ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
The Prevalence of Chlamydophila pneumoniae in the Blood Samples of Patients with Primary Cutaneous Lymphomas
Nedoszytko B1, Wierzbicki PM2, Karenko L1, Maciejewska-Radomska A3, Stachewicz P2, Zablotna M1, Glen J1, Vakeva L4, Nowicki R1 and Sokołowska-Wojdyło M1
1Department of Dermatology, Venereology and Allergology, Medical University of Gdańsk, Poland
2Department of Histology, Medical University of Gdańsk, Poland
3Individual Specialist Medical Practice, Gdynia, Poland
4Department of Dermatology and Allergology, University of Helsinki and Helsinki University Central Hospital, Helsinki, Finland
- *Corresponding Author:
- Sokolowska-Wojdylo M
Department of Dermatology
Venereology and Allergology
Medical University of Gdańsk, Gdansk
Tel: 48583492598
Fax: 48583492586
E-mail: mwojd@gumed.edu.pl
Received Date: December 12, 2016; Accepted Date: December 27, 2016; Published Date: January 05, 2017
Citation: Nedoszytko B, Wierzbicki PM, Karenko L, et al. The Prevalence of Chlamydophila pneumoniae in the Blood Samples of Patients with Primary Cutaneous Lymphomas. J Transm Dis Immun. 2017, 1:1.
Abstract
Microbial infection and associated super antigens have been implicated in the pathogenesis of CTCL, and many patients die from complicating bacterial infections. It has been postulated that Chlamydophila pneumoniae infection may be involved in the pathogenesis of Mycosis fungoides (MF) but published data are limited and controversial. Aim of the study: The aim of the study was to analyze the frequency of C. pneumoniae DNA presence in blood samples of cutaneous T-cell and B-cell lymphomas (CTCL, CBCL) cases. Material and Methods: Using Q-PCR method we analyzed the presence of C. pneumoniae DNA in the blood samples obtained from 57 patients with CTCL (55-MF/Sézary Syndrome (SS), 1-primary cutaneous anaplastic large cell lymphoma (CD30+) and 1-NKT cell lymphoma) and 3 patients with cutaneous B-cell lymphomas and 120 individuals from control groups (40 patients with psoriasis, 40 patients with atopic dermatitis and 40 healthy controls). Results: C. pneumoniae DNA was identified in 13 of 55 cases in MF/SS group (23, 6%), in one patient with CD30+ large cell lymphoma and in 1 of 3 patients with B-cell lymphoma. The presence of C. pneumoniae was confirmed in 1 of 40 psoriatic patients (2, 5%), in 5 of 40 patients with atopic dermatitis (12, 5%) and in none of 40 healthy individuals. The frequency of C. pneumoniae DNA occurrence in MF patients group was strongly associated with the progression of the disease; rs = 0.756; p=0.0123 for groups IA→IVB, also for MF + SS patients divided by stages, the presence of C. pneumoniae was noted more frequently in advanced (III + IV) stages than in early (I-II) stages (p=0.0139). There are no differences in the main age of MF/SS patients with and without infection. Conclusion: Our results indicated that the presence of C. pneumoniae DNA in the blood cells is frequent event in late stages of MF/SS and may be explained by Th2 shift and suppression of immune system during the course of the disease.
Keywords
Mycosis fungicides; Cutaneous T-cell lymphoma; Chlamydia pneumoniae
Abbreviations
ATCC: American Type Culture Collection; bFGF: Basic Fibroblast Growth Factor; C. pneumoniae: Chlamydophila pneumoniae; C. trachomatis: Chlamydia trachomatis; CBCL: Cutaneous B-cell Lymphoma; CD: Cluster of Differentiation; CTCL: Cutaneous T-cell Lymphoma; CXCL- 10: C-X-C Motif Chemokine Ligand 10; DNA: Deoxyribonucleic Acid; E. coli: Escherichia coli; EDTA: Ethylene-Diamineteraacetic Acid; HELA line: Henrietta Lacks Cell Line; IFN-γ: Interferon Gamma; IL- Interleukin; kDa: Kilodalton; MHC-I: Major Histocompatibility Complex Class I; MF: Mycosis fungoides; NK: Natural Killer; ompA: Outer Membrane Protein A; PBMC: Peripheral Blood Mononuclear Cells (PBMC); PCR: Polymerase Chain Reaction; pUC19: Plasmid Cloning Vector from University of California 19; RFLP: Restriction Fragments Length Polymorphism; RNA: Ribonucleic Acid; SAF: Sézary Cell Activation Factor; SS: Sezary Syndrome; Th: T helper; TNFa: Tumor Necrosis Factor Alpha
Introduction
Antigen stimulation by pathogens such as bacteria’s and viruses has been considered as possible predisposing factor to uncontrolled cell proliferation and development of lymphoid and other tissue neoplastic. Helicobacter pylori precedes the development of gastric B-cell lymphoma, Mycoplasma-like organisms and hepatitis C virus have been suggested to be associated with Hodgkin’s disease and Chlamydia trachomatis infection may associate with rectal and cervical cancer. Although several an etiologies have been postulated for Mycosis fungicides (MF) and Sézary syndrome (SS) their causes remain unknown. Based on the limited experimental and clinical data it has been speculated that chronic local antigen stimulation by Staphylococcus aureus as well as Chlamydophila pneumoniae (C. pneumoniae) may play a role in the pathogenesis of cutaneous T-cell lymphoma (CTCL) [1-3] C. pneumoniae is a common intracellular microorganism. Seroepidemiological studies indicate that C. pneumoniae infection is by far the most common human chlamydial infection in different cohorts, with seropositivity in at least 50% of the general population over age 20 [4-10]. But PCR studies on asymptomatic healthy adults (more than 1000) had established only 1% of positivity in nasopharyngeal swabs specimens [11] In addition to pneumonia, pharyngitis, bronchitis and asthma is also associated with arteriosclerosis, lung cancer, multiple sclerosis and Alzheimer’s disease [12-18] C. pneumoniae can infect, reside and replicate in various cells types including smooth muscle cells, fibroblasts, endothelial cells, bronchial epithelial cells, keratinocytes as well as various immune cells such as macrophages, lymphocytes and natural killers cells (NK) [19,20] It induces the increased release of pro-inflammatory mediators including tumor necrosis factor alpha (TNF-α), interleukin 6 and 8 (IL-6, IL-8), basic fibroblast growth factor (bFGF) and up regulates adhesion molecules [21]. Recently it has been suggested that C. pneumoniae infection may also stimulate the IL-10 production which down regulates the expression of major histocompatibility complex class I (MHC-I), inhibits apoptosis and increases the longevity of the host cell, enhancing the survival of bacteria itself [22,23]. The role of C. pneumoniae in the aetiology of CTCL is controversial. It has been suspected that a localized bacterial infection increases local production of inflammatory cytokines including interferon gamma (IFN-γ) (critical in immunity and immunopathology of chlamydial infection) and CXCL-10, a cytokine chemo attractive for epidermotropic T lymphocytes [24] Studies on the growth requirements of the abnormal T lymphocytes in MF/SS lead to the identification of a so-called Sézary cell activation factor (SAF) that stimulates the growth of both malignant and non-malignant T cells. SAF was originally defined as an inducer of functional interleukin-2 receptors. It is postulated that combination of SAF and IL-2 stimulates the propagation of oligoclonal T-cell populations from the peripheral blood mononuclear cells (PBMC) of patients with SS, with approximately one third of those cell clones containing the predominant malignant clone [25]. Using a monoclonal antibody inhibitory for SAF activity Abrams et al. demonstrated that SAF is present in more than half skin biopsies taken from patients with MF It was also confirmed that SAF determinant is not of eukaryotic origin and is associated with C. pneumoniae bacteria SAF is a protein of approximately 30 kDa, resembling the C. pneumoniae T cell activation factor originally described by Halme et al. [26] Abrams et al. confirmed the presence of C. pneumoniae DNA and RNA in the skin by PCR and reverse transcription- PCR and by sequence analysis of the PCR products. The authors showed that C. pneumoniae antigen expression was associated with active disease and was not found after psoralen and ultraviolet a therapy.
Materials and Methods
Study and control groups
In this study 60 patients with skin lymphomas (48 from Poland-30 man and 18 women, main age 60.7 ± 13.5 and 12 from Finland-8 man and 4 woman, main age 61.9 ± 21.6) were included, of whom 57 patients were diagnosed with primary cutaneous T-cell lymphomas (CTCL) and 3 patients with B-cell lymphoma according to WHO criteria. The control groups consisted of 40 patients with psoriasis (22 man and 18 women, main age 49.3 ± 14.3), 40 with atopic dermatitis (21 man and 19 women, main age 13.8 ± 7.7) and 40 healthy individuals (21 men, 19 women, 10 from Finland and 30 from Poland, main age 39 ± 15, 0). From the 57 CTCL patients 55 have Mycosis fungoides or Sézary syndrome, 1 patient had primary cutaneous anaplastic large cell lymphoma CD (30+), and 1 had NK/T cell lymphoma. From 55 MF/SS patients 20 were in early clinical stages (IA - IIA) and 35 patients were in advanced stages (IIB-IVB). Additionally from 6 MF/SS patients skin biopsies were obtained.
Material collection and DNA samples
From all patients and control groups a peripheral whole blood samples (EDTA-K2 (Medlab Products, Raszyn, Poland) was collected and stored at -80°C. Additionally from 6 examined patients skin biopsies was collected and stored at -80°C. DNA was extracted using Blood Mini or Mini AX Tissue (A&A Biotechnology, Gdynia, Poland) (A&A Biotechnology, Gdynia, Poland) according to manufacturer’s protocols. Further, DNA was precipitated using common sodium acetate-ethanol technique in a final volume of 20 μl and stored at -20°C for further analyses. DNA concentration and purity was assessed by NanoDrop ND1000 (ThermoScientific, Wilmington, DE, USA).
C. pneumoniae strain and cell lines C. pneumoniae TW183 strain was purchased from American Type Culture Collection (ATCC) (Rockville, MD, USA) and stored at -80°C. In order to propagate C. pneumoniae for PCR optimization, HELA 229 line (ATCC) cells were infected by TW183 lysate in 24-cell plate, followed by centrifugation (15 min, 400 x G) and incubated at 37°C and 5% of CO2 for 2 days, according to ATCC protocol. After incubation, cells were harvested and stored at -80°C. Presence of C. pneumoniae in cells was further confirmed by diagnostic nested-PCR assay (BLIRT-DNA Gdańsk, Poland).
Quantitative PCR Analysis
In order to create a specific quantitative assay for C. pneumoniae detection we checked (BLAST N database) 15 reference sequences of the C. pneumoniae strains and for diversification C. trachomatis strains and the ompA gene was chosen as a good molecular target for detection and quantification.
Optimisation and validation of QPCR assay
We amplified 805 bp fragment of C. pneumoniae TW183 strain based on following primers: 5’CCGGCCTACAATAAGCATTTAC and 5’GAGCTTCTGCAGTAAGTGACCA. Sequence was confirmed by RFLP method. After purification, the PCR product was cloned into pUC19 plasmid, followed by propagation in E. coli strain, isolation, purification and spectrophotometric quantification of plasmid. This construct was applied as a positive control for calibration curve. The primers for QPCR assay were designed using VNTI software (Invitrogen, Life Technologies, Carlsbad, CA, USA): 5’AACAAAGTCTGCGACCATCAATTAC, 5’GGCTGAGCAATGCGGATGTTATCAC. The conditions of PCR reaction: 2 x SybrGreen Supermix (Bio-Rad, Hercules, CA, USA), 170 nm of each primer, 4 mm MgCl2 and ddH2O were mixed with 2 μl of template DNA to a final volume of 17 μl. QPCR was performed in iCycler and fluorescence data were automatically collected and analyzed by iCycler iQ Optical Sofware ver. 3.0a (Bio-Rad, Hercules, CA, USA). QPCR conditions were: initial denaturation - 95°C/3 min; 40x (95°C/20s, 65°C/30s, 72°C/20s, 77°C /5 s-fluorescence reading step). A calibration curve was performed in triplicates using 6 ten-fold dilutions (10E+8 to 10E+3) of pUC19 plasmid DNA containing ompA gene. The slope was = -3,497; efficiency = 93.2% with a correlation coefficient R2=0.997. Dynamic melt-curve analysis and agarose-gel electrophoresis were used for all post-PCR reaction tubes to confirm the size of expected amplicon (147 bp). All reactions containing analyzed DNA were performed in duplicates. If the ΔCt between replicates was >0.3 and/or we found melting peak with a different melting point >1°C from expected, the reactions were repeated. As a method of quantification we chose relative quantity method and for histographs we applied ΔCt method, where ΔCt= (mean Ct of analyzed group)-(mean Ct of calibration points).
Statistical analyses
All statistical analyses were done using the Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA) Pearson and Fisher’s exact test. For all statistical tests, we used a comparison related significance level of P < 0.05.
Results
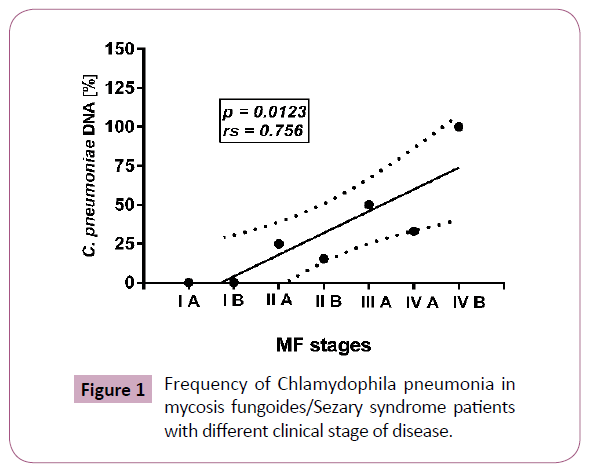
The presence of C. pneumoniae DNA was detected in 14 of 57 (24.5%) CTCL patients and in 1 of 3 patients with B-cell lymphomas. In the CTCL group positive results were found in 13 of 55 (23.6%) MF/SS patients and in one patient with CD30 (+) anaplastic large cell lymphoma. C. pneumonia was detected only in 1 of 6 analyzed skin biopsies taken from MF/SS patients (Table 1). From the controls, bacterial DNA was present in the blood samples in 1 of 40 (2.5%) psoriasis patients, in 5 of 40 (12.5%) patients with atopic dermatitis, and in none of 40 healthy individuals. No differences between MF and SS patients was observed (p=0.266). The frequency C. pneumoniae DNA in MF/SS group correlated with disease progression (rs=0.756, p=0.0123). None of 16 samples from MF patients with disease (IA or IB) were positive while C. pneumonia was detected in 3 of 17 (17, 6%) cases in stage IIA or IIB, and in 6 of 11 (54, 5%) MF patients in stages III or IV of disease. In SS patients C. pneumoniae DNA was found in 4 of 11 (36.4%) patients with stage IVB. Cumulatively in MF/SS group C. pneumoniae infection was found in 1 of 20 (5.0%) patients in early clinical stage (IA-IIA) and in 12 of 35 (34.3%) patients in advanced clinical stage (IIB-IVB). The results were statistically significant (p=0.0139). No differences in mean age between MF/SS patients with and without infection were observed (Figure 1).
Table 1: Frequency of Chlamydophila pneumoniae DNA in blood samples from patients with various types of lymphomas, psoriasis, atopic dermatitis and healthy control groups.
| Diagnose/Clinical stage | Frequency of Chlamydophila pneumoniae DNA | ||||
|---|---|---|---|---|---|
| Polish patients (N=48) | Finnish patients (N=12) | Both patients groups (N=60) | |||
| MF patients | |||||
| IA | 0/2 | 0/1 | 0/3(0%) | 0/16 (0%) | 1/20 (5%) |
| IB | 0/13 | 0/0 | 0/13 (0%) | ||
| IIA | 1/3 | 0/1 | 1/4 (25%) | 3/17 (17.6%) | |
| IIB | 1/11 | 1/2 | 2/13 (15%) | 12/35 (34.3%) p=0,0139 | |
| III | 3/6 | 0/0 | 3/6 (50%) | 6/11 (54.5%) | |
| IVA | 0/0 | 1/3* | 1/3 (33%) | ||
| IVB | 2/2 | 0/0 | 2/2 (100%) | ||
| MF summary | 9/44 (20.5%) | ||||
| SS patients | |||||
| IVA | 0/1 | 0/0 | 0/1 | ||
| IVB | 4/5 | 0/5 | 4/10 (40%) | ||
| SS summary | 4/11 (36.4%) (MF vs SS,ns, p=0,266) | ||||
| All MF/SS patients | 11/43 (25.6%) | 2/12 (16.7%) | 13/55 (23.6%) | ||
| c-ALCL (CD30+) | 1/1 | 0 | 1/1 | ||
| NK/T lymphoma | 0/1 | 0 | 0/1 | ||
| All CTCL patients | 14/57 (24.5%) | ||||
| B-cell lymphoma | 1/3 | 0 | 1/3 (33%) | ||
| All lymphomas | 15/60 (25%) | ||||
| Controls | |||||
| AD patients | 5/40 (12.5%) | ||||
| Psoriasis patients | 1/40 (2.5%) | ||||
| Healthy individuals | 0/40(0%) | ||||
*positive results obtained only from skin biopsy material; MF: Mycosis Fungoides; SS: Sezary Syndrome; cALCL: Cutaneous Anaplastic Large Cell Lymphoma; AD: Atopic Dermatitis.
Discussion
CTCL is a malignancy of skin-homing Th2 T cells; however the reason for Th2 bias remains unclear. A prominent feature of CTCL is immunosuppression, which increases the risk of bacterial and viral infections in patients especially in the advanced stages of disease. The pathophysiology of this immunodeficiency is probably multifactorial. Data from experimental studies suggest that the T-cell repertoire in CTCL patients is significantly contracted [27] Immunological abnormalities in CTCL are typically associated with depressed ability of peripheral blood cells to produce the Th1 cytokines, interferon gamma and IL-2 as a result of Th2 skewing [28-37]. During CTCL progression, reduced T-cell-mediated cellular immune responses and diminished natural killer cell activity also develop [38,39] Wysocka et al. have demonstrated a direct relationship between the extend of the pool of circulating malignant T-cells and an impaired immune response [40]. Also the functions of natural killer (NK) cells, including cellular cytotoxicity and production of interferon IFN-γ, become increasingly impaired as the circulating tumor burden increases. The authors showed an inverse correlation between circulating clonal T cells and activation status of both NK cells and CD8 T cells with a diminishing expression of number the activation markers CD69 and CD25, as well as decreased intracellular IFN-γ production. The following observations have two main pathophysiological implications. The impaired cellular immune response, which is pivotal for direct antitumor responses, leads to further acceleration of growth of the malignant T-cells population. Another consequence of decline in cytotoxic T-cell and NK cell functions is impaired activity against opportunistic infectious pathogens. This theory has been supported by the clinical observations [41-43]. It is suggested that the Th2 cytokine pattern may create a permissive environment for C. pneumoniae infection. This concept is supported by few experimental studies and clinical observations [44]. Referring to our results, C. pneumoniae DNA was found in 25% of all patients with CTCL. The frequency of chlamydial infection correlated with the stage of disease. The positive results were stated in 5% and 34% of patients in low and high clinical stages of disease respectively. This observation may confirm the important role of impaired cellular immunological response in the control of opportunistic infections such as C. pneumoniae in CTCL patients. The association between Th1 response impairment and C. pneumoniae infection may be also supported by increased incidence of bacterial DNA in patients with atopic dermatitis (12.5%) (Th2 cytokines pattern) vs. psoriatic patients (2.5%) (Th1 cytokines pattern) and healthy controls (0%) (immunocompetent individuals). C. pneumoniae DNA was detected only in one skin biopsy in our study group, in accordance with the results of German and Italian investigators, who not detected bacterial in the skin biopsies from MF/SS patients, and confirm that C. pneumoniae infection could not be estimated as a primary event in the pathogenesis of CTCL, in spite of Casselli et al. case describing skin presence of Chlamydia spp (and HHV8) in all recurrences of CD30+CTCL as well as in routine control blood samples [45-47].
In the summary, our results suggest that C. pneumoniae infection is not a primary event in the pathogenesis of CTCL, but may be estimated as a risk factor complicating advanced stages of the disease associated with Th2 shift and deficiency of antibacterial defence mechanisms.
Acknowledgement
The study was approved by the local research ethics committee of the Medical University of Gdańsk. The study is financed by Polish Ministry of Science and Higher Education grant 02-0066/07/253".
References
- Tan RSH, Butterworth CM, Mclaughlin H, Malka S, Samman PD (1974) Mycosis fungoides: a disease of antigen persistence. Br J Dermatol 91: 607-616.
- Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, et al. (1997) Association of erythrodermic cutaneous T-cell lymphoma, superantigen – positive Staphylococcus aureus and oligoclonal T-cell receptor V beta gene expansion. Blood 89: 32-40.
- Abrams JT, Balin BJ, Vonderheid EC (2001) Association between Sezary T-cell activating factor, Chlamydia pneumoniae, and cutaneous T cell lymphoma. Annals of the New York Academy of Sciences 941: 69-85.
- Kanamoto Y, Ouchi K, Mizui M, Ushio M, Usui T (1991) Prevalence of antibody to Chlamydia pneumoniaeTWAR in Japan. J Clin Microbiol 29: 816-818.
- Marin A, Karolyi A, Szalka A (1992) Prevalence of Chlamydia pneumoniae antibodies in Hungary. Eur J Clin Microbiol Infect Dis 11: 139-142.
- Montes M, Cilla G, Alcorta M, Perez-Trallero E (1992) High prevalence of Chlamydia pneumoniae infection in children and young adults in Spain Pediatr Infect Dis J 11: 972-973.
- Koivisto AL, Isoaho R, Von Hertzen L, Toyryla M, Laippala P(1999) Chlamydial antibodies in an eldery Finnish population. Scand J Infect Dis 31: 153-159.
- Ni AP, Lin GY, Yang L, He HY, Huang CW, et al. (1996) A seroepidemiologic study of Chlamydia pneumoniae, Chlamydia trachomatis and Chlamydia psittaci in different populations on the mainland of China. Scand J Infect Dis 28: 553-557.
- Ferrari M, Poli A, Olivieri M, Tardivo S, Biasin C, et al. (2000) Seroprevalence of Chlamydia pneumoniae antibodies in a young adult population sample living in Verona. European Community Respiratory Health Survey (ECRHS) Verona. Infection 28: 38-41.
- Freidank HM, Brauer D (1993) Prevalence of antibodies to Chlamydia pneumoniae TWAR in a group of German medical students. J Infect 27: 89-93.
- Miyashita N, Niki Y, Nakajima M, Fukano H, Matsushima T (2001) Prevalence of asymptomatic infection with Chlamydia pneumoniae in subjectively healthy adults. Chest 119: 1416-1419.
- Grayston JT, Aldous MB, Easton A (1993) Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J Infect Dis 168: 1231-1235.
- Kuo CC, Jackson LA, Campbell LA, Grayston JT (1995) Chlamydia pneumoniae (TWAR). Clin Microbiol Rev 8: 451-461.
- Bauriedel G, Andrie R, Likungu JA, Welz A, Braun P (1999) Persistence of Chlamydia pneumoniae in coronary plaque tissue. A contribution to infection and immune hypothesis in unstable angina pectoris. Dtsch Med Wochenschr 124: 1408-1413.
- Fortini A, Corti G, Cappelletti C (1999) Chlamydia pneumoniae and atherosclerosis. Ann Ital Med Int 14: 253-263.
- Balin BJHC, Gerard EJ, Arking DM, Appelt PJ, Branigan JT, et al. (1998) Whittum-Hudson Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med. Microbiol. Immunol 187: 23-42.
- Budak F (2007) The investigation of Chlamydophila pneumoniae in patients with multiple sclerosis. Int J Neurosci 117: 409-415.
- West SK (2009) Detection of circulating Chlamydophila pneumoniae in patients with coronary artery disease and healthy control subjects. Clin Infect Dis, 48: 560-567.
- Rodel J, Woytas M, Groh A, Schmidt KH, Lehmann M, et al. (2000) Production of Basic Fibroblast Growth Factor and Interleukin 6 by human Smooth Muscle Cells following infection with Chlamydia pneumoniae. Infect Immun 68: 3635-3641.
- Kol A, Bourcier T, Lichtman AH, Libby P (1999) Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells and macrophages. J Clin Invest 103: 571-577.
- Kaukoranta-Tolvanen SS, Ronni T, Leinonen M, Saikku P, Laitinen K (1996) Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog 21: 407.
- Caspar-Bauguil S, Puissant B, Nazzal D, Lefcvre JC, Thomsen M, et al. (2000) Chlamydia pneumoniae induces interleukin - 10 production that down - regulates Major Histocompatibility Complex class I expression. J Infect Dis 182: 1394-1401.
- Geng Y, Shane RB, Berencsi K, Gonczol E, Zaki MH, et al. (2000) Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol 164: 5522-5529.
- Byrne GI, Ojcius DM (2004) Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol 2: 802-808.
- Sarris AH, Esgleyes-Ribot T, Crow M, Broxmeyer HE, Karasavvas N, et al. Cytokine loops involving interferon-gamma and IP-10, a cytokine chemotactic for CD4+ lymphocytes: an explanation for the epidermotropism of cutaneous T-cell lymphoma? Blood 86: 651-658.
- Abrams JT, Vonderheid EC, Kolbe S, Appelt DM, Arking DJ, et al. (1999) Sezary T-cell activating factor is Chlamydia pneumoniae-associated protein. Clinical and diagnostic laboratory immunology 6: 895-905.
- Halme S, Saikku P, Surcel HM (1997) Characterization of Chlamydia pneumoniae antigens using human T cell lines. Scandinavian Journal of Immunology 45: 378-384.
- Yawalkar N, Ferenczi K, Jones DA (2003) Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood 102: 4059-4066.
- Hahtola S, Tuomela S, Elo L, Häkkinen T, Karenko L, et al. (2006) Th1 response and cytotoxicity genes are down-regulated in cutaneous T-cell lymphoma. Clin Cancer Res 12: 4812-4821.
- Okamura H, Tsutsi H, Komatsu T (1995) Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378: 88-91.
- Naik SM, Cannon G, Burbach GJ (1999) Human keratinocytes constitutively express interleukin-18 and secrete biologically active interleukin-18 after treatment with pro-inflammatory mediators and dinitrochlorobenzene. J Invest Dermatol 113: 766-772.
- Yoshimoto T, Takeda K, Tanaka T (1998) IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol 161: 3400-3407.
- El-Mezayen RE, Matsumoto T (2004) In vitro responsiveness to IL-18 in combination with IL-12 or IL-2 by PBMC from patients with bronchial asthma and atopic dermatitis. Clin Immunol 111: 61-68.
- Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, et al. (1999) Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 94: 902-908.
- Yoshimoto T, Tsutsui H, Tominaga K (1999) IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA 96: 13962-13966.
- Hwang ST, Janik JE, Jaffe ES, Wilson WH (2008) Mycosis fungoides and Sezary syndrome. Lancet 371: 945-957.
- Adams AE, Zwicker J, Curiel C (2004) Aggressive cutaneous T-cell lymphomas after TNFalpha blockade. J Am Acad Dermatol 51: 660-662.
- Kaplan EH, Rosen ST, Norris DB, Roenigk HH Jr, Saks SR, et al. (1990) Phase II study of recombinant human interferon gamma for treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst 82: 208-212.
- Laroche L, Kaiserlian D (1983) Decreased natural-killer-cell activity in cutaneous T-cell lymphomas. N Engl J Med 308: 101-102.
- Vowels BR, Cassin M, Vonderheid EC, Rook AH (`1992) Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol 99: 90-94.
- Wysocka, M (2002) Sezary syndrome patients demonstrate a defect in dendritic cell populations: effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood 100: 3287-3294.
- Axelrod PI, Lorber B, Vonderheid EC (1992) Infections complicating mycosis fungoides and Sezary syndrome. JAMA 267: 1354-1358.
- Lee J, Richardson S, Melhem ER, Rook AH, Kim EJ (2007) Progressive multifocal leukoencephalopathy from JC virus in a patient with advanced mycosis fungoides. JAAD 57: 893-895.
- Park CS, Kim TB, Moon KA, Bae YJ, Lee HR, et al. (2010) Chlamydophila pneumoniae enhances secretion of VEGF, TGF-b, and TIMP-1 from human bronchial epithelial cells under Th2 dominant microenvironment. Allergy Asthma Immunol Res 2: 41-47.
- Rößler MJ, Rappl G, Muche M, Hasselmann DO, Sterry W, et al. (2003) No evidence of skin infection with Chlamydia pneumoniae in patients with cutaneous T cell lymphoma. Clinical microbiology and infection 9: 721-723.
- Ferreri AJ, Ponzoni M, Govi S, Pasini E, Mappa S, et al. (2012) Prevalence of chlamydial infection in a series of 108 primary cutaneous lymphomas. Br J Dermatol 166: 1121-1123.
- Caselli E, Borghi A, Maritati M, Gafà R, Lanza G, et al. (2016) Relapses of primary cutaneous anaplastic large-cell lymphoma in a female immunocompetent patient with persistent chlamydophila pneumoniae and human herpesvirus 8 infection. Infect Agent Cancer 5: 31.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences