ISSN : 2573-4466
Insights in Enzyme Research
The Active Role of Spray-Dried Phytase Produced by Rhizopus microsporus var. microsporus Biofilm in Feeding Broiler Chickens
Vanessa Sayuri Sato1, Gabriel Vilella Dessimoni Pinto2, Cecilia Maria Costa do Amaral3, Ana Rosa Crisci3, Wanderley Pereira de Oliveira4 and Luis Henrique Souza Guimarães1,5*
1Institute of Chemistry of Araraquara – UNESP, 14800-900 Araraquara, Brazil
2Department of Animal Science, Faculty of Agriculture and Veterinary Sciences - UNESP, 14884-900 Jaboticabal, Brazil
3University Center Barão de Mauá, 14090-180 Ribeirão Preto, Brazil
4Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo (USP), 14040-901 Ribeirão Preto, Brazil
5Faculty of Philosophy, Sciences and Letters of Ribeirão Preto – USP, 14040- 901 Ribeirão Preto, Brazil
- *Corresponding Author:
- Luis Henrique Souza Guimarães
Departamento de Biologia, Faculdade de Filosofia
Ciências e Letras de Ribeirão Preto, USP
Avenida Bandeirantes, 3900, Monte Alegre
14040-901 Ribeirão Preto, São Paulo, Brazil
Tel: +551633154682
Email: lhguimaraes@ffclrp.usp.br
Received Date: December 09, 2016 Accepted Date: February 18, 2017 Published Date:February 25, 2017
Citation: Sato VS, Pinto GVD, do Amaral CMC, et al. The Active Role of Spray-Dried Phytase Produced by Rhizopus microsporus var. microsporus Biofilm in Feeding Broiler Chickens. Insights Enzyme Res. 2017, 1:1. doi: 10.21767/2573-4466.100006
Abstract
Objective: Phytases are enzymes that can catalyze the hydrolysis of phytic acid, and have potential for use in the formulation of broiler chickens’ diets. As such, we evaluated the effects of supplementation of broiler chicken diets with a spraydried phytase derived from the fungal Rhizopus microsporus var. microsporus biofilm on the animal performance.
Methods: Cobb 500 male chicks were housed for 42 days under controlled environment conditions and provided with three different dietary regimes. These included a control diet meeting nutritional requirements, a diet with phosphorus reduction of 0.175% over days 1-21 and 0.225% over days 22-42, and diets with spray-dried phytase added at 250 FTU/kg, 500 FTU/kg and 750 FTU/kg. Animal performance was assessed based on their body weight, feed intake, weight gain, feed conversion rate, and viability.
Results: At the first phase of development (1-21 days of age), performance was not improved by phytase addition, but at the latter phase (22-42 days of age); performance improved significantly, especially in birds fed phytase at 750 FTU/ kg. In addition, phytase promoted an increase in villous height for animals of both 21- and 42 day-old animals, improving nutrient absorption and performance at the end phase of development.
Conclusion: Phytase supplementation positively affected animal development and jejunum morphology, highlighting its potential to be used as an additive for broiler chicken diet formulation.
Keywords
Broiler chickens; Diet formulation; Jejunum morphology; Phosphorus; Phytase; Rhizopus microsporus var. microsporus biofilm
Introduction
Phytic acid, or inositol hexaphosphate, is a large phosphate storage molecule in most plants, grains, and oilseed [1], and can be used for feed formulation. Due to its lack of molecular specificity, it has high potential to complex with positively charged molecules such as proteins [2,3], carbohydrates, and amino acids [2,4], as well as with cations (Zn+2, Fe+2, Fe+3, Mg+2, and Ca+2) in the intestine, promoting negative effects on animal development and performance. Physical and chemical methods can be employed to remove the phytic acid from food, but the use of these methods can reduce its nutritional value [5]. Alternatively, the anti-nutritional effects of phytic acid can be minimized by the use of enzymes such as phytases, which can catalyze the hydrolysis of phytate to myo-inositol and inorganic phosphate, especially when added in the diets of non-ruminants such as pigs, fishes, poultries and humans [5]. Phytases can be applied in numerous fields such as the animal feed industry, the food industry, bread making, production of plant protein isolates; corn wet milling, and fractionation of cereal bran [6].
During the last two decades, phytases have attracted the attention of scientists and entrepreneurs of different areas such as biotechnology, environmental protection and nutrition. Despite the potential application of phytase in different fields, it’s most important commercial value is associated with the food and feed industries [5]. Different works have examined the usefulness of fungal phytases for swine and poultry nutrition. Several fungal strains have been used for the commercial production of phytases, such as Aspergillus niger, Aspergillus oryzae, Trichoderma reesei, and Penicillium funiculosum. For this purpose, submerged fermentation is the main process for enzyme production, using both wild-type and recombinant strains [5]. The effects of fungal phytase supplementation to feeds on performance and nutrient utilization by poultry, especially chickens, have been reported in different studies [7-9]. Improvement of growth and performance, as well as improvement in the absorption capacity of the intestine and retention of phosphorus, were observed for broiler chickens. Fukuyama et al. found positive effects on different parameters during animal development when a commercial phytase (Phytase 5000) from Ouro Fino (Brazil) was added to diets with reduced amounts of phosphate [10]. This strategy may reduce the cost of animal production and the environmental impact caused by phosphate pollution [5,7,8].
Animal diets have been experimentally supplemented with different phytase levels, in order to achieve the same or better performance of a standard nutritional diet with normal levels of nutrients and to promote the disruption of the maximum P-phytic acid complex and allow better mineral absorption [10-12]. Brazil has an important stake in this effort because of its strong production in the agricultural sector. The poultry industry has experienced intense development in recent years, which makes the search for new sources of phytases with biotechnological properties promising for generating new products for improving animal nutrition. Economic and environmental contexts make the use of phytase increasingly relevant in modern poultry production [13]. However, the use of spray-dried phytase in feed formulation for broiler chickens has not been previously investigated. Sato et al. [14] reported that spray-drying of phytase produced by Rhizopus microsporus var. microsporus biofilm yielded high enzyme activity. Accordingly, we aimed to investigate the active role of spray-dried phytase (using cornmeal as adjuvant) from the filamentous fungus R. microsporus var. microsporus, added to a corn and soybean meal diet, on the performance and jejunum morphology of broiler chickens.
Materials and Methods
Microorganism and culture conditions
A record of the filamentous fungus R. microsporus var. microsporus is deposited in the fungal culture collection of the Laboratory of Microbiology from the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo - USP, Ribeirão Preto, São Paulo, Brazil. Fungal colonies were maintained on potatodextrose- agar slants held at 4°C. After cultivation, slants were scraped, and the scrapings were added to distilled water to obtain a (106 spores/mL) spore suspension for use in producing a fungal biofilm as described by Sato et al. [14]. The Rhizopus biofilm was obtained by immersing polyethylene sheets in the spore suspension for 2 h at 30°C under 100 rpm agitation. After spore adhesion, sheets were washed twice with distilled water to remove unadhered spores, then incubated for 96 h at 30°C under agitation 50 rpm in NBRIP modified medium [15], using sugarcane bagasse as a carbon source.
Obtaining the enzymatic fraction
After cultivation, the fungal biofilm was removed from the culture medium and the free cell solution containing the enzyme was used to quantify phytase activity and for spray drying, as described by Sato et al. [14], using a bench-top spray dryer (model SD-05; Lab- Plant, Huddersfield, UK), with a concurrent flow regime. Air was heated to 100°C and injected into the spray dryer, whereas crude extract, in presence of cornmeal as adjuvant, was fed past at a flow rate of 4 g/min. The atomizing air was adjusted at a pressure of 141 kPa and flow rate of 17.0 L/min.
Animals and housing
A total of 1,080 Cobb 500 male chicks, one day old at the beginning of the experiment, were housed for 42 days in an experimental facility with a tunnel-type system to control environmental conditions, in the Poultry sector of the Department of Animal Science of the Faculty of Agriculture and Veterinary Sciences of Jaboticabal, São Paulo, Brazil. Temperature, humidity and air exchange were automatically regulated by exhaust fans and climate control system, according to the age of the animals (Broiler Management Guide COBB 500, 2008). The air temperature values remained between 32.33 ± 0.57°C and 26.66 ± 1.15°C and relative humidity between 84.33 ± 13.21% and 24.66 ± 4.93%. For the period from 1 to 21 days of age, the ideal temperature recommended for breeding of broilers is 32-34°C. In the second week of life, the recommended temperature varies from 28°C to 32°C, and in the third week, from 26°C to 28°C [16]. For older animals, the indicated temperature is between 20°C and 27°C [17].
Chicks were provided ocular vaccination against coccidiosis on the 1st day of life. Water-administered vaccines were given against low strain Gumboro disease at 6 days of age, New Castle disease at 11 days of age, and strong strain Gumboro at 16 days of age. All experimental procedures were approved by the Ethics Committee on Animal Use of the Faculty of Agriculture and Veterinary Sciences of Jaboticabal - UNESP (authorization nº 0184337/13).
Diets and experimental design
All experimental diets were based on corn and soybean meal, and were formulated according to the Brazilian Tables for Poultry and Swine [18]. Diet composition and nutritional levels fed during the starter (1 to 21 days of age) and grower (22 to 42 days of age) phases are presented in Table 1.
| Item | 1-21 days of age | 22-42 days of age | ||||
|---|---|---|---|---|---|---|
| NC | PC1 | PC2 | NC | PC1 | PC2 | |
| Ingredients, % | ||||||
| Corn | 63.050 | 62.802 | 62.553 | 65.095 | 64.846 | 64.597 |
| Soybean meal (45%) | 32.888 | 32.939 | 32.990 | 29.367 | 29.418 | 29.469 |
| Soy oil | 0.610 | 0.694 | 0.778 | 2.768 | 2.851 | 2.935 |
| Dicalcium phosphate | 0.606 | 0.931 | 1.256 | 0.363 | 0.688 | 1.013 |
| Limestone | 1.543 | 1.333 | 1.123 | 1.337 | 1.127 | 0.917 |
| NaCl | 0.433 | 0.433 | 0.433 | 0.399 | 0.400 | 0.400 |
| DL-Methionine | 0.321 | 0.321 | 0.322 | 0.255 | 0.255 | 0.255 |
| L-Lysine | 0.317 | 0.316 | 0.315 | 0.234 | 0.233 | 0.232 |
| L-Threonine | 0.122 | 0.122 | 0.122 | 0.072 | 0.072 | 0.072 |
| Premix2,3 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Phytase | - | - | - | - | - | - |
| Inert | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritional levels, % | ||||||
| ME, kcal/kg | 3,000 | 3,000 | 3,000 | 3,170 | 3,170 | 3,170 |
| Crude protein | 21.6 | 21.6 | 21.6 | 20.0 | 20.0 | 20.0 |
| Calcium | 0.867 | 0.867 | 0.867 | 0.717 | 0.717 | 0.717 |
| Phosphorus | 0.225 | 0.285 | 0.345 | 0.175 | 0.235 | 0.295 |
| Sodium | 0.213 | 0.213 | 0.213 | 0.198 | 0.198 | 0.198 |
| Potassium | 0.794 | 0.794 | 0.794 | 0.735 | 0.735 | 0.735 |
| Methionine | 0.606 | 0.606 | 0.606 | 0.524 | 0.524 | 0.524 |
| Methionine+Cystine | 0.902 | 0.902 | 0.902 | 0.804 | 0.804 | 0.804 |
| Lysine | 1.253 | 1.253 | 1.253 | 1.101 | 1.101 | 1.101 |
| Threonine | 0.814 | 0.814 | 0.814 | 0.715 | 0.715 | 0.715 |
| Tryptophan | 0.218 | 0.218 | 0.218 | 0.199 | 0.199 | 0.199 |
1PC – positive control (diet meeting the nutritional requirements of the birds); NC – negative control (phosphorus reduction of 0.175% for 1-21 days and phosphorus reduction of 0.225% for 22-42 days). 2Inicial: folic acid 1,000 mg; pantotenico acid 15,000 mg; antioxidant 0.5 g; niacin 40,000 mg; selenium 300 mg; biotin 60 mg; VB1 1,800 mg; VB12 12,000 mg; VB2 6,000 mg; VB6 2,800 mg; VD3 2,000,000 IU; VE 15,000 mg; VK3 1,800 mg; Mn 150,000 mg; Zn 100,000 mg; Fe 100,000 mg; Cu 16,000 mg; I 1,500 mg. 3Final: folic acid 700 mg; pantotenico acid 13,000 mg; antioxidant 0.5 g; niacin 3,5000 mg; selenium 300 mg; VB1 1,600 mg; VB12 10,000 mg; VB2 5,000 mg; VB6 2,600 mg; VD3 1,500,000 IU; VE 12,000 mg; VK3 1,500 mg; Mn 150,000 mg; Zn 100,000 mg; Fe 100,000 mg; Cu 16,000 mg; I 1,500 mg.
Table 1: Composition of experimental diets for the phases from 1 to 21 and 22 to 42 days of age of the birds1.
All broiler chicks were weighed (± 45 g each) and randomly distributed into each treatment group according to the similarities of their body weight means, described as follows. Six treatments were analyzed, with 6 replicates for each, totaling 36 treatments housed in boxes of 3.0 m2 with 30 animals per box.
Treatments were applied as follows. PC diets, or positive control diet, met the nutritional requirements of the animals. Two positive controls were used: PC1, or the basal diet+inorganic phosphate at 0.06%, and PC2, or the basal diet+inorganic phosphate 0.12%. NC diets, or negative control diets, had phosphorus content reduced by 0.175% for the initial phase (1 to 21 days) and by 0.225% for the final phase (22 to 42 days). T diets, or treatment diets, consisted of T1, with NC+phytase at 250 FTU/kg; T2, with NC+phytase at 500 FTU/kg; and T3, with NC+phytase at 750 FTU/kg.
Performance
Performance was assessed at 21 and 42 days of age, taking into account animal weight and leftover feed mass, as well as body weight (BW), feed intake (FI), body weight gain (BWG), feed conversion rate (FCR), and viability (VIA). Mortality was recorded daily and used to adjust the feed intake calculation and feed conversion ratio as described by Sakomura and Rostagno [19].
Analysis of the intestinal morphology
Jejunum samples from 21- and 42-day-old broilers were collected and stored in formal saline until required for analysis, as follows. After collecting, samples were fixed in Bouin’s solution for 4 h, and then added to 10% formalin for 24 h. Then, samples were dehydrated in different alcohol solutions (70%, 80%, 90%, 95%, and 100%) for 1 h each. They were then cleared with xylene and impregnated with paraffin wax. Tissue sections of 4-5 μm depth were cut on a microtome (LUPETEC Modelo MRP2015), fixed on plain glass slides (26 mm × 76 mm), and stained using hematoxylin and eosin for 5 min. Slides were then washed in running water for 5 min and stained with eosin acid dye for 30 s.
Histological preparations were observed using a light microscope (Leica DM5000 B) and imaged using a digital camera (Leica 5000 B). Morphometric analysis was performed using Leica QWin software (Leica Image Processing and Analysis Software, Leica Microsystems Ltd, Heerbrugg, Switzerland). Villus height measurements, defined as the villus tip to the villus-crypt junction, were obtained from six consecutive intact villi in each sample. Crypt depth was estimated by measuring six crypts per section.
Statistical Analysis
Statistical analysis were performed using SAS version 9.1 [20], using three replicates. Significant effects were determined based on analysis of variance with a P<0.05 threshold. Linear and quadratic polynomial regression analyses were carried out, and Tukey’s test at 5% probability was performed when significant linear effects were detected.
Results and Discussion
Influence of the spray-dried phytase on broilers’ performance
Initial phase of development (1-21 days): For broiler chickens in the initial phase of development (1-21 days), maximum BWG was achieved in animals receiving a diet with an appropriate nutritional level of phosphorus (PC2: inorganic phosphate 0.12%) and consequently high media for body weight (BWG) (Table 2). Addition of different proportions of spray-dried phytase did not yield improvement in the BWG and BW, with animals receiving a phosphorus-deficient diet showing similar values (NC: phosphorus reduction of 0.175%) and differing from the positive controls (P<0.05). In addition, no significant differences were observed among the treatments containing phytase at different concentrations (250, 500 and 750 FTU/kg) (P>0.05). These results indicate that spray-dried phytase, independent of the proportion used, had no positive effect on animal development during the initial period (1-21 days). Previous researchers have similarly found no significant effect for the addition of phytase in diets on the performance of animals prior to 21 days of age [4,21,22]. Similarly, Murata et al. [23] found that adding 750 and 1,000 FTU of phytase/kg of feed did not yield significant effects on broiler performance during the initial phase. In the same study, no difference in weight gain was observed when 1,500-10,000 FTU of phytase/kg of feed was used in comparison to controls.
| Treatments1 | Body weight (kg) | Body weight gain, kg | Feed conversion | Feed intake, kg | Viability, % |
|---|---|---|---|---|---|
| PC2 | 0.76a | 0.72a | 1.54 | 1.11a | 98.3 |
| PC1 | 0.71a | 0.67a | 1.58 | 1.06ab | 99.4 |
| NC | 0.60b | 0.56b | 1.63 | 0.91b | 100.0 |
| T1 | 0.61b | 0.57b | 1.72 | 0.98ab | 97.2 |
| T2 | 0.61b | 0.56b | 1.71 | 0.97ab | 99.4 |
| T3 | 0.60b | 0.56b | 1.64 | 0.92b | 98.9 |
| MSD | 0.04 | 0.04 | 0.20 | 0.14 | 3.13 |
| F value2 | 55.38** | 56.19** | 2.15ns | 5.21** | 1.90ns |
| CV, % | 3.5 | 3.7 | 7.0 | 8.2 | 1.8 |
MSD=Minimal significant difference; CV=Coefficient of variation. a, b, c Values with the same letter for each column do not differ by Tukey test at 5% probability (P = 0.05). 1PC – Positive control (diet meeting the nutritional requirements of the birds); NC – Negative control (phosphorus reduction of 0.175%); T1-NC+250 FTU/kg; T2-NC+500 FTU/kg; T3-NC+750 FTU/kg. 2 ns=not significant, **=Significant at 1% probability.
Table 2: Effect of spray-dried phytase from R. microsporus var. microsporus on the performance of broilers at 1-21 days.
In terms of feed intake (FI), animals that received appropriate phosphorus level (PC2: inorganic phosphate 0.12%) showed optimum values for this parameter (Table 2). However, there was no significant difference (P>0.05) among the animals fed phytase treatments and those fed positive control diets, or among animals fed phytase treatments and those fed negative control diets. The same was also observed for feed conversion ratio (FCR) parameter, indicating that the spray-dried phytase added to the diet did not affect these parameters. Previous studies [2,4,24-31] have similarly found no significant improvement in feed conversion parameters for broilers fed with phytase dietary supplements. According to Sebastian et al. [4] and Viveros et al. [32], diets with lower nutritional level, especially those deficient in phosphorus content, cause a reduction in the feed intake, which could explain the lower feed intake observed in animals receiving phytase-supplemented diets. In this case, addition of phytase was not able to recover the FI values such that they would be similar to the FI values obtained for animals receiving the appropriate levels of phosphorus (PC2: inorganic phosphate 0.12%) in spite of the absence of statistical significant difference. Reduced feed intake caused reduction in the BWG as also observed for the animals 1-21 days old. Viability was not affected by the addition of inorganic phosphate (controls) or phytase at different concentrations.
Latter phase of development (21-42 days): BWG for animals receiving PC diets was significantly higher than in those receiving NC and NC+250 FTU/kg diets (P<0.05). Diet supplementation with 500 FTU/kg and 750 FTU/kg of spray-dried phytase significantly affected BWG and BW in comparison to diets with a low nutritional level of phosphorus (NC) (P<0.05). The diet containing 750 FTU of phytase/kg of feed also induced an increase in BWG, similar to that observed in positive controls. In terms of FI, similar effects were observed for the treatments using different phytase concentrations and both positive controls. According to Santos et al. [12], when feed diets were supplemented with phytase, animals showed feed intake and weight gain similar to those of the positive control. In our study, addition of spray-dried phytase promoted a positive effect (P<0.05) on FI when compared to NC (with reduced inorganic phosphate amount), indicating its potential to eliminate the negative FI effect of the lower nutritional level of the diet. Addition of 750 FTU of phytase/ kg of feed promoted the highest FI. We suggest that addition of appropriate levels of phytase can overcome the suppressive effect of the poorer nutritional diet by disrupting the P-phytic acid complex, releasing phosphorus for absorption and reducing the negative effect of its deficiency on feed intake [4,10]. The FI values observed for treatments using 250 and 500 FTU of phytase/kg of feed were lower than that observed for positive controls, though not significantly so. This fact could be due to inadequate levels of phytase for achieving maximal hydrolysis of the phytate complex.
The highest FCR value was observed in animals fed a diet supplemented with 750 FTU/kg of spray-dried phytase (Table 3). This result reinforces that an appropriate level of phytase should be used to obtain maximal hydrolysis of phytate. Statistically, no significant difference was observed among the NC, PC and spray-dried phytase treatments. Similar to the initial phase of development, viability of the animals was not affected in the final phase by the addition of phytase, or inorganic phosphate in the positive controls. However, viability (± 84.3%) was reduced in the final phase (21-42 days) in comparison with the initial phase (1- 21 days) (± 98.6%).
| Treatments1 | Body weight, kg | Body weight gain, kg | Feed conversion | Feed intake, kg | Viability, % |
|---|---|---|---|---|---|
| PC2 | 2.80a | 2.77a | 1.25 | 3.44ab | 87.8a |
| PC1 | 2.55abc | 2.52abc | 1.29 | 3.23bc | 89.5a |
| NC | 2.17d | 2.12d | 1.25 | 2.64d | 87.8a |
| T1 | 2.41cd | 2.37cd | 1.35 | 3.20bc | 82.8a |
| T2 | 2.53bc | 2.49bc | 1.24 | 3.07c | 81.7a |
| T3 | 2.69ab | 2.65ab | 1.39 | 3.63a | 79.5a |
| MSD | 0.26 | 0.26 | 2.07 | 0.34 | 10.65 |
| F value2 | 14.03** | 14.01** | 0.18ns | 18.22** | 2.74* |
| CV, % | 5.77 | 5.87 | 8.02 | 6.07 | 7.06 |
MSD=Minimal significant difference; CV=Coefficient of variation. a, b, c, d Values with the same letter for each column do not differ by Tukey test at 5% probability (P = 0.05). 1PC- positive control (diet meeting the nutritional requirements of the birds); NC- negative control (phosphorus reduction of 0.225%); T1-NC+250 FTU/kg; T2-NC+500 FTU/kg; T3-NC+750 FTU/kg. 2ns=not significant, *=Significant at 5% probability, **=significant at 1% probability.
Table 3: Effect of phytase produced by biofilm R. microsporus var. microsporus on the performance of broilers at 22-42 days.
Overall, the results obtained for the final phase of development differed from those obtained for the initial phase of development. Similarly, Lelis et al. [28] observed that supplementation with 250 and 500 FTU of phytase/kg of feed in diets with reduced nutritional levels improved broiler performance in the final phase of development. Other studies found positive effects of increasing levels of phytase in the diet on growth in broiler performance [21,29]. For example, Santos et al. [30] observed an improvement in the broiler performance using 500, 1000, 1500 FTU/kg in diets with reduced levels of phosphorus and calcium, similar to the results obtained using diets containing normal levels of nutrients in feed. According to Tejedor et al. [21] the optimum amount of phytase inclusion is 500-700 FTU of phytase/kg of feed. Strada et al. [31] added an enzymatic complex in corn-based feed and soybean meal, and found no significant improvements in performance indices for broilers up to 42 days old.
In general, when an improvement of animal performance is observed for broilers receiving diets supplemented with phytase, a multifactorial explanation can be presented, such as the utilization of discharged minerals from the mineral-phytate complex, usage of inositol obtained from phytate hydrolysis, improvement of starch digestibility and protein availability, and impediment of the saponification reaction among lipids and mineral-phytate complex [10]. Additionally, for phytase to be effective, the presence of the specific substrate in the diet formulation as well as its addition at ideal level must be determined.
Influence of phytase supplementation on the morphology of jejunum cells
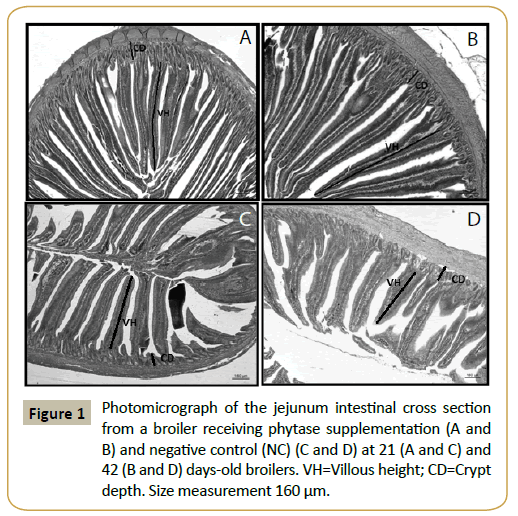
At 21 days of age (Figure 1A and 1C), villus length increased significantly with the addition of phytase (P<0.05), especially in animals receiving 500 FTU of phytase/kg of feed, similar to the values observed under PC treatment (Table 4). Shorter villus length was observed under NC treatment, while crypt length was not affected by the various diets. At 42 days of age (Figure 1B and 1D), there was significant difference in villus length between treatments with reduction of phosphorus content (NC) and PC treatment (P<0.05). Addition of 250 and 500 FTU of phytase/ kg of feed also promoted an increase in villus height compared with the NC treatment, although not significantly so. Depth of the crypts under supplementation with 250 FTU of phytase/kg of feed was greater than that obtained under the NC treatment, but this was also not statistically significant.
| Treatments1 | Villous | Crypts | ||
|---|---|---|---|---|
| 21 days | 42 days | 21 days | 42 days | |
|
PC2 |
512.27a | 750.75a | 87.61 | 82.82ab |
|
PC1 |
491.02a | 670.85ab | 102.56 | 78.55ab |
|
NC |
397.82b | 607.39b | 92.63 | 77.73b |
|
T1 |
467.95ab | 680.35ab | 103.14 | 95.50a |
|
T2 |
524.93a | 700.82ab | 85.47 | 84.23ab |
|
T3 |
495.99a | 596.32b | 97.60 | 86.48ab |
|
MSD |
90.19 | 131.65 | 25.27 | 17.22 |
|
F value2 |
4.81** | 3.70* | 1.67ns | 2.67* |
|
CV (%) |
10.53 | 11.09 | 14.98 | 11.50 |
MSD=Minimal significant difference; CV=Coefficient of variation. a, b Values with the same letter for each column do not differ by Tukey test at 5% probability (P = 0.05). 1PC - positive control (diet meeting the nutritional requirements of the birds); NC - negative control (phosphorus reduction of 0.175% for 1-21 days and phosphorus reduction of 0.225% for 22-42 days); T1-NC+250 FTU/kg; T2-NC+500 FTU/kg; T3-NC+750 FTU/kg. 2 ns=not significant, *=significant at 5% probability, **=significant at 1% probability.
Table 4: Measurements of villous height (µm) and crypts (µm) height obtained from broilers slaughtered at 21 and 42 days of age receiving different dietary phytase treatments.
It seems likely that improved performance resulted from the increased digestibility of nutrients based on a positive effect of phytase on the morphology of the intestine, with increased the villi height and crypt depth. According to Smulikowska et al. [33], morphological analysis of the mucosa of the small intestines of chicks after phytase supplementation indicates increases of villus height and crypt depth. The surface of the small intestine contains finger-like projections known as villi, which increase the surface area and therefore increases its absorptive capacity (Kadhim et al.) [34]. Phytase supplementation in poultry diets breaks the phytate-mineral complex to release minerals for absorption, which reduces mineral excretion and improves digestibility, as well as reduced intestinal stress (Sebastian et al.) [4].
There are few studies reporting the effect of phytase on intestinal morphology of animals. According to Pirgozliev et al. [35], the supplementation of feed with Escherichia coli-derived phytase did not affect morphometry of ileal villi of broiler chickens. This was further demonstrated by Langhout et al. [36] and Pirgozliev et al. [37]. However, Emami et al. [38] reported an increase in villus height and villus height/crypt depth ratio in the duodenum and jejunum, with decreased jejunum crypt depth, under a maize/soybean-based det poor in available phosphorus but supplemented with phytase. The addition of 1,000 units of dietary phytase and citric acid stimulated villus and crypt modifications, suggesting an increased rate of nutrient absorption and a reduced rate of enterocyte cell migration from crypts to villi [39]. Smulikowska et al. [33] reported that jejunum and ileum villi were shorter, and crypt depth was greater, in chickens fed with diets with lower level of dietary phosphorus compared to diets with normal phosphorus levels. Organic acids further suppressed jejunum villus height, but phytase supplementation was able to increase both villus height and crypt depth. Similar to our study, Wu et al. [40] also observed an increase in villus height in the duodenum of broilers given phytase supplementation. They suggested that the effects of exogenous phytase on the morphology of the gastrointestinal tract may have contributed to improved animal performance. This suggests that the phytase from Rhizopus microsporus var. microsporus contributed to a good performance of broilers.
Conclusion
Supplementation with spray-dried phytase from R. microsporus var. microsporus promoted an improvement in the performance of animals at the final phase of development, especially when 750 FTU of phytase/kg of feed was used, but did not have effect on chickens in the initial phase of development. Phytase supplementation also positively affected jejunum morphology by increasing villus height, allowing better nutrient absorption and consequently improving performance. The spray-dried phytase from R. microsporus var. microsporus presented good biotechnological potential for application in broilers’ diets, and future studies can potentially develop its use for industrial employment
Conflict of Interest
All authors declare that they have no conflict of interest.
Acknowledgements
We thank the financial support of Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP- grant number 11/50880-1) and CAPES (Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior), Fátima Aparecida Harnich and Maurício de Oliveira for the technical assistance. This manuscript is part of the VSS PhD Thesis presented to the Institute of Chemistry of Araraquara- UNESP, Araraquara, São Paulo, Brazil.
References
- Pandey A, Szakacs G, Soccol CR, Rodriguez-Leon JA, Soccol VT (2001) Production, purification and properties of microbial phytases. Biores Technol 77: 203-214.
- Ravindran V, Cabahug S, Ravindran G, Bryden WL (1999) Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult Sci 78: 699-706.
- Cowieson AJ, Acamovic T, Bedford MR (2006) Using the precision-feeding bioassay to determine the efficacy of exogenous enzymes-a new perspective. Animal Feed Sci Technol 129: 149-158.
- Sebastian S, Touchburn SP, Chavez ER, Lague PC (1996) Efficacy of supplemental microbial phytase at different dietary calcium levels on growth performance and mineral utilization of broiler chickens. Poult Sci 75: 1516-1523.
- Singh B, Satyanarayana T (2015) Fungal phytases: characteristics and amelioration of nutritional quality and growth of non-ruminants. J Anim Physiol Anim Nutr 99: 646-660.
- Dahiya S (2016) Industrial applications of phytases. Int J Appl Res 2: 95-98.
- Mansoori B, Modirsanei M, Nodeh H, Rahbari S (2010) The interactive effect of phytase and coccidia on the gross lesions as well as the absorption capacity of intestine in broilers fed with diets low in calcium and available phosphorous. Vet Parasitol 168: 111-115.
- Tiwari SP, Gendley MK, Pathak AK, Gupta R (2010) Influence of an enzyme cocktail and phytase individually or in combination in Ven Cobb broiler chickens. Br Poult Sci 51: 92-100.
- Pillai UP, Manoharan V, Lisle A, Li X, Bryden W (2009) Phytase supplemented poultry diets affect soluble phosphorus and nitrogen in manure and manure-amended soil. J Environ Qual 8: 1700-1708.
- Fukayama EH, Sakomura NK, Dourado LRB, Neme R, Fernandes JBK, et al. (2008) Effect of phytase supplementation on performance and nutrient digestibility in diets of broilers. R Bras Zootec 37: 629-635.
- Dilger RN, Onyango EM, Sands JS, Adeola O (2004) Evaluation of microbial phytase in broiler diets. Poult Sci 83: 962-970.
- Santos FR, Sakomura NK, Mendonça MO (2005) Efeito da suplementação com fitase em dietas de frangos decorte sobre a viabilidade econômica e desempenho. Rev Bras Cienc Avic Supl. 7: 119.
- Kaur P, Kunze G, Satyanarayana T (2007) Yeast phytases: Present scenario and future perspectives. Crit Rev Biotechnol 27: 93-109.
- Sato VS, Jorge JA, Oliveira WP, Souza CRF, Guimarães LHS (2014) Phytase production by Rhizopus microsporus var. microsporus biofilm: characterization of enzymatic activity after spray drying in presence of carbohydrates and nonconventional adjuvants. J Microbiol Biotechnol 24: 177-187.
- Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170: 265-270.
- Oliveira RFM, Donzele JL, Abreu MLT, Ferreira RA, Vaz RGMV, et al. (2006) Effects of temperature and relative humidity on performance and yield of noble cuts of broilers from 1 to 49 days old. Rev Bras Zootec 35: 797-803.
- Abreu PG, Abreu VMN, Coldebella A, Jaenisch FRF, Paiva DP (2007) Environmental thermal conditions and performance of broilers raised in aviaries with and without the use of polyethylene lining. Arq Bras Med Vet Zootec 59: 1014-1020.
- Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RFM, et al. (2011) Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 3rd edn., Universidade Federal de Viçosa, Viçosa, Minas Gerais - Brazil.
- Sakomura NKHS, Rostagno HS (2007) Métodos de pesquisa em nutrição de monogástricos. Funep Ed., Jaboticabal, São Paulo, Brazil.
- SAS Institute Inc. (2004) Base SAS 9.1 Procedure Guide. Cary, NC, USA, pp: 1440.
- Tejedor AA, Albino LFT, Rostagno HS, Vieites FM (2001) Effect of phytase supplementation on the performance and heal digestibility of nutrients. Rev Bras Zootec 30: 802-808.
- Brandão PA, Costa FGP, Brandao JS, Silva JHV (2007) Effect of phytase addition in broiler rations, during grower and final phases. Cienc Agrotec 31: 492-498.
- Murata LS, Oliveira ASC, Cordeiro CDC, Mcmanus CM, Rezende MJM, et al. (2006) Desempenho de frangos de corte consumindo dieta com diferentes níveis de fitase. Rev Bras Cienc Avic supl. 8: 133.
- Denbow DM, Ravindran V, Kornegay ET, Yi Z, Hulet RM (1995) Improving phosphorus availability in soybean-meal for broilers by supplemental phytase. Poult Sci 74: 1831-1842.
- Sohail SS, Roland DA (1999) Influence of supplemental phytase on performance of broilers four to six weeks of age. Poult Sci 78: 550-555.
- Schoulten NA, Teixeira AS, Freitas RTF, Bertechini AG, Conte AJ, et al. (2003) Levels of calcium in diets supplemented with phytase for broilers in the initial phase. R Bras Zootec 32: 1190-1197.
- Santos R, Zanella I, Bonato EL, Rosa AP, Magon L, et al. (2004) Decrease of the levels of calcium and phosphorus in diet with whole rice meal and enzyme on the performance of broilers. Cienc Rural 34: 517-521.
- Lelis GR, Albino LFT, Calderano AA, Tavernari FC, Rostagno HS, et al. (2012) Diet supplementation with phytase on performance of broiler chickens. R Bras Zootec 41: 929-933.
- Pintar J, Homen B, Gazic K, Grbesa D, Sikiric M, et al. (2004) Effects of supplemental phytase on performance and tibia ash of broilers fed different cereals based diets. Czech J Animal Sci 49: 542-548.
- Santos TT, Srinongkote S, Bedford MR, Walk CL (2013) Effect of high phytase inclusion rates on performance of broilers fed diets not severely limited in available phosphorus. Asian Australas J Animal Sci 26: 227-232.
- Strada ESO, Abreu RD, Oliveira GJC, Costa MCMM, Carvalho GJL, et al. (2005) Enzymes in the broiler diets. R Bras Zootec 34: 2369-2375.
- Viveros A, Brenes A, Arija I, Centeno C (2002) Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poult Sci 81: 1172-1183.
- Smulikowska S, Czerwinski J, Mieczkowska A (2010) Effect of an organic acid blend and phytase added to a rapeseed cake-containing diet on performance, intestinal morphology, caecal microflora activity and thyroid status of broiler chickens. J Anim Physiol Anim Nutr 94: 15-23.
- Kadhim KK, Abu-Bakar MZ, Noordin MM, Babjee MA, Saad MZ (2014) Light and scanning electron microscopy of the small intestine of young malaysian village chicken and commercial broiler. Pertanika J Tropical Agric Sci 37: 51-64.
- Pirgozliev V, Oduguwa O, Acamovic T, Bedford MR (2008) Effects of dietary phytase on performance and nutrient metabolism in chickens. Br Poult Sci 49: 144-154.
- Langhout DJ, Schutte JB, Van Leeuwen P, Wiebenga J, Tamminga S (2009) Effect of dietary high-and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Braz J Poult Sci 40: 340-347.
- Pirgozliev V, Oduguwa O, Acamovic T, Bedford MR (2007) Diets containing Escherichia coli-derived phytase on young chickens and turkeys: Effects on performance, metabolizable energy, endogenous secretions and intestinal morphology. Poult Sci 86: 705-713.
- Emami NK, Naeini SZ, Ruiz-Feria CA (2013) Growth performance, digestibility, immune response and intestinal morphology of male broilers fed phosphorus deficient diets supplemented with microbial phytase and organic acids. Livestok Sci 157: 506-513.
- Nourmohammadi R, Afzali N (2013) Effect of citric acid and microbial phytase on small intestinal morphology in broiler chicken. Ital J Anim Sci 12: e7.
- Wu YB, Ravindran V, Thomas DG, Birtles MJ, Hendriks WH (2004) Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolisable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. Br Poult Sci 45: 76-84.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences