ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Study of the Effect of Extracts from the Seeds "Trigonella foenum-gracecum, Nigella sativa" on Oxalo-Calcium Crystallization
Aoumria Ouldmoumna1*, Fatima Zohra Kiari3, Naouel Bouregba4 and Abdelkrim Gueffai2
1LMAE Laboratory, Faculté des Sciences Exactes, Université de Mustapha Stambouli de Mascara, Mascara, Algeria
2LGPCS Laboratory, Faculté des Sciences techniques, Université de Mustapha Stambouli de Mascara, Mascara, Algeria
3LRSBG Laboratory, Faculté des Sciences et de la Technologie, Université de Mustapha Stambouli de Mascara, Mascara, Algeria
4LSTE Laboratory, Faculté des Sciences techniques, Université de Mustapha Stambouli de Mascara, Mascara, Algeria
- *Corresponding Author:
- Aoumria Ouldmoumna
LMAE Laboratory, Faculté des Sciences Exactes,
Université de Mustapha Stambouli de Mascara, Mascara,
Algeria,
E-mail: Aoumria.ouldmoumna@univ-mascara.dz
Received date: November 25, 2023, Manuscript No. AJPSKY-23-18158; Editor assigned date: November 28, 2023, PreQC No. AJPSKY-23-18158 (PQ); Reviewed date: December 12, 2023, QC No. AJPSKY-23-18158; Revised date: December 19, 2023, Manuscript No. AJPSKY-23-18158 (R); Published date: January 01, 2024, DOI: 10.36648/2249-7412.14.1.312
Citation: Ouldmoumna A, Kiari FZ, Bouregba N, Gueffai A (2023) Study of the Effect of Extracts from the Seeds "Trigonella foenum-gracecum, Nigella sativa" on Oxalo-Calcium Crystallization. Asian J Plant Sci Vol:14 No:1: 312.
Abstract
The main objective of this study is to evaluate the effect of extracts of medicinal plants “Trigonella foenumgracecum and Nigella sativa”, on oxalocalcic crystallization in vitro. The measurement of the concentration is chosen to follow the kinetics of crystallization of calcium oxalate in the absence and in the presence of extract of the plants “Trigonella foenum-gracecum and Nigella sativa” we have chosen different concentrations for the extracts. Plants (10%, 5% and 1%). The qualitative analysis of these extracts as well as of the powders was carried out by CCM and by IRTF analysis which revealed the presence of numerous constituents. The presence of inhibitors at various concentrations allows us to give information on the percentage of inhibition. Extracts from the seeds of Trigonella foenum-gracecum and the Nigella sativa were beneficial on crystallization of crystals in vitro. The Nigella sativa extract recovered by the two Soxhlet and maceration techniques revealed percentages of inhibition of: 73.65% and 78.19% successively in comparison with the extract of Trigonella foenum-gracecum which gave percentages of: 95.79% and 33.59%.
Keywords
Trigonella foenum-gracecum; Nigella sativa; Oxalocalcium; Cristallizations; Inhibition
Introduction
The lithiasis disease, from the Greek "lithos" which means stone, merges with the history of mankind since the first known bladder calculus dates back to around 4800 years before Jesus-Christ and was discovered in the remains of a mummy in Upper Egypt [1]. Urolithiasis is a common pathology which affects, depending on the country, 4% to 20% of the general population [2].
Since the dawn of time, man has always sought to use plants to feed himself first but also to heal himself. Herbal medicine constitutes a serious alternative or at least an appreciable complement to the classic pharmacy resulting from modern chemistry. And many prescribed remedies have active ingredients of natural origin. In popular tradition, many plants are mentioned as remedies for various diseases, including urolithiasis.
Modern research is only rediscovering this knowledge acquired over the centuries. Indeed, many studies on the activity and therapeutic modes of action of medicinal plants [3,4]. Medicinal plants allow treatment to be approached in a comprehensive and less aggressive way by eliminating most of the side effects.
Our vision is to analyze the activity of the crude extracts obtained from the seeds of Trigonella foenum-gracecum and the seeds of Nigella sativa on oxalocalcic crystallization, using two extraction methods: Soxhlet and maceration for both plants.
Materials and Methods
The objective of this work is the study of the inhibitory effect by extracts of seeds of Nigella sativa and Trigonnella foenum gracecum on oxalocalcic crystallization, to achieve this objective two steps are followed:
The first step is the extraction "the preparation of crude extracts from the seeds of Nigella sativa and Trigonnella foenum gracecum.
The second step is the study of the inhibitory effect of extracts from the seeds of Nigella sativa and Trigonnella foenum gracecum on the growth of calcium oxalate like crystals which were followed by measurement of the absorbance using a spectrophotometer.
The second step is the study of the inhibitory effect of extracts from the seeds of Nigella sativa and Trigonnella foenum gracecum on the growth of calcium oxalate like crystals which were followed by measurement of the absorbance using a spectrophotometer.
Preparation of extracts
The choice falls on these two types of seeds, the black seed under the name of Nigella sativa and the Trigonnella foenum gracecum because they can represent a good precursor for the preparation of the extracts.
Plant material: The seeds of Nigella sativa and Trigonnella foenum used in this study were procured from the local Mascara market (Algeria), these seeds are cleaned and crushed by the device "analysis grinder 150 ml with stainless steel knife N35107" then were passed through a 0.45 mm porosity sieve.
Chemical products: The solvents used (methanol, distilled water) during the various extraction steps, as well as the CaCl2 (Calcium Chloride) and Na2C2O4 (Sodium Oxalate), NaCl (Sodium Chloride) supplied by the company (MERCK).
Methods
Extraction of Nigella sativa and Trigonnella foenum gracecum seeds by the Soxhlet technique: The extraction of the seeds of Nigella sativa and Trigonnella foenum gracecum were carried out according to the method described [5], which consisted in obtaining aqueous extracts using an organic solvent, from which the seeds are extracted by the "Soxhlet" extractor. The extract obtained is recovered after rotary evaporation (Heidolph) at temperature 40°C then the extraction yield is calculated according to following equation.
The extracts are mixed and stored in a sterile glass tube at refrigeration temperature (4°C).
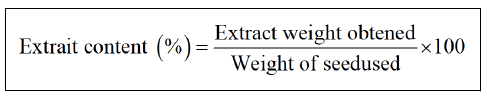
Extraction of Nigella sativa and Trigonella foenum gracecum seeds by the maceration technique: It is a method of preparation that is used in particular with seeds; in this case, the seeds of Nigella sativa and Trigonnelle foenum gracecum are ground before maceration to ensure better distribution of the active ingredients [6]. The extracts obtained are recovered after distillation at a temperature of 64.7°C and stored in sterile hermetic bottles at 4°C. The extraction yield is calculated using the above equation.
Characterization techniques
The characterization methods suitable for our study are: The Fourier transform spectrophotometer method, optical microscope, thin layer chromatography.
Analysis of Nigella sativa and Trigonella foenum gracecum extracts by Thin Layer Chromatography (TLC): To ensure the migration and then the chromatographic separation of the different chemical constituents of the extracts, we deposited a drop of each extract prepared on aluminum support silica gel plates (stationary phase) at reference points 1.5 cm from the lower edge of the plate. This stationary phase is then traversed by a mobile phase contained in tanks whose flow causes migration and separation of the chromatographic compounds between the two phases. For each extract, several mobile phases (eluent) were used (Table 1). After development in a glass tank and drying by a hot plate, the TLC plates are observed under a UV lamp (LANGWELLE) 264 nm and 366 nm. Spot colors were recorded.
| Extracts | Eluant proportions |
|---|---|
| Raw extract | Chloroform:Ethyl acetate:Formic acid (50%:40%:10%) |
| Hexane:Ethyl ether:Acetone (77.66%:19.41%:2.91%) | |
| Acetone: Hexane (1 v:2 v) | |
| Hexane:Methanol:Distilled water:Acetone (1:1:1:1 v/v/v/v) |
Table 1: Proportions of eluent used for each extract.
Analysis of plants by infrared spectrometry: The powders of Nigella sativa and Trigonnelle foenum gracecum, the extracts obtained (methanolic and aqueous) and the residues are characterized by the Fourier transform spectrometer method. The acquisition of infrared data is carried out by an Agilent Technologies Fourier transform spectrometer at the LMAE laboratory, University of Mascara (Algeria). The prepared samples (Nigella sativa and Trigonnelle foenum gracecum,) are analyzed directly by the infrared device under operational conditions, the most intense vibration bands of the material are recorded.
Preparations of urinary crystals of calcium oxalate type
Artificial urinary crystals are prepared from Calcium Chloride (CaCl2) and Sodium Oxalate (Na2C2O4). In our study, artificial urine of known concentration was prepared by dissolving the exact quantities of CaCl2 and Na2C2O4 in NaCl. Our objective is to test the effect of methanoic and aqueous extracts on the crystals of calcium oxalates monohydrate; for this reason, we have prepared different solutions. A measurement of the absorbance of the solution as a function of time by a spectrophotometer at 620 nm is determined. At the end of each experiment, the solutions are filtered and then dried and the filtrates recovered are subjected to characterization by FTIR. Table 2 groups the different preparation of the solutions.
| CaCl2 | Na2C2O4 | NaCl | Extract Nigella.s by Soxhlet | Extract Nigella.s by maceration | Extract Trigonnelle.f by Soxhlet | Extract Trigonnelle.f by maceration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 5% | 1% | 10% | 5% | 1% | 10% | 5% | 1% | 10% | 5% | 1% | ||||
| Solution 1 | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - |
| Solution 2 | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - |
| Solution 3 | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - |
| Solution 4 | + | + | + | - | - | + | - | - | - | - | - | - | - | - | - |
| Solution 5 | + | + | + | - | - | - | + | - | - | - | - | - | - | - | - |
| Solution 6 | + | + | + | - | - | - | - | + | - | - | - | - | - | - | - |
| Solution 7 | + | + | + | - | - | - | - | - | + | - | - | - | - | - | - |
| Solution 8 | + | + | + | - | - | - | - | - | - | + | - | - | - | - | - |
| Solution 9 | + | + | + | - | - | - | - | - | - | - | + | - | - | - | - |
| Solution 10 | + | + | + | - | - | - | - | - | - | - | - | + | - | - | - |
| Solution 11 | + | + | + | - | - | - | - | - | - | - | - | - | + | - | - |
| Solution 12 | + | + | + | - | - | - | - | - | - | - | - | - | - | + | - |
| Solution 13 | + | + | + | - | - | - | - | - | - | - | - | - | - | - | + |
Table 2: The different preparations of the solutions.
Monitoring of the kinetics of the evolution of calcium oxalate by UV visible spectrophotometer
Study without inhibitor: We prepared the solutions of CaCl2, 2H2O (10 mM) and Na2C2O4 (4 mM) using a solvent: Sodium chloride solution (0.15 M). A volume of 1.5 ml of dehydrated calcium chloride is transferred into the empty cell with 1.5 ml of sodium oxalate, at the previous volume, the measurement is started immediately and the absorbance is launched for a time interval of 20 mins for each experiment. At the end of the experiment, the solution is filtered and then dried at 60°C and the filtrate undergoes characterization by FTIR.
Study with inhibitor: We prepared inhibitor solutions (10%, 1%, 5%) of each extract of the seeds of Nigella sativa and Trigonella foenum gracecum. 1 ml of dehydrated calcium chloride is mixed with 1 ml of inhibitor solution in the measuring cell. Take a blank reading, then add a volume of 1 ml of sodium oxalate and the measurement is immediately started for a time of 20 min. The percent inhibition was calculated using the following equation.
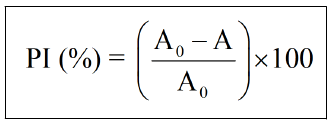
Note: A0: Absorbance of solution without inhibitor; A: Absorbance of solution with inhibitor.
Microscopic study of urinary crystals
The morphological analysis of the prepared solutions was carried out by an optical microscope (PHYWE) with a 40X objective. The photographs are taken using a type camera (LG.E. Nexus 05-8 MP 3264 406 KB) in each experiment, at the beginning and at the end. A drop of the mixture is placed in the Mallasses cell, which is immediately placed under the microscope objective.
Monitoring of the pH of the solutions
We measured the pH for each solution using a pH meter (Inolab) during the study of the kinetics which determines the absorbance of the solution as a function of time by spectrophotometry at 620 nm.
Results and Discussion
Characterization of powders and extracts
Yield of extracts: The extraction by the method of Soxhlet (methanolic) of the seeds of the Trigonelle a makes it possible to obtain a viscous extract of brown color with a yield of 40.46% of the weight of the seed, in comparison with other works this value is lower than that obtained by Indian Trigonella foenum gracecum (45%) [7]. This result is superior to that obtained by maceration which results in a yield of 22.98% of the weight of the seed.
The seeds of Nigella sativa recorded a yield of 34.28% of the weight of the seed by the Soxhlet method. The crude extracts are brown in color, with a strong odor, in comparison with other ecotypes of the nigella, this yield of the Algerian ecotype is comparable to that of the Egyptian nigella (34.78%) [8] and higher than that obtained by the Tunisian nigella which revealed a yield of 28.48% but it is lower than the Iranian nigella (40.35%) [9], moreover the extraction by Maceration gave a yellowish brown extract with a yield of 18.66%, the latter is less than 27% which is reported by Houcher and collaborators [10] having used the same extraction technique but whose percentage of methanol in the extraction mixture is different, the yield of extraction found in this work, it is comparable to that of 21.5% reported by MEZITI ASMA [11].
Analysis of extracts of Nigella sativa and Trigonnelle foenum gracecum by Thin Layer Chromatography (TLC): Qualitative analysis of the extracts (methanolic and aqueous) of Nigella sativa and Trigonnelle foenum gracecum by thin layer chromatography revealed that the two samples of Nigella sativa have almost the same qualitative composition and contain a number considerable amount of constituents visible on the chromatographic profiles. We opted for this technique in order to characterize the different constituents of Nigella sativa seed extracts.
The migration system allowed to have a very good chromatographic separation and an acceptable visibility of the spots. The revelation was carried out by observation under ultraviolet light (at 366 nm). The comparison of the Rfs of the spots resulting from the separation of the extracts with those of the controls used makes it possible to have an idea of the compounds present in the seeds of Nigella sativa. Elagic acid; quercitrin; morina; thymol; carvacrol; gallic acid. Are probably present in the methanolic extract of Nigella sativa. catechin; elagic acid; quercitrin; gallic acid; thymol. Are probably present in the aqueous extract of Nigella sativa.
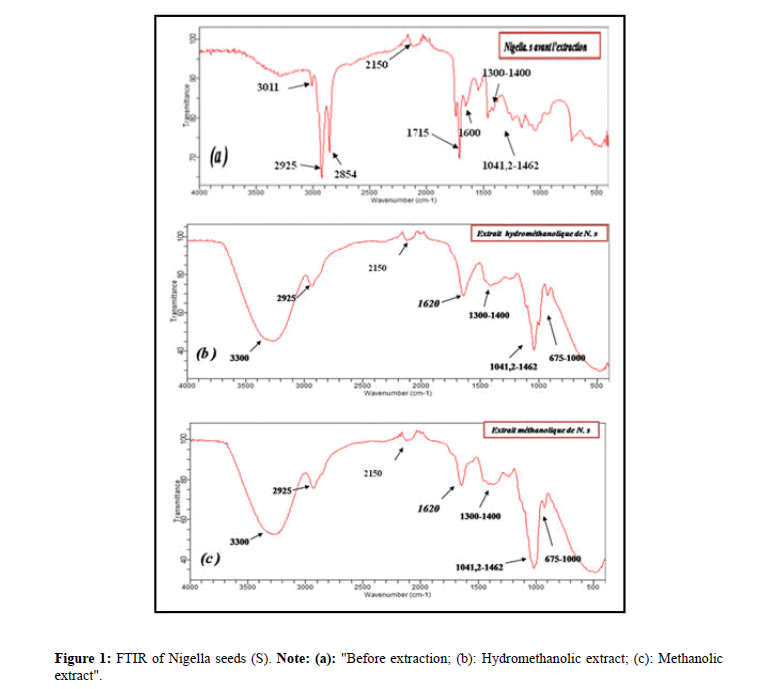
Analysis of powders and extracts of Nigella sativa and Trigonella foenum gracecum by FTIR: Figure 1 shows the Fourier transform infrared spectra of Nigella sativa seeds "before extraction (a) Aqueous extract (b) Methanolic extract (c) the spectra are similar but with different intensities.
A broad absorption band at 3000-3600 cm-1 with a maximum at about 3011 cm-1 is characteristic of the stretching vibration of bonded hydroxyl groups (from the water). This band is intense for the hydromethanolic extract because of the presence of water.
The FTIR spectra of the seed spectral bands at 2925 cm-1 and 2854 cm-1 resulting in the middle aliphatic C-H band of elongation (in an aromatic methoxyl group, in the methyl and methylene groups of side chains) of aromatic CH. A faint band of low intensity observed at 2150 cm-1 corresponds to C-C groups of alkynes. Respectively a broad band observed in all spectra at ~1041.2 cm-1 to 1462 cm-1 can be attributed to an elongation band in the C-O moiety of acids, alcohols, phenols, ethers or ester groups [12]. In the region of 1300-1400 cm-1 several absorption bands are observed corresponding to an elongation band of C-N vibration bonds. A peak observed at 1600 cm-1 corresponds to a deformation in the N-H grouping plane of the amides and at 1620 cm-1 for the methanolic and hydromethanolic extracts and in the region of 675-1000 cm-1 weak bands are observed corresponding to an elongation band of the C=C-H vibration bonds for the methanolic and hydromethanolic extracts. The band located at 1715 cm-1 indicates the presence of a C=O elongation band of aldehydes.
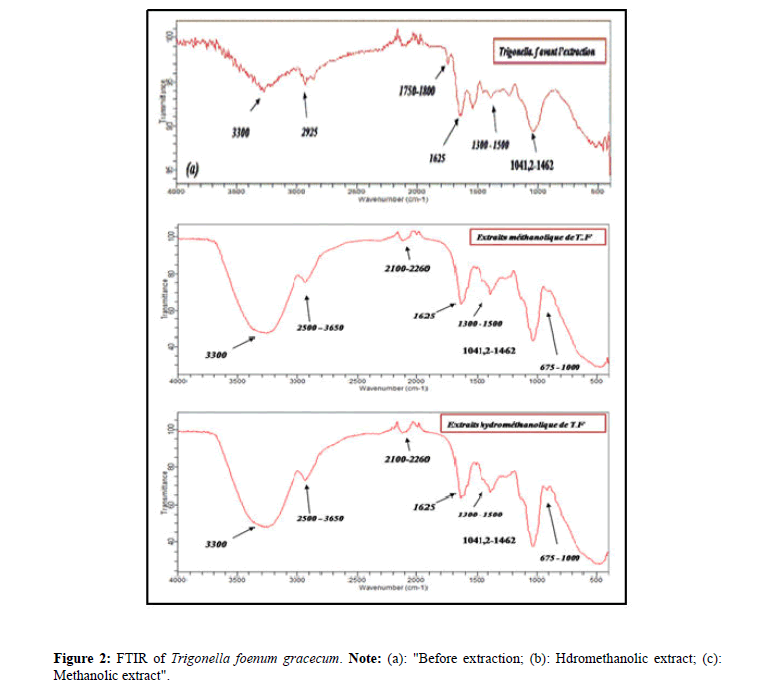
Figure 2 presents the Fourier transform infrared spectra of the seeds of Trigonella foenum gracecum. Note: (a): "Before extraction; (b): Hydromethanolic extract; (c): Methanolic extract; (d): After extraction".
A broad band of O-H absorption at 3000-3500 cm-1 with a maximum at around 3300 cm-1 is characteristic of the stretching vibration and can be attributed to alcohols or phenols. This band is intense for the hydromethanolic extract because of the presence of water. The FTIR spectra of seed spectral bands at 2921 cm-1 and 2854 cm-1 resulting in the middle aliphatic C-H stretching band (in the side chain methyl and methylene groups) of aromatic CH, respectively. Two peaks observed around 1500 to 1700 cm-1 correspond to the C=O group of esters, lactones. In the region of 1300 to 1500 cm-1 an absorption band is observed corresponding to an elongation band of the bonds of aromatic C=C vibrations. And in the region of 675 to 1000 cm-1 weak bands are observed corresponding to an elongation band of C=C-H vibrational bonds for methanolic and hydromethanolic extract. A peak observed at 1246 cm-1 corresponds to an elongation band of C-O bonds of aromatic ethers. The band located at 1015 cm-1 indicates the presence of a common primary alcohol C-O elongation band for the three spectra (a,b,c).
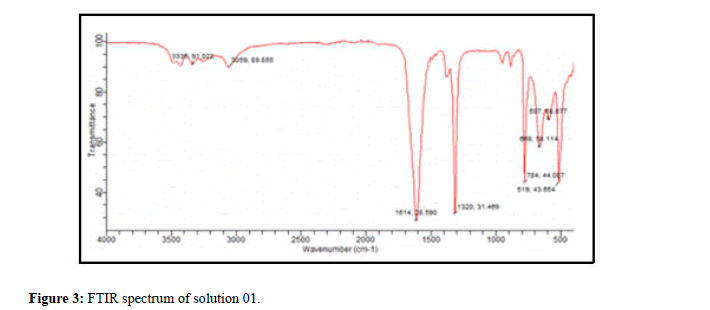
Analysis of the filtrate of solution 01 by FTIR: The spectrum (Figure 3) represents the identity card of calcium oxalate monohydrate, the characteristic peaks of this type of crystals are gathered in Table 3.
| Mineralogical name | Formula | Abbreviation | Characteristic peaks |
|---|---|---|---|
| Whewellite | CaC2O4 | COM, Cl, Wew | TF:1614 cm-1; F:1318 cm-1; M:784 cm-1 |
| Peak observed (cm-1) | |||
| 3335F-3059F-1614TF-888TF-658TF-784M-519F-597F | |||
| Investigations in the identification of constituent. | |||
|
|||
|
|||
|
|||
|
|||
Table 3: Fact sheet for calcium oxalate monohydrate (solution 01).
For the interpretation, we systematically give a file corresponding to the compound including the mineralogical name, the formula, the abbreviation and the characteristic and observed peaks as well as the investigations.
The absence of the spectra of the other solutions studied shows the significant effect of the plant extracts used which presented a total inhibition of the crystals of calcium oxalates which is in agreement with the results of the tests carried out by morphology using microscopy optics.
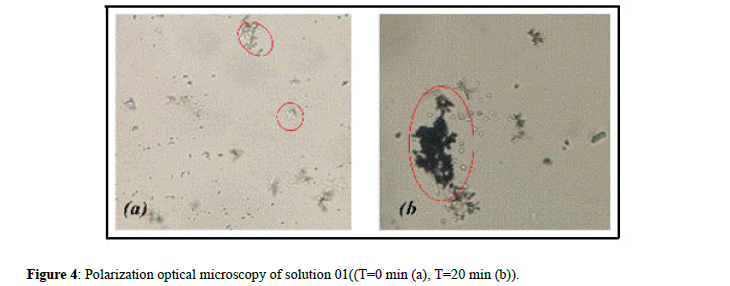
Analysis by Optical Microscope (OM): Microscopic examination can quickly provide information on the type of urinary crystals and their size.
The results of the photographs give an overview of the nature of the crystals at T=0 (a) and at T=20 min (b) for urinary crystals of calcium oxalates (Figure 4).
From Figure 4a, we notice that the size and the number of crystals are small: At T=0 min which shows a step corresponding to crystal germination, in comparison with Figure 4b, which presents a significant number of crystals and its consistent with the last step which is crystal aggregation.
It has been recorded that the extracts of the seeds of Nigela sativa and Trigonella foenum gracecum have an inhibiting effect on crystal aggregation in the two main phases of crystallization (growth and aggregation) in the tests carried out by all the extracts used (1%, 5% and 10%) of the two types of seeds, can also be explained by an important conversion of the crystals from the form of calcium oxalates monohydrate to the dihydrate form, a very significant cause for the absence of the infrared spectra for all the solutions studied.
Application of extracts to crystal inhibition
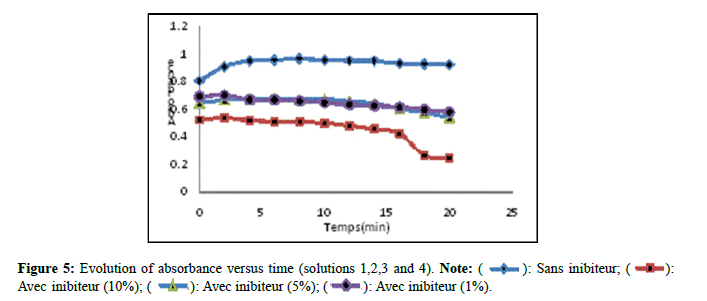
Monitoring the effect of extracts by spectrophotometry: Figure 5 presents the evolution of the absorbance as a function of time of solutions 1,2,3 and 4. According to (Figure 5) which represents the kinetics of absorbance as a function of time, an increase was observed absorbance as a function of time in solution 01 (without inhibitor), but for solution 02 a significant decrease was recorded, solutions 03 and 04 revealed a slight decrease in comparison with solution 01. This decrease may be explained by a slight effect of the extract of Nigella sativa on the crystallization of monohydrated calcium oxalates. This agrees with the work done by Barros et al., [13].
According to Figure 6, solution 05 marked a decrease, solutions 06 and 07 revealed a slight decrease in comparison with solution 01 which indicates that the variation of the absorbance decreases progressively with the increase in the concentration of the inhibitor. These results explain that the extract fraction of Nigella sativa by maceration exerts an inhibitory activity on the growth of the crystals in comparison with the extract by Soxhlet. The results found are confirmed by other works which found that the extracts of medicinal plants react to the stage of growth aggregates [14,15].
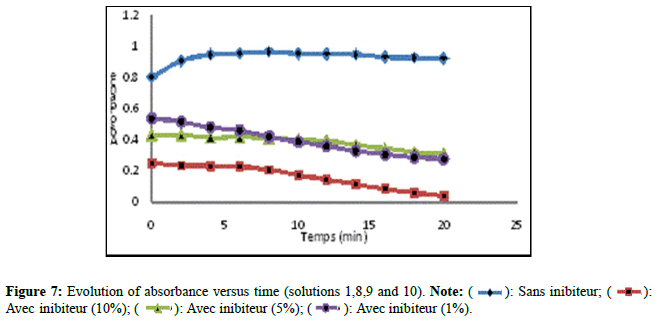
The results of the absorbance of the solutions prepared without and with extract of Trigonella foenum gracecum by Soxhlet are shown in Figure 7. According to (Figure 7), solution 10 recorded a significant decrease in comparison with solutions 08 and 09 which revealed a slight decrease. The Trigonella foenum gracecum extract recovered by Soxhlet extraction, rich in acid compounds, has an inhibitory activity; these acid functions; probably did a direct action of complexing calcium ions. This contributed to a slowing (prolongation) in the growth stage of the crystals and the photographs obtained by microscopy, clearly show that the inhibition by extract manifests itself at the growth stage. The results found are comparable with other researchers [15]. Figure 8 shows the variation in absorbance as a function of time (min) of calcium oxalate crystals without and with inhibitor (extract from Trigonella foenum gracecum by maceration (10%, 5% and 1%)). Solutions 11,12 and 13 recorded a significant decrease (Figure 8), compared to solution 01 which indicates that the change in absorbance gradually decreases with increasing inhibitor concentration. This decrease shows that the extract of Trigonella foenum gracecum recovered by the maceration method marked a slight effect on the crystallization of monohydrated calcium oxalates in comparison with the extract (Soxhlet).
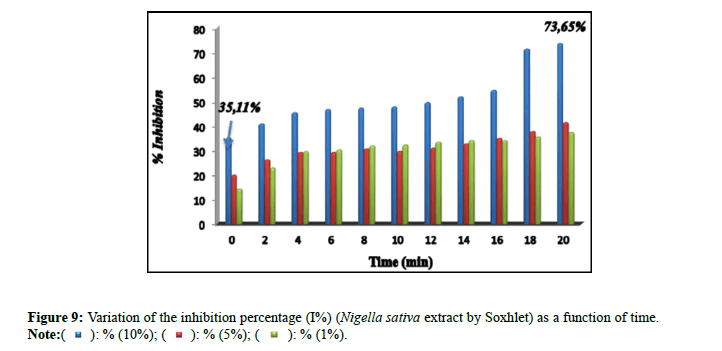
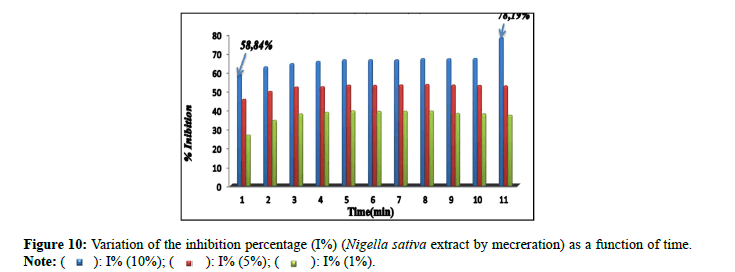
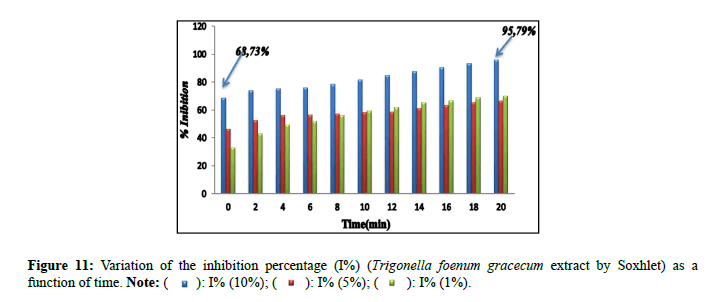
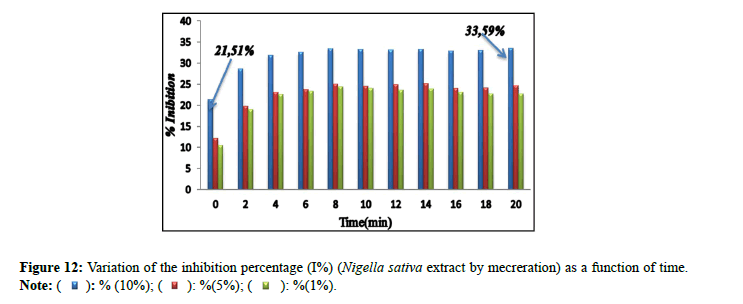
Percentage of inhibitions: The inhibitory effect of the extract of Nigella sativa (Soxhlet). It is confirmed by the percentage of inhibition (Figure 9), which revealed 73.65% by the extract (10%) in comparison with the extracts (5% and 1%) which marked inhibition percentages of: 14.47% and 37.26% successively. The percentages of inhibition (I%) of the solutions prepared for the extract of Nigella sativa by maceration (10%, 5% and 1%) are presented in Figure 10. The results found of the percentage of the inhibition (I%) of the solutions prepared for the extract of Trigonella foenum gracecum by Soxhlet (Figure 11) revealed very significant percentages with respect to the inhibition of monohydrated calcium oxalate crystals, in particular for the percentage of extract of 10% which recorded 95.79%. This result is encouraged and is comparable to those found by Sharma et al., (I%=84.71%) [16]; and the researcher Bensatal et al., (I%=58.33) [17]. From the calculated inhibition percentages of the (aqueous) extracts of the seed of Trigonella foenum gracecum which recorded 33.59% for the 10% extract. (Figure 12). It can be said that the methanolic extract has a very significant inhibitory effect compared to the aqueous extract for the seed of Trigonella foenum gracecum, which agrees with the results found by Priya et al., [18].
Behavior of the pH as a function of the composition of the solutions studied
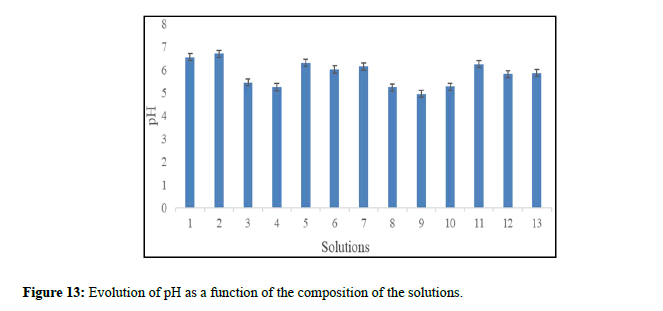
Among the factors responsible for the risk of crystallization. Changes in urine pH: They cause the ionic product to vary [19]. A pH that is too acidic (<5.5) promotes the transformation of sodium urate into uric acid, which is much less soluble. Too alkaline pH (>6.5) reduces the solubility of phosphate salts. Precipitation, however, occurs at a pH that varies widely from subject to subject. Bacteria (especially proteus). They promote lithogenesis [19]. We should also note the excessive intakes of vitamin D (calcium oxalate stones) [20]. The solubility of calcium oxalate stones is less affected by the pH value, the use of acidifying diets could promote their formation. Calcium oxalate stones are in fact preferentially formed at acidic pH, between 6.3 and 6.7 [21]. This agrees with the results found in our study, it was recorded decreases in the pH of the solutions studied depending on the composition of the solutions (depending on the concentration and type of inhibitors used) (Figure 13).
Conclusion
Within the framework of the valorization of the seeds of Nigella sativa and Trigonella foenum gracecum the study of their preventive effects against the crystallization of calcium oxalates, we prepared by two methods of extraction "Soxhlet and maceration" extracts which recorded significant yields by the Soxhlet method (40.46% for Trigonella foenum gracecum and 34.28% for Nigella sativa) compared to those recovered by the maceration technique (22.98% for Trigonella foenum gracecum and 18, 66% concerning Nigella sativa).
Qualitative analysis of these extracts by TLC revealed the presence of numerous constituents. The spectrophotometric technique was chosen to follow the kinetics of the crystallization of calcium oxalate in the absence and in the presence of plant extracts "Nigella sativa and Trigonella foenum gracecum" for different concentrations of plant extracts (10%, 5% and 1%).
To confirm the reliability and reproducibility of our experimental results, we calculated the coefficient of inhibition in all the tests performed. The values obtained were very encouraging. This very beneficial result prompted us to make an infrared and microscopic analysis of the solutions after crystallization tests in the absence and in the presence of inhibitors, which showed a significant conversion of the crystals from the form of calcium oxalates monohydrates to the dihydrate form. The microscopic analysis of the solutions recovered after tests in the absence and in the presence of the extract (inhibitor), shows the very significant absence of crystals in the test in the presence of extract compared to the test in the absence. From the results obtained, we find that the extract of the plant Nigella sativa and Trigonella foenum gracecum has an inhibitory effect on the aggregation of crystals in the two main phases of crystallization (growth and aggregation). The pH of the crystal medium is influenced by the crystal composition and the concentration and type of inhibitor.
References
- Daudon M (2005) Epidémiologie actuelle de la lithiase rénale en France. Ann Urol 39: 209-231.
[Crossref], [Google Scholar]
- Oussama A, Hilmi A, Semmoud A, Daudon M (2000) Analyse des calculs urinaires de l’adulte dans le Moyen Atlas marocain par spectrophotométrie infrarouge à transformée de fourier. Prog Urol 10: 404-410.
- Falleh H, Hafsi C, Mohsni I, Ksouri R (2021) Evaluation of different procedures for the extraction of phenolic compounds from a medicinal plant: Verbena officinalis. Biol Aujourdhui 215: 133-142.
[Crossref], [Google Scholar], [Indexed]
- Gueffai A, Gonzalez-Serrano DJ, Christodoulou MC, Orellana-Palacios JC, Ortega MLS, et al. (2022) Phenolics from defatted black cumin seeds (Nigella sativa L.): Ultrasound-assisted extraction optimization, comparison, and antioxidant activity. Biomolecules 12: 1311.
[Crossref], [Google Scholar], [Indexed]
- Naima B (2006) Etude phytochimique des plantes médicinales algérienne: Ruta montana, Matricaria pubescens et Hypercum perfoliatum.
- Sophie A, Ehrhart N (2011) La phytothérapie se soigner par les plantes. Eyrolles, 1er edition, France, 1-192.
- Ciftci ON, Przybylski R, Rudzinska M, Acharya S (2011) Characterization of fenugreek (Trigonella foenum-graecum) seed lipids. J Am Oil Chem Soc 88: 1603-1610.
[Crossref], [Google Scholar]
- Atta MB (2003) Some characteristics of nigella (Nigella sativa L) seed cultivated in Egypt and its lipid profile. Food Chem 83: 63-68.
[Crossref], [Google Scholar]
- Cheikh-Rouhou S, Besbes S, Hentati B, Blecker C, Deroanne C, et al. (2007) Nigella sativa L: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem 101: 673-681.
[Crossref], [Google Scholar]
- Houcher Z, Boudiaf K, Benboubetra M, Houcher B (2007) Effects of méthanolic extract and commercial oil of Nigella sativa L. on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines 18: 8-18.
- Meziti A (2009) Activité antioxydant des extraits des graines de Nigella sativa L. Étude in vitro and in vivo. Universite de BATNA, pp: 73.
- Zawadzki J (1989) Infrared spectroscopy in surface chemistry of carbons. In: Thrower, PA. Ed., chemistry and physic of carbon, Marcel Dekker, New York 21: 147-386.
- Barros ME, Schor N, Boim MA (2003) Effects of an aqueous extract from Phyllanthus niruri on calcium oxalate crystallization in vitro. Urol Res 30: 374-379.
[Crossref], [Google Scholar], [Indexed]
- Joshi VS, Parekh BB, Joshi MJ, Vaidya AB (2005) Herbal extracts of Tribulus terrestris and Bergenia ligulata inhibit growth of calcium oxalate monohydrate crystals in vitro. J Cryst Growth 275: 1403-1408.
[Crossref], [Google Scholar]
- Suzuki K, Kawamura K, Tsugawa R (1999) Formation and growth inhibition of calcium oxalate by Takusha (Alismatis rhizoma). Scanning Microsc 13: 183-189.
- Sharma D, Dey YN, Sikarwar I, Sijoria R, Wanjari MM, et al. (2016) In vitro study of aqueous leaf extract of Chenopodium album for inhibition of calcium oxalate and brushite crystallization. Egypt J Basic Appl Sci 10: 164-171.
[Crossref], [Google Scholar]
- Bensatal A, Ouahrani MR (2008) Inhibition of crystallization of calcium oxalate by the extraction of Tamarix gallica L. Urol Res 36: 283-287.
[Crossref], [Google Scholar], [Indexed]
- Priya T (2003) Antimicrobial studies of methanol and ethanol extracts (Soxhlet extraction) of Fenugreek (Trigonella Foenum). J Pharmatutor.
- Fourcade J (2006) Néphrologie: Lithiase urinaire. Faculté de Médecine Montpellier Nîmes, France.
- Eurobiologiste (2001) Lithiase urinaire: Numéro thématique (Partie 1). 44/253: 1-64.
- Lulich JP, Osborne CA, Lekcharoensuk C, Kirk CA, Bartges JW (2004) Effects of diet on urine composition of cats with calcium oxalate urolithiasis. J Am Anim Hosp Assoc 40: 185-191.
[Crossref], [Google Scholar], [Indexed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 ): Sans inibiteur; (
): Sans inibiteur; ( ):
Avec inibiteur (10%); (
):
Avec inibiteur (10%); ( ): Avec inibiteur (5%); (
): Avec inibiteur (5%); (  ): Avec inibiteur (1%).
): Avec inibiteur (1%).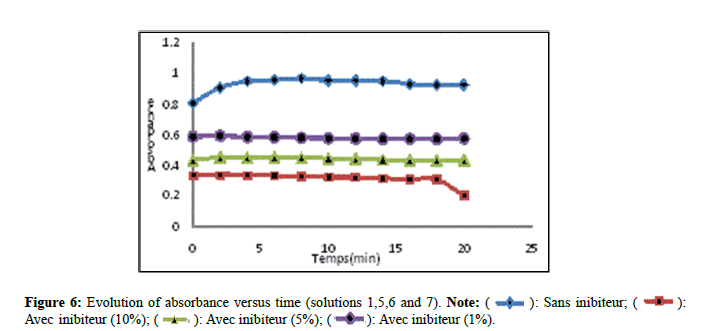
 ): Sans inibiteur; (
): Sans inibiteur; (  ):
Avec inibiteur (10%); (
):
Avec inibiteur (10%); ( ): Avec inibiteur (5%); (
): Avec inibiteur (5%); (  ): Avec inibiteur (1%).
): Avec inibiteur (1%).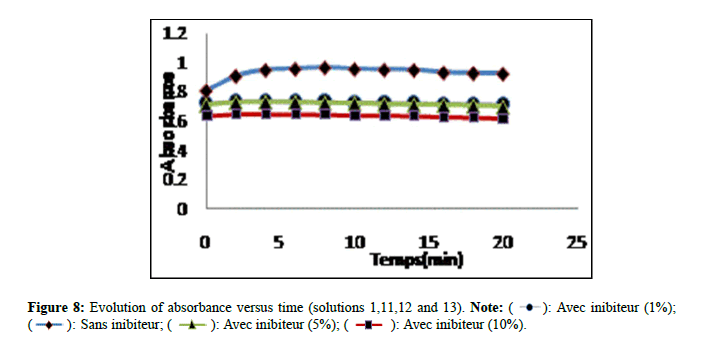
 ): Avec inibiteur (1%);
(
): Avec inibiteur (1%);
(  ): Sans inibiteur; (
): Sans inibiteur; ( ): Avec inibiteur (5%); (
): Avec inibiteur (5%); (  ): Avec inibiteur (10%).
): Avec inibiteur (10%).