through suppliers recognized by the National Honey Board (NHB), India. Details pertaining to the region of honey procurement and associated longitude and latitude of these regions have been mentioned in Table 1.
| Honey | Collection site | Latitude, Longitude | Diastase zone (mm) | Diastase number (DN) | Protein content (µg/g) |
|---|---|---|---|---|---|
| NER-1 | Imphal, Manipur | 24.8170° N, 93.9368° | 27 ± 1.1 | 26.7 | 195 ± 4.2 |
| IGR-1 | East Champaran, Bihar | 26.6098° N, 84.8568° | 29 ± 1.0 | 31.0 | 1691 ± 21.2 |
| IGR-2 | Muzaffarpur, Bihar | 26.1209° N, 85.3647° | 28 ± 1.0 | 29.0 | 2489 ± 32.4 |
| IGR-3 | Samastipur, Bihar | 25.8630° N, 85.7810° | 25 ± 1 | 21.5 | 175 ± 3.1 |
| IGR-4 | Begusarai, Bihar | 25.5184° N, 86.1752° | 18 ± 1 | 2.0 | 810 ± 15.3 |
| IGR-5 | Bharatpur, Rajasthan | 27.2170° N, 77.4895° | 26 ± 1.0 | 24.5 | 218 ± 2.2 |
| IGR-6 | Neemuch, Madhya Pradesh | 24.4764° N, 74.8624° | 27 ± 0.7 | 24.0 | 477 ± 2.5 |
| SPR-1 | Baroda, Gujrat | 22.3072° N, 73.1812° | 19 ± 0.7 | 3.5 | 561 ± 3.2 |
| SPR-2 | Sahyadri, Maharashtra | 18.9838° N, 73.7695° | 12 ± 0.8 | 1.65 | 882 ± 7.4 |
| SPR-3 | Bhimashankar, Maharashtra | 19.0720° N, 73.5357° | 30 ± 1.1 | 32.5 | 502 ± 6.1 |
| SPR-4 | Kastalav, Maharashtra | 17.6805° N, 74.0183° | 25 ± 0.6 | 21.0 | 1096 ± 10.2 |
| SPR-5 | Mahabaleshwar, Maharashtra | 17.9307° N, 73.6477° | 32 ± 1.3 | 45 | 1650 ± 21.2 |
| SPR-6 | Kolhapur, Maharashtra | 16.7050° N, 74.2433° | 28 ± 1.0 | 29.7 | 632 ± 4.3 |
| SPR-7 | Nagpur, Maharashtra | 21.1458° N, 79.0882° | 30 ± 0.8 | 34.0 | 703 ± 5.7 |
| SPR-8 | Nanded, Maharashtra | 19.1383° N, 77.3210° | 19 ± 1.1 | 4.5 | 334 ± 2.4 |
| SPR-9 | Akola, Maharashtra | 20.7059° N, 77.0219° | 16 ± 0.8 | 1.65 | 200 ± 1.8 |
| SPR-10 | Warangal, Andhra Pradesh | 17.9689° N, 79.5941° | 15 ± 0.5 | 1.5 | 230 ± 21.2 |
Table 1: Unprocessed honey collections and their associated diastase profile and protein content.
Diastase activity in terms of Diastase Number (DN)
The diastase activity (Diastase number, DN) was calculated following the Schade method of the International Honey Commission [8]. The unit of Diastase Activity, the Gothe unit, is defined as that amount of enzyme which will convert 0.01 gram of starch to the prescribed end-point in one hour at 40°C under the conditions of test. A standard solution of starch, capable of developing, with iodine, a colour in a defined range of intensity, is acted upon by the enzyme in the sample under standard conditions. The diminution in the blue colour is measured at intervals. A plot of absorbance against time, or a regression equation, is used to determine the time tx required to reach the specified absorbance i.e., 0.235. The Diastase Number is calculated as 300 divided by tx, provided the method is followed precisely. The total protein content was determined by the standard Lowry’s method using bovine serum albumin (0-100 μg/ ml) as a standard [9].
The newly developed: In-vitro Starch Hydrolysis Plate Assay (ISHPA)
Modified starch minimal medium (pH 6.8) containing starch (1%), K2HPO4·3H2O (0.3%), KH2PO4 (0.15%), ammonium sulfate (0.2%), and agar (2%) was poured into glass petri plates and allowed to solidify. Later, wells were formed using a sterile cork borer, and 225 μL of filter sterilized aqueous honey extract (50% w/v) was dispensed into the well and the plates were incubated in upright position at 37°C for 24 h. Plates were later stained with iodine solution (Resublimed Iodine: 0.0088%; potassium iodide: 4.0176%) and photographed in a TLC visualizer (CAMAG, Switzerland) and also the zone of clearance (mm) around the well was measured.
Results
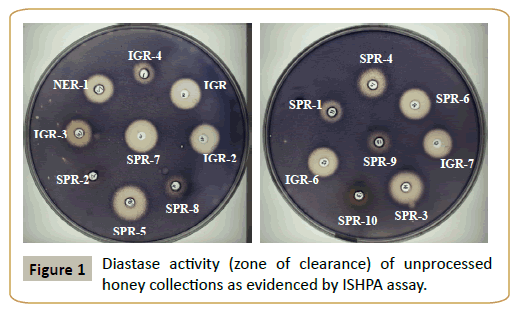
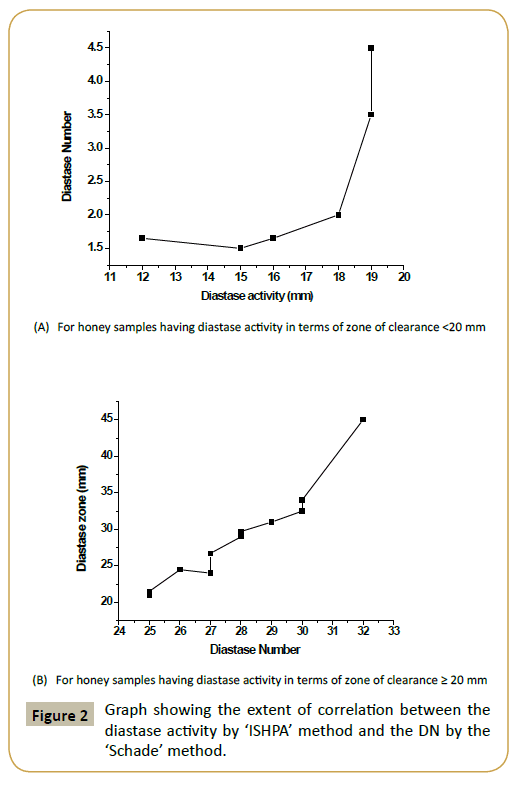
The honey collections displayed varying diastase activity when assessed by Schade method as well as newly developed method called as In-vitro Starch Hydrolysis Plate Assay (ISHPA) (Figure 1). A prominent variation was noticed among honey of same floral type (S. cumini). Of the 17 honey collections, 11 honey samples had diastase number (DN) >8 [8]. However, 6 samples had diastase value <8. Of these, 4 samples (IGR-4, SPR-2, SPR-10, SPR- 9) had DN values ≤ 3 and thus did not agree with characteristics established in European and Codex standards relative to the diastase activity, although the other physico-chemical parameters were within the range of the allowable limits. Interestingly, these 6 honey samples had a low HMF value (≤ 15 mg/kg of honey, data not shown). The diastase activity of honey collections in terms of zone of clearance as determined by the proposed methodology varied significantly and it ranged from 15 mm (SPR-10) to 32 mm (SPR-5) (Figure 1). Diastase activity obtained through both the methodologies were compared (using Origin Pro 8 software) and found to display a nearly straight line equation for the samples having diastase activity (in terms of zone of clearance) ≥ 20 mm (Figure 2). In this condition both the methods of diastase assessment were found to well correlated (R2=0.919). As good diastase activity is considered as a positive quality attribute for the honey samples, the proposed method offers a promising alternate method for such samples. However, for honey samples with low diastase activity positive correlation is there but linear relation was not prominent (R2=0.4274). It could be also due to the variable sensitivities of both the existing as well as proposed methods. However, a low correlation (0.3) was observed between the protein content and diastase activity in terms of zone of clearance indicating that honey contains much other type of nonenzymatic proteins and the relative proportion of diastase may be low.
Discussion
The ‘ISHPA’ assay is a simple and cost effective method that is based upon the development and enumeration of the zone of clearance that results due to the breakdown of starch by the diastase present in honey. The assay offers various advantages such as it is simple to perform, easy data interpretation, and multiple samples could be handled simultaneously with practical ease thus minimizing the restriction imposed in other standard assays. Correlation exists between the diastase activity (zone of clearance, mm) and the diastase number (DN). Quantifying the zone of clearance could indicate about the DN of the honey sample under investigation.
Besides, the assay involves least dependency on costly instrumentation thus making it a cost effective methodology whereas standard methods of diastase assessment requires at least a spectrophotometer and a thermostated water bath. ISHPA assay displayed less sensitivity to biological variations. The DN of honey samples varied between 1.65 to 34 whereas most of the other physical and biochemical parameters were quite comparable. This could not be explained and it seems that these methods are quite sensitive to minor biological sample variations whereas such discrepancy was found to be significantly reduced in case of the ISHPA assay. In this case the end points of assay system ranged between 15 mm (SPR-10) to 32 mm (SPR-5) in terms of the zone of clearance (hydrolysis) and it thus nullifies the fluctuation due to sample variation which should be a pre-requisite for a standard analytical method. Most of the honey samples had DN value ≥ 8, except for some honey samples such as IGR-4, SPR-2, SPR-10, SPR-9 having DN values ≤ 3. The diastase activity in honey in terms of zone of clearance may vary significantly depending on the age of the bees, the nectar collection period, the amount of nectar processing by the bees, and the physiological period of the colony [6]. Besides, honeys with lower enzyme content are also produced from young nectars in early spring owing to the reduced activity of the bees during their growth. A high flow of concentrated nectar leads to a lower enzyme content and pollen consumption [6]. Therefore honey from very rich nectar sources e.g., often show low natural enzyme activities therefore certain unifloral honeys have a naturally low diastatic activity.
Conclusion
A novel ‘ISHPA’ In-vitro Starch Hydrolysis Plate Assay (ISHPA) is proposed for diastase assessment of honey. The assay is quite easy to perform, cost effective, has better reliability and shows fewer fluctuations. A good correlation exists between ISHPA and Schade method.
References
- Begum SB, Roobia RR, Karthikeyan M, Murugappan RM (2013) Validation of nutraceutical properties of honey and probiotic potential of its innate microflora. LWT Food Sci Technol. 60: 743-750.
- Manyi-Loh CE, Clarke AM, Munzhelele T, Green E, Mkwetshana NF, et al. (2010) Selected South African honeys and their extracts possess in vitro anti-Helicobacter pylori activity. Arch Med Res. 41: 324-331.
- Mayer A, Slezak V, Takac P, Olejnik J, Majtan J (2014) Treatment of non-healing leg ulcers with honeydew honey. J Tissue Viability 23: 94-97.
- Oryan A, Alemzadeh E, Moshiri A (2016) Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability 25: 98-118.
- da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R (2016) Honey: Chemical composition, stability and authenticity. Food Chem 196: 309-323.
- White JW (1994) The role of HMF and diastase assay in honey quality evaluation. Bee World 75: 104-117.
- Tosi E, Martinet R, Ortega M, Lucero H, Re E (2008) Honey diastase activity modified by heating. Food Chem 106: 883-887.
- International Honey Commission (2009) Harmonised methods of the International Honey Commission. Available from: https://www.bee-hexagon.net/en/network.htm.
- Saxena S, Gautam S, Sharma A (2010) Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem 118: 391-397.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences