ISSN : 0976-8505
Der Chemica Sinica
Synthesis and Antimicrobial Activity of 2-Benzylidene-1,3 Indandiones: A Structure-Reactivity Study
Radhakrishnan K1, Mohandass P1, Sankaralingam S2 and Chandra Mohan S3
1Department of Chemistry, Saraswathi Narayanan College, Perungudi, Madurai, Tamil Nadu, India
2Department of Botany, Saraswathi Narayanan College, Perungudi, Madurai, Tamil Nadu, India
3Division of Phytochemistry, Shanmuga Centre for Medicinal Plants Research, Thanjavuru, Tamil Nadu, India
Abstract
Substituted 2-benzylidene-1,3-indandiones have been prepared and characterized by UV, IR, 1H and 13C NMR spectral analysis. The antimicrobial activities and structure reactivity correlation of the compounds have been studied.
Keywords
Substituted 2-benzylidene-1,3-indandiones; Antimicrobial; Correlation studies
Introduction
Different methods have been used for the synthesis of 1,3-indandione derivatives with substitution at position 2. Previous studies [1,2] reported phenylation of 1,3-indandione with diaryliodonium salts and α-alkenylation of β-dicarbonyl compounds with alkenyltriarylbismuthonium salts. The Friedel-Crafts methods were also reported for the derivatization of 1,3-indandione at position 2 [3]. In addition to these conventional methods, the electrochemical synthesis has also been used for preparation of indandione derivatives with catechol or 2,3-dimethylhydroquinone ring on their position 2 [4-6].
Studies of substituent effects on zone of inhibition against the growth of microorganisms in various substituted N-(1-piperidino benzyl) nicotinamide [7] and substituted N-(1-piperidinobenzyl) acetamide and substituted N-(1- morpholinobenzyl) acetamide [8] have been reported. The literature reveals that there is a little work done on the antimicrobial study of activated olefinic compounds. As a part of our interest in the structure-reactivity study, we have synthesized 2-benzylidene-1,3-indandiones and studied the antimicrobial activity to find out the substituent effect on 2-benzylidene-1,3-indandione.
Materials and Methods
All chemicals used were purchased from Sigma Aldrich. Purity of the compounds was checked by TLC on silica gel G plates. UV-Visible spectra were recorded on a CARY VARION (V 550), CHCl3 was used as a solvent. Infrared spectra were obtained on a PARAGON 500 spectrometer on KBr pellets. 1H and 13C spectra were obtained on a BRUKER AMX 400 MHz spectrometer. Chemical shift of 1H were measured with the peak of CDCl3 at δ 7.29 as the internal reference, while those of 13C were recorded with the central peak of CDCl3 at δ 77.03 as the internal reference.
General procedure for the synthesis of 2-benzylidene-1,3-indandiones (1 to 6)
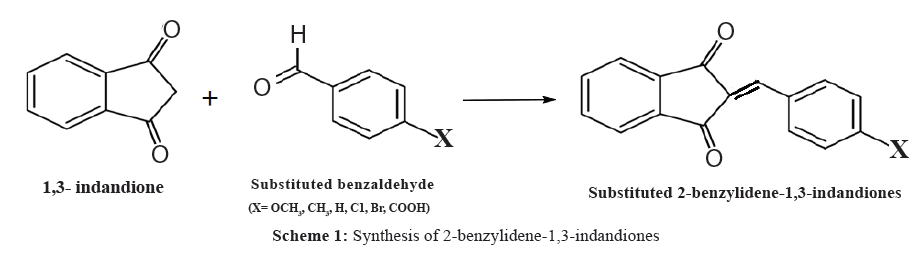
Preparation of 2-Benzylidene-1,3-indandione and its substituted compounds (1 to 6) were done as per the reported procedure [9]. To the calculated amount of the appropriate benzaldehyde (12.5 mM) and 1,3-indandione (12.5 mM) in warm ethyl alcohol was added a 10% solution of sodium hydroxide (catalytic amount) and the reaction mixture stirred for 2 h to 3 h and left overnight (Scheme 1). The solvent was removed in vacuum.
Spectral analysis of compounds (1 to 6)
Compound 1: 2-(4’-Methoxybenzylidene)-1,3-indandione
UV: 252,386 nm; IR: 526.21, 731.99, 839.46, 1022.34, 1174.91, 1266.32, 1505.89, 555.70, 1581.14, 2839.62, 3439.07 cm-1; 1H NMR: δ 3.917(s,3H), 6.998-7.027(m,2H), 7.766-7.842(m,2H), 7.958-8.00(m,2H), 8.529-8.558(m,2H); 13C NMR: δ 55.60,114.38, 123.07, 126.46, 134.87, 146.86, 189.54, 190.86.
Compound 2: 2-(4’-Methylbenzylidene)-1,3-indandione
UV: 264,357 nm; IR: 739.74, 818.12, 989.52, 1080.52, 1189.24, 1332.42, 1351.89, 1376.46, 1588.08, 1683.93, 1725.29, 3489.89 cm-1; 1H NMR: δ 2.343(s,3H), 7.178(d,2H), 7.689-7.710(m,2H), 7.766(s,1H), 7.899(dd,1H), 8.287(d,1H); 13C NMR: δ 22.01, 123.22, 123.22, 128.17, 135.04, 147.10, 189.20, 190.53.
Compound 3: 2-Benzylidene-1,3-indandione
UV: 264,345 nm; IR: 568.09, 682.38, 732.69, 981.07, 1198.94, 1244.02, 1345.96, 1376.53, 1586.17, 1683.75, 1724.93, 2364.96, 3063.50, 3783.09 cm-1; 1H NMR: δ 7.519-7.580(m,4H), 7.824-7.846(m,2H), 7.9249(s,1H), 8.020- 8.047(m,1H), 8.465-8.4869(dd,2H); 13C NMR: δ 123.26, 128.82,129.17, 135.25, 135.45, 189.04, 190.32.
Compound 4: 2-(4’-Chlorobenzylidene)-1,3-indandione
UV: 265,351 nm; IR: 830.56, 1074.32, 1092.42, 1203.63, 1411.93, 1486.02, 1582.35, 1609.80, 1689.99, 1726.37, 2363.08, 3092.11, 3696.20 cm-1; 1H NMR: δ 7.474-7.495,(m,2H), 7.827(s,1H), 7.834-7.848(m,2H), 7.997- 8.018(m,2H), 8.417-8.438(m,2H); 13C NMR: δ 123.44, 129.14, 131.54, 135.39, 145.20, 189.01, 189.98.
Compound 5: 2-(4’-Bromobenzylidene)-1,3-indandione
UV: 265,353 nm; IR: 736.35, 828.32, 1072.53, 1162.92, 1202.37, 1248.20, 1408.55, 1485.35, 1577.50, 1612.90, 1689.61, 1726.98, 3086.69 cm-1; 1H NMR: δ 7.640-7.662(dd,2H), 7.811(s,1H), 7.829-7.851(m,2H), 8.011- 8.035(m,2H), 8.322-8.353(dd,2H); 13C NMR: δ 123.44, 129.63, 132.16, 135.57, 145.27, 189.01, 189.96.
Compound 6: 2-(4’-Carboxybenzylidene)-1,3-indandione
UV: 263,340 nm; IR: 734.01, 988.89, 1156.73, 1202.53, 1248.65, 1293.26, 1428.79, 1592.13, 1618.67, 1687.05, 1731.80, 2549.02, 2825.28 cm-1; 1H NMR: δ 7.851(s,1H), 7.902-7.923(m,2H), 7.955-7.992(m,2H), 8.039(d,2H), 8.465(d,2H); 13C NMR: δ 39.05, 123.64, 129.70, 134.37, 136.36, 144.36, 188.60, 189.34.
Antimicrobial activity
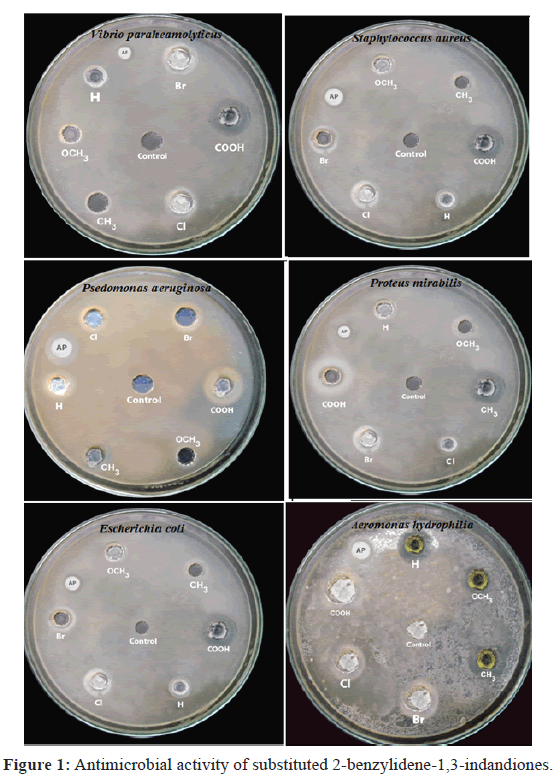
Agar well-diffusion method was followed to determine the antimicrobial activity [10]. Nutrient agar (NA) and potato dextrose agar (PDA) plates were swabbed (sterile cotton swabs) with 8 h old broth culture of respective bacteria. Wells (6 mm) were made in each of these plates using sterile cork borer. Briefly, agar plates were inoculated with bacterial strain under aseptic conditions and wells (diameter=6 mm) were filled with 50 μl of the test samples and incubated at 37°C for 24 h. After the incubation period, the radii of the growth of inhibition zones were measured. The distance between centers of the well to the edge of the zone was determined to be the inhibition zone radius. Three inhibition zone radii measurements were taken for each well and averaged, for each replicates the readings were taken in three different fixed directions and the average values were recorded. The average inhibition zone radii for the various bacteria are shown in Table 1.
| S.N. | Name of the microorganisms | Inhibition zone radius (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Standard (Amphotericin – B) |
-OCH3 | -CH3 | -H | -Cl | -Br | -COOH | ||
| 1 | Aeromonas hydrophilia | 21 | 5 | 6 | 7 | 8 | 9 | 12 |
| 2 | Escherichia coli | 16 | 6 | 7 | 8 | 9 | 10 | 12 |
| 3 | Psedomonas aeruginosa | 21 | 5 | 6 | 8 | 8 | 9 | 11 |
| 4 | Proteus mirabilis | 18 | 5 | 6 | 7 | 9 | 10 | 12 |
| 5 | Staphylococcus aureus | 16 | 6 | 7 | 8 | 9 | 9 | 11 |
| 6 | Vibrio paraheamolyticus | 18 | 5 | 7 | 9 | 10 | 10 | 12 |
Table 1: Antimicrobial activity (zone of inhibition (mm) values) of substituted 2-benzylidene-1, 3-indandiones.
Results and Discussion
In this study, gram-positive bacteria (Staphylococcus aureus) and five gram-negative bacteria (Aeromonas hydrophila, Escherichia coli, Psedomonas aeruginosa, Proteus mirabilis, and Vibrio paraheamolyticus) were used. The result of the present study showed a broad range of antimicrobial activity. The data found in the literature, that the compounds with halogen substituent are the most efficient against gram-positive bacteria, particularly against S. aureus [11,12]. But in this study, we found more or less equal zone of inhibition values for all gram-positive and gram-negative bacteria (Figure 1). It shows that the antibacterial activity depends upon substituent only. Compound 6 exhibited excellent antibacterial activity. It has been established that the –COOH group has an excellent metal-binding capacity [13]. This explains the higher antibacterial activity. The results also reveal that the antibacterial activity is affected by the nature of the substituent group (X) found in the aryl ring. The chloride derivative is characterized by greater antibacterial activity than that of the methyl and methoxy derivatives. According to Mohamed et al. [13] this may be attributed to the electron-withdrawing character of the chlorine group that decreases the electron density in the indandiones group, increasing its cationic character. The derivatives with electron withdrawing groups showed strong antibacterial activity than those of electron donating group [14]. Electron-withdrawing substituent increases acidity also. Bacterial growth is inhibited by increasing the acidity of the substituents. The order of antibacterial activity of compounds (1 to 6) for all the microorganism were in the following sequence:
–OCH3<–CH3<–H<–Cl<–Br<–COOH
If atom or group attracts electrons less strongly than hydrogen, it is said to have +I effect (electron repelling or electron releasing) viz., –OCH3, –CH3 groups showing lesser zone inhibition values compared to unsubstituted phenyl ring (–H).
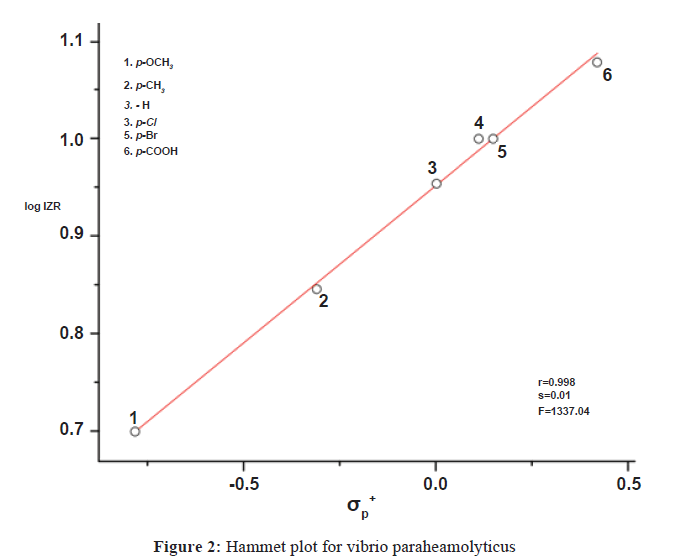
In order to express the effect of substituents quantitatively it was considered worthwhile to correlate the logarithm of inhibition zone radius (IZR) of 1 to 6 at the same concentration with the Hammett substituent constants for all the microorganisms. The results of statistical SSP analysis are given in Table 2. The corresponding Hammett plot for V. paraheamolyticus is shown in Figure 2.
| S.N. | Bacteria | Scale | ρ | r | s | F | Log(IZR)o | N |
|---|---|---|---|---|---|---|---|---|
| 1 | Aeromonashydrophila | σP | 0.48 ± 0.04 | 0.981 | 0.03 | 104.12 | 0.839 ± 0.012 | 6 |
| σP o | 0.50 ± 0.11 | 0.915 | 0.06 | 20.56 | 0.815 ± 0.028 | 6 | ||
| σP+ | 0.30 ± 0.05 | 0.95 | 0.05 | 37.47 | 0.897 ± 0.019 | 6 | ||
| σP+/σP | 0.29 ± 0.06 | 0.927 | 0.06 | 24.42 | 0.878 ± 0.023 | 6 | ||
| σP+/σP- | 0.26 ± 0.03 | 0.981 | 0.03 | 102.65 | 0.879 ± 0.012 | 6 | ||
| σP+/σP/σP- | 0.25 ± 0.03 | 0.972 | 0.04 | 67.47 | 0.865 ± 0.015 | 6 | ||
| 2 | Escherichia coli | σP | 0.39 ± 0.03 | 0.985 | 0.02 | 134.03 | 0.896 ± 0.008 | 6 |
| σP o | 0.40 ± 0.09 | 0.918 | 0.05 | 21.5 | 0.877 ± 0.022 | 6 | ||
| σP+ | 0.25 ± 0.04 | 0.96 | 0.03 | 47.81 | 0.944 ± 0.014 | 6 | ||
| σP+/σP | 0.23 ± 0.04 | 0.94 | 0.04 | 30.76 | 0.928 ± 0.017 | 6 | ||
| σP+/σP- | 0.20 ± 0.02 | 0.975 | 0.03 | 78.24 | 0.929 ± 0.011 | 6 | ||
| σP+/σP/σP- | 0.20 ± 0.03 | 0.97 | 0.03 | 64.73 | 0.917 ± 0.012 | 6 | ||
| 3 | Pseudomonas aeruginosa | σP | 0.45 ± 0.05 | 0.976 | 0.03 | 81.07 | 0.853 ± 0.013 | 6 |
| σP o | 0.47 ± 0.10 | 0.916 | 0.06 | 21.05 | 0.83 ± 0.026 | 6 | ||
| σP+ | 0.30 ± 0.03 | 0.986 | 0.02 | 140.74 | 0.909 ± 0.01 | 6 | ||
| σP+/σP | 0.28 ± 0.04 | 0.963 | 0.04 | 52.47 | 0.89 ± 0.015 | 6 | ||
| σP+/σP- | 0.24 ± 0.03 | 0.968 | 0.04 | 61.35 | 0.891 ± 0.014 | 6 | ||
| σP+/σP/σP- | 0.24 ± 0.03 | 0.965 | 0.04 | 54.32 | 0.877 ± 0.015 | 6 | ||
| 4 | Proteus miratrilis | σP | 0.52 ± 0.03 | 0.992 | 0.02 | 257.61 | 0.852 ± 0.008 | 6 |
| σP o | 0.55 ± 0.10 | 0.943 | 0.05 | 32.39 | 0.824 ± 0.025 | 6 | ||
| σP+ | 0.32 ± 0.05 | 0.952 | 0.05 | 38.53 | 0.915 ± 0.02 | 6 | ||
| σP+/σP | 0.31 ± 0.06 | 0.94 | 0.05 | 38.53 | 0.895 ± 0.022 | 6 | ||
| σP+/σP- | 0.27 ± 0.04 | 0.953 | 0.05 | 30.39 | 0.895 ± 0.02 | 6 | ||
| σP+/σP/σP- | 0.27 ± 0.04 | 0.956 | 0.05 | 40.009 | 0.88 ± 0.019 | 6 | ||
| 5 | Staphylococcusaureus | σP | 0.33 ± 0.03 | 0.987 | 0.02 | 162.4 | 0.887 ± 0.007 | 6 |
| σP o | 0.35 ± 0.07 | 0.931 | 0.04 | 25.9 | 0.869 ± 0.018 | 6 | ||
| σP+ | 0.21 ± 0.02 | 0.979 | 0.02 | 92.15 | 0.927 ± 0.009 | 6 | ||
| σP+/σP | 0.20 ± 0.03 | 0.962 | 0.03 | 49.6 | 0.914 ± 0.011 | 6 | ||
| σP+/σP- | 0.18 ± 0.14 | 0.988 | 0.16 | 164.59 | 0.914 ± 0.006 | 6 | ||
| σP+/σP/σP- | 0.18 ± 0.01 | 0.986 | 0.02 | 143.3 | 0.904 ± 0.007 | 6 | ||
| 6 | Vibrio paraheamolyticus | σP | 0.47 ± 0.08 | 0.945 | 0.05 | 33.62 | 0.893 ± 0.023 | 6 |
| σP o | 0.48 ± 0.14 | 0.865 | 0.08 | 11.91 | 0.87 ± 0.036 | 6 | ||
| σP+ | 0.32 ± 0.01 | 0.998 | 0.01 | 1337.04 | 0.952 ± 0.003 | 6 | ||
| σP+/σP | 0.31 ± 0.02 | 0.99 | 0.02 | 200.93 | 0.932 ± 0.009 | 6 | ||
| σP+/σP- | 0.26 ± 0.04 | 0.964 | 0.04 | 52.37 | 0.932 ± 0.017 | 6 | ||
| σP+/σP/σP- | 0.26 ± 0.03 | 0.973 | 0.03 | 72.13 | 0.917 ± 0.014 | 6 |
Table 2: Results of statistical treatment of log IZR (mm) with σP, σP o, σP +, σP +/σP, σP +/σP-, σP+/σP/σP- substituent constants using single parameter Equation 1.
The positive value of the reaction constant (ρ) equation 1:
log IZR=(0.32 ± 0.01)σP++(0.952 ± 0.003)
(r=0.998, n=6, F=1337.04)
Indicates that electron withdrawing substituents increase the antimicrobial activity and electron releasing substituents retard it.
DSP analysis has been performed for each of the resonance scale (σR, σR+ , σR –). The best fit of DSP analysis for A. hydrophila is taken from satisfactory correlation coefficient (R) and least standard error (SE) of the regression equations (2) and (3) and the result obtained given in Table 3.
| S.N. | Bacteria | Scale | ρI | ρR | R | SE | F | Log(IZR)o | N | λ=ρR/ρI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aeromonashydrophila | σI ,σR | 0.44 ± 0.17 | 0.50 ± 0.17 | 0.89 | 0.08 | 5.72 | 0.87 ± 0.05 | 6 | 1.14 |
| σI ,σR+ | 0.08 ± 0.18 | 0.17 ± 0.06 | 0.876 | 0.08 | 4.97 | 0.92 ± 0.07 | 6 | 2.12 | ||
| σI ,σR- | 0.61 ± 0.05 | 0.68 ± 0.06 | 0.991 | 0.02 | 84.98 | 0.86 ± 0.02 | 6 | 1.11 | ||
| F,R | 0.41 ± 0.08 | 0.46 ± 0.08 | 0.979 | 0.03 | 22.27 | 0.84 ± 0.02 | 5 | 1.12 | ||
| 2 | Escherichia coli | σI ,σR | 0.37 ± 0.12 | 0.42 ± 0.12 | 0.918 | 0.06 | 8.04 | 0.92 ± 0.04 | 6 | 1.14 |
| σI ,σR+ | 0.08 ± 0.15 | 0.14 ± 0.05 | 0.88 | 0.07 | 5.17 | 0.96 ± 0.05 | 6 | 1.75 | ||
| σI ,σR- | 0.50 ± 0.06 | 0.53 ± 0.06 | 0.986 | 0.02 | 50.92 | 0.91 ± 0.02 | 6 | 1.06 | ||
| F,R | 0.36 ± 0.07 | 0.39 ± 0.07 | 0.978 | 0.03 | 21.88 | 0.90 ± 0.02 | 5 | 1.08 | ||
| 3 | Pseudomonas aeruginosa | σI ,σR | 0.43 ± 0.10 | 0.53 ± 0.11 | 0.956 | 0.05 | 16.19 | 0.89 ± 0.03 | 6 | 1.23 |
| σI ,σR+ | 0.06 ± 0.14 | 0.17 ± 0.05 | 0.92 | 0.06 | 8.35 | 0.94 ± 0.05 | 6 | 2.83 | ||
| σI ,σR- | 0.56 ± 0.1 | 0.62 ± 0.11 | 0.966 | 0.04 | 20.96 | 0.87 ± 0.03 | 6 | 1.11 | ||
| F,R | 0.43 ± 0.22 | 0.56 ± 0.23 | 0.999 | 0.009 | 341.28 | 0.88 ± 0.007 | 5 | 1.3 | ||
| 4 | Proteus miratrilis | σI ,σR | 0.54 ± 0.13 | 0.53 ± 0.14 | 0.944 | 0.06 | 12.26 | 0.87 ± 0.04 | 6 | 0.98 |
| σI ,σR+ | 0.18 ± 0.19 | 0.17 ± 0.07 | 0.883 | 0.09 | 5.29 | 0.91 ± 0.07 | 6 | 0.94 | ||
| σI ,σR- | 0.69 ± 0.09 | 0.66 ± 0.09 | 0.98 | 0.04 | 36.34 | 0.86 ± 0.02 | 6 | 0.96 | ||
| F,R | 0.54 ± 0.80 | 0.54 ± 0.08 | 0.984 | 0.03 | 31.31 | 0.85 ± 0.03 | 5 | 1 | ||
| 5 | Staphylococcus aureus | σI ,σR | 0.31 ± 0.09 | 0.37 ± 0.10 | 0.931 | 0.04 | 9.72 | 0.91 ± 0.03 | 6 | 1.19 |
| σI ,σR+ | 0.05 ± 0.12 | 0.12 ± 0.04 | 0.889 | 0.05 | 5.65 | 0.95 ± 0.04 | 6 | 2.4 | ||
| σI ,σR- | 0.41 ± 0.05 | 0.47 ± 0.05 | 0.985 | 0.02 | 47.3 | 0.90 ± 0.01 | 6 | 1.15 | ||
| F,R | 0.29 ± 0.19 | 0.36 ± 0.19 | 0.998 | 0.007 | 216.31 | 0.90 ± 0.006 | 5 | 1.24 | ||
| 6 | Vibrio paraheamolyticus | σI ,σR | 0.42 ± 0.08 | 0.61 ± 0.08 | 0.98 | 0.03 | 36.82 | 0.95 ± 0.02 | 6 | 1.45 |
| σI ,σR+ | 0.02 ± 0.19 | 0.18 ± 0.07 | 0.865 | 0.09 | 4.45 | 0.99 ± 0.07 | 6 | 9 | ||
| σI ,σR- | 0.55 ± 0.15 | 0.68 ± 0.16 | 0.934 | 0.06 | 10.3 | 0.93 ± 0.05 | 6 | 1.24 | ||
| F,R | 0.42 ± 0.03 | 0.66 ± 0.03 | 0.998 | 0.01 | 314.17 | 0.95 ± 0.008 | 5 | 1.57 |
Table 3: DSP analysis of log IZR (mm) with dual parameter Equations 2 and 3.
log IZR=(0.61 ± 0.05) σI+(0.68 ± 0.06)σR-+(0.86 ± 0.02) (2)
(R=0.991, SE=0.02, n=6, F=84.98)
log IZR=(0.41 ± 0.08)F+(0.46 ± 0.08)R+(0.84 ± 0.02) (3)
(R=0.979, SE=0.03, n=5, F=22.27)
The sign of ρI and ρR are positive, reveals that the normal substituent effects operates on IZR, i.e. An electron releasing substituents decrease the IZR and electron withdrawing substituents increase the IZR. The ρR values are rather smaller than ρI values and this reveals the importance of polar component.
The Yukawa-Tsuno equation 4 and Table 4 for S. aureus proved the less contribution of resonance effect.
| S. N. |
Bacteria | Scale | ρ | r | R | SE | F | N |
|---|---|---|---|---|---|---|---|---|
| 1 | Aeromonashydrophila | σP,(σP+- σP) | 0.45 ± 0.07 | 0.07 ± 0.01 | 0.984 | 0.03 | 45.17 | 6 |
| σPo,(σP+- σPo) | 0.41 ± 0.09 | 0.2 ± 0.9 | 0.97 | 0.04 | 23.99 | 6 | ||
| 2 | Escherichia coli | σP,(σP+- σP) | 0.35 ± 0.04 | 0.07 ± 0.04 | 0.992 | 0.02 | 94.85 | 6 |
| σPo,(σP+- σPo) | 0.33 ± 0.06 | 0.17 ± 0.06 | 0.978 | 0.03 | 32.96 | 6 | ||
| 3 | Pseudomonas aeruginosa | σP,(σP+- σP) | 0.38 ± 0.04 | 0.12 ± 0.05 | 0.993 | 0.02 | 110.24 | 6 |
| σPo,(σP+- σPo) | 0.37 ± 0.03 | 0.23 ± 0.03 | 0.996 | 0.02 | 173.63 | 6 | ||
| 4 | Proteus miratrilis | σP,(σP+- σP) | 0.50 ± 0.04 | 0.04 ± 0.05 | 0.994 | 0.02 | 122.79 | 6 |
| σPo,(σP+- σPo) | 0.46 ± 0.07 | 0.18 ± 0.07 | 0.983 | 0.03 | 44.05 | 6 | ||
| 5 | Staphylococcus aureus | σP,(σP+- σP) | 0.29 ± 0.02 | 0.07 ± 0.02 | 0.0998 | 0.008 | 311.31 | 6 |
| σPo,(σP+- σPo) | 0.28 ± 0.02 | 0.15 ± 0.02 | 0.995 | 0.01 | 145.43 | 6 | ||
| 6 | Vibrio paraheamolyticus | σP,(σP+- σP) | 0.34 ± 0.04 | 0.22 ± 0.05 | 0.993 | 0.02 | 115.03 | 6 |
| σPo,(σP+- σPo) | 0.33 ± 0.02 | 0.31 ± 0.02 | 0.998 | 0.009 | 556.9 | 6 |
Table 4: Results of multiple regression analysis of log IZR (mm) with σP, (σP+-σP) and σP o, (σP+- σP o) constants using Yukava-Tsuno Equation 4.
log IZR=(0.29 ± 0.02)σP+(0.07 ± 0.02)(σP+-σP)+(0.90 ± 0.06) (4)
(R=0.998, SE=0.008, n=6, F=311.31)
Conclusion
To summarize, substituted 2-benzylidene-1,3-indandiones have been synthesized and evaluated for their antibacterial activities. This reaction protocol offers a simple, easier work-up procedure and good yields. The compounds have been characterized by their spectral data. The antimicrobial activities of all synthesized compounds have been studied. The inhibition zone radii of these compounds have been correlated with Hammett substituent constants, F and R parameters. From the results of statistical analysis, the effects of substituent on the antimicrobial activity of compounds have been studied.
References
- Beringer FM, Galton SA, Huang SJ (1962) Diaryliodonium Salts XVII The Phenylation of 1,3-Indandiones. J Am Chem Soc 84: 2819-2823.
- Matano Y, Imahori H (2004) A New efficient method for direct α-Alkenylation of β-Dicarbonyl compounds and phenols using alkenyltriarylbismuthonium salts. J Org Chem 69: 5505-5508.
- Song HH, Lee HJ, Kim HR, Ryu EK, Kim JN (1999) Unusual behaviour of N-tosyl pipecolinic acid in Friedel-Crafts reaction conditions. Synth Commun 29: 3303-3311.
- Shamsipur M, Davarani SSH, Bayandori-Moghaddam A, Nematollahi D, Fakhari AR (2007) Facile one-pot synthesis of 2-(3,4-dihydroxyphenyl)-2-phenyl-2H-indene derivatives via electrochemical oxidation of catechols in the presence of 2-phenyl-1,3-indandione. Polish J Chem 81: 237-247.
- Hosseiny Davarani SS, Nematollahi D, Shamsipur M, Mashkouri Najafi N, et al. (2006) Electrochemical oxidation of 2, 3-dimethylhydroquinone in the presence of 1, 3-dicarbonyl compounds. J Org Chem 71: 2139-2142.
- Bayandori-Moghaddam A, Ganjali MR, Norouzi P, Latifi M (2006) A green method for the electro organic synthesis of new 1,3-indandione derivatives. Chem Pharm Bull 54: 1391-96.
- Raman N, Ravichandran S (2002) Synthesis and Antimicrobial Activity of Substituted N-(1-piperidinobenzyl)- Nicotinamide: A Structure-Reactivity Study. Asian J Chem 14: 1766-1768.
- Raman N, Ravichandran S (2003) Effect of Substituents on N-(1-Piperidinobenzyl)acetamide and N-(1-Morpholinobenzyl)acetamide and Their Antimicrobial Activity. Asian J Chem 15: 1848-1850.
- M Islam, AK Khalam (1969) Indian J Chem 7: 546.
- Chandra Sekar V, Knabel SJ, Anantheswaran RJ (2015) Modeling development of inhibition zones in an agar diffusion bioassay. Food Sci Nutri 3: 394-403.
- Smart BE (2001) Fluorine substituent effects (on bioactivity). J Fluor Chem 109: 3-11.
- Dipankar B, Panneerselvam P, Asish B (2012) Synthesis, characterization and antimicrobial activities of some 2-Pyrazoline derivatives. Asian J Pharm Clin Res 5: 42-46.
- Mohamed NA, Sabaa MW, El-Ghandourb AH, Abdel-Azizc MM, Abdel-Gawad OF (2013) Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int J Biol Macromol 60: 156-164.
- Martins AF, Facchi SP, Follmann HDM, Pereira AGB, Rubira AF, et al. (2014) Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A Review. Int J Mol Sci 15: 20800-20832.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences