ISSN : 0976-8505
Der Chemica Sinica
Solvent Based Variations in Yield of Bioactive Extracts from the Sclerotium of Pleurotus tuber-regium
Reginald C Ohiri*, Mercy O Ifeanachor, Keku Preye
Department of Biochemistry, Faculty of Science, University of Port Harcourt, Nigeria
Abstract
Background: The therapeutic effectiveness of herbs and fungi used for medicinal purposes is not only a factor of their bioactive constituents but also a factor of both the extraction solvent and extraction method.
Objective: The objective of this study is to extract and analyze the bioactive components in the sclerotum of Pleurotus tuber-regium using different solvents, as to ascertain the solvent that gives a better yield.
Method: A quantity of 10.0 kg of fresh sclerotia of P. tuber-regium purchased at Zarama Market in Southern Nigeria was washed, peeled and the white inner parts were sliced using a sterilized knife. The sliced samples were dried at room temperature for fourteen days. After grinding, the bioactive components were extracted by weighing 10 g of sample into three well stopper bottles and each was extracted in 20 mL of specific extraction solvent (methanol, hexane and dichloromethane), while that of soxleth extraction was done in a soxleth apparatus, using ethanol as the solvent. The process was repeated twice and the combined aliquot obtained were concentrated to 5.0 mL and purified. Two milliliters of the extracts were used for gas chromatographic and mass spectroscopy analysis.
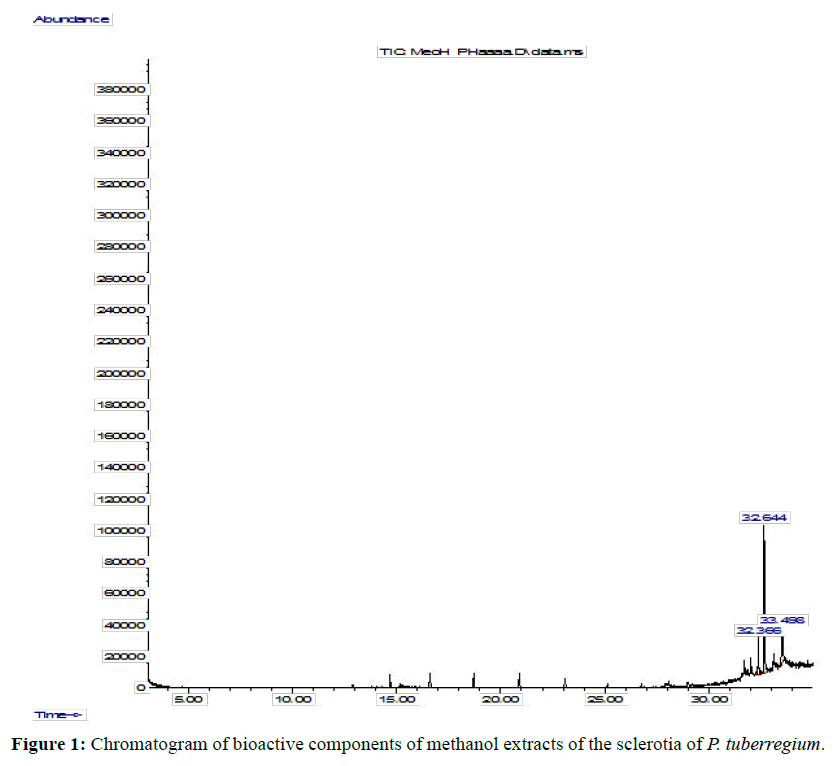
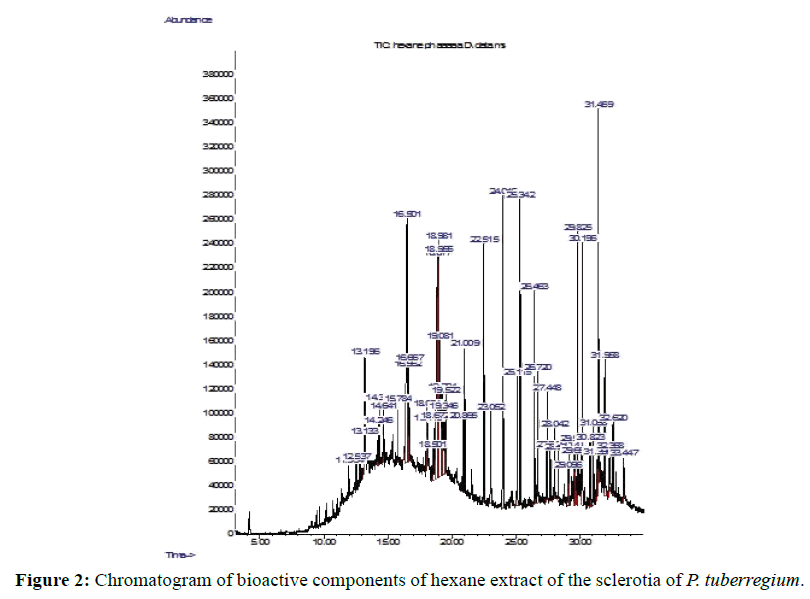
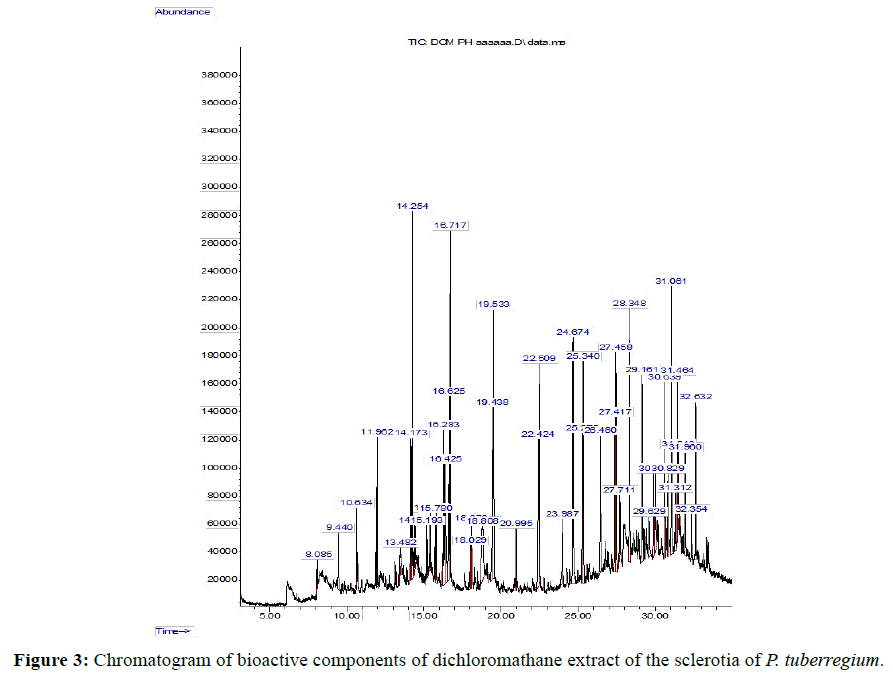
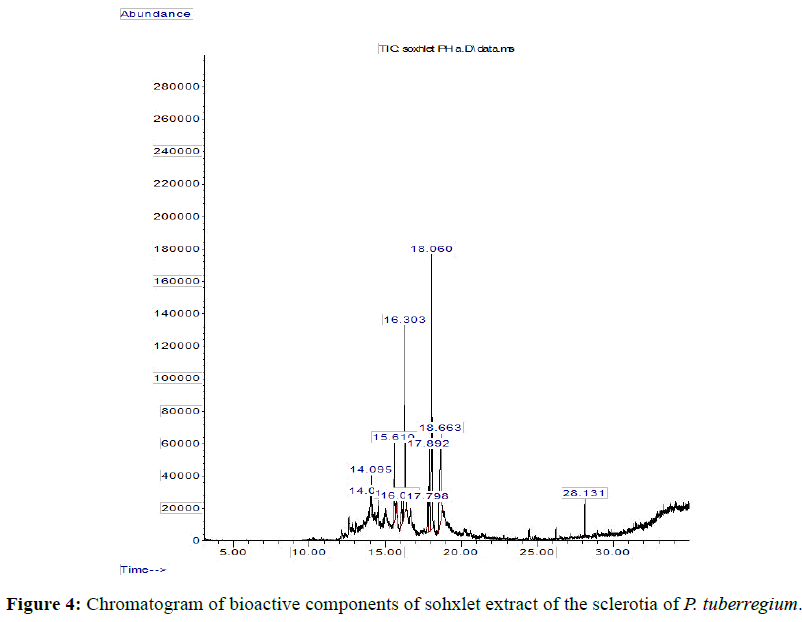
Result: The highest peak on the chromatogram for the methanol extract was observed at 32.644 min., while hexane, dichloromethane and soxhlet extracts had their highest peaks at 31.459 min., 14.254 min. and 18.060 min. respectively. The highest bioactive component in methanol extract was (3aR,4R,7R)-1,4,9,9-Tetramethyl-3, 4,5,6,7,8-hexahydro- 2H-3a,7-methane with a value of 62.856 %, while hexane, dichloromethane and soxhlet extracts had Hexasiloxane, tetradecamethyl- , Bis(2-ethylhexyl) phthalate and Phthalic acid, 3-chlorobenzyl butyl ester with values of 29.170 %, 5.092 % and 25.490 % respectively.
Conclusion: Hexane and dichloromethane extracts yielded more bioactive components with better nutriceutical and medicinal properties and may be regarded as better solvents for mushroom and fungi extractions.
Keywords
Mushroom, Bioactive components, Extraction methods, Solvents, extracts
Introduction
Different plants and fungal materials have been used in traditional medicine to solve varieties of health problems. The effectiveness of various combinations of herbs and other ingredients used by traditional medical practitioners depends on the constituents of the selected plant or fungi materials. The availability of the constituents is a factor of the adopted extraction method. The extraction of a given compound from a substrate depends on both the solubility of the compound and the polarity of the solvent. Modern extraction techniques such as accelerated solvent extraction, supercritical fluid extraction, microwave-assisted extraction and ultrasound-assisted extraction are presently explored in nutraceuticals extractions from plants. These modern techniques are mainly targeted at decreasing extraction time, while increasing yield and enhancing extract’s quality with a reduced solvent consumption. Dadi et al. [1] improve the total phenolic content, total flavonoid content, and antioxidant activity assessed from Moringa stenopetala leaves by optimizing ultrasonic-assisted extraction using response surface methodology. They recorded an extraction time of 26 min, 68 % ethanol concentration, and solvent-to-sample ratio of 42 mL/g.
Oil extraction is mainly a diffusion process in which the solvent penetrates into the lipid containing cells of a raw sample material, thereby forming a solution of the oil in the solvent. Though some of these plants and fungal materials are still very relevant in traditional medicine for treatment of diseases mainly of microbial related infections [2], most of their isolates have shown potential anti-inflammatory and antidiabetic properties in preliminary studies [3].
Pleurotus tuberregium is a notable saprotroph that produces a food storage sclerotium upon its consumption of decaying wood [4]. It is one mushroom that both its basidiocarp and sclerotium are of economic importance due to their nutritive and medicinal properties [4]. The extract of this notable fungus has been used in traditional combinations for the treatment and management of diseases ranging from skin infections, inflammation, childhood malnutrition, headache, stomach problem, cold, asthma, fever, high blood pressure, diabetes and small pox [5]. Isikhuehmen and LeBauer [6] reported the use of this mushroom and its extract in Eastern Nigeria as a thicker and flavoring agent for soups and for the treatment of heart related ailments, while it is used for the treatment and management of asthma, cough and obesity in the Southern part of Nigeria [6]. Aside Nigeria and Africa, P. tuber-regium also grows in Asia and Australia [7].
Other therapeutic activities such as antitumour, immunomodulatory, antioxidant, anti-inflammatory, hypocholesterolaemic, antihypertensive, antihyperglycaemic, antimicrobial and antiviral activities Pleurotus spp. has also been reported [8]. Though these therapeutic activities are exhibited by the extracts or isolated compounds, fermentation broth, mycelia and fruiting bodies of Pleurotus spp. [9], the biochemical mechanisms are still elusive due poor characterization and identification of the bioactive components. The aim of this present study is to extract the bioactive components in the sclerotum of P. tuber-regium using different solvents, to analyze the extracts obtained for identification of the bioactive components and to ascertain the solvent that gives a better yield.
Materials and Methods
Sample collection, preparation and extraction
A quantity of 10.0 kg of fresh sclerotia of Pleurotus tuberregium purchased at Zarama Market in Southern Nigeria was washed, peeled and the white inner parts were sliced using a sterilized knife. The sliced samples were allowed to dry at room temperature in a dust free environment for a period of fourteen days. The samples were ground in a warring blender into a fine powder. Using an analytical weighing balance, 10 g of the ground sample was weighed into three well stopper bottles. A volume of 20 mL of a specific extraction solvent (methanol, hexane and dichloromethane) was added to each stopper bottle. The mixtures were vigorously agitated and were left to stand for 5 days, while that of soxleth extraction was done in a soxleth apparatus, using ethanol as the solvent. The crude extract from each process was collected by filtering into a quartz beaker in a fume hood. The process was repeated twice and the combined aliquot collected from each extraction solvent were separately concentrated on a steam berth to 5.0 mL and purified by passing through a pasture pipette packed with silica gel and anhydrous sodium sulphate. The purified samples were air dried to 2.0 mL for gas chromatographic analysis.
GC-MS analysis of extracts
The extracts were analysed using a combined gas chromatograph model HP 6890 and mass spectrometer model 5973 (Agilent Tech.) fitted with a capillary column HP-5 MS (5% phenylmethylsiloxane) 30.0 m × 250 μm × 0.25 μm, using Helium as a carrier gas at initial column temperature 120°C for 5 minutes. Thereafter, the column temperature was increased at 5°C per minutes to 320°C and held for 5 minutes. Electron impact ionization for mass spectroscopy was done at ionization energy of 70 eV. The oil was diluted with 98% hexane and 2 μL of the diluted sample was automatically injected into Agilent Tech. model 5973 mass spectrometer. The constituent compounds were identified using the Chem-Office software attached tothe MS library. The names and structures of the component oils were confirmed using the database of National Institute of Standard and Technology (NIST).
Results
The Chromatogram of bioactive components of methanol, hexane, dichloromethane and soxhlet extracts of the sclerotia of P. tuberregium are shown in Figures 1-4, respectively. The highest peak for the methanol extract was observe at 32.644 min. Hexane extract has its highest peak at 31.459 min., while dichloromethane and soxhlet extracts have their highest peaks at 14.254 min. and 18.060 min. respectively.
The retention time, percentage concentration, molecular formula, molecular weight and structures of the bioactive components of methanol, hexane, dichloromethane and soxhlet extracts of the sclerotia of P. tuberregium are shown in Tables 1-4. (3aR, 4R, 7R)-1,4,9,9-Tetramethyl-3, 4,5,6,7,8-hexahydro-2H-3a,7-methane was the highest bioactive component in the methanol extract with a value of 62.856 % followed by Urs-12-en-28-oic acid, 3-hydroxy-,methyl ester, (3.beta.)- With a value of 23.151 %. Hexane extract has it highest bioactive component as Hexasiloxane, tetradecamethyl- with a value of 29.170 % followed by Hexacosane, 13-dodecyl- with a value of 28.339 %, while Bis(2-ethylhexyl) phthalate and Eicosane were the highest bioactive components observed in dichloromethane extract with values of 5.092% and 4.721% respectively. The highest bioactive component observed in the sohxlet extract was Phthalic acid, 3-chlorobenzyl butyl ester with a value of 25.490 % followed by 1,4-Naphthalenedione, 5,8-dihydroxy- 2,7-dimethoxy- with a value of 23.513 %.
| S/N | Compound | Retention Time (min) | Percentage of the total | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|---|---|
| 1 | 2H-Cyclopropa[a]naphthalen-2-one | 32.366 | 13.992 | C15H24 | 204.3511 | 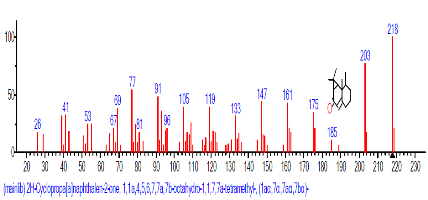 |
| 2 | (3aR,4R,7R)-1,4,9,9-Tetramethyl-3, 4,5,6,7,8-hexahydro-2H-3a,7-methane |
32.644 | 62.856 | C8H15N3 | 153.22 | 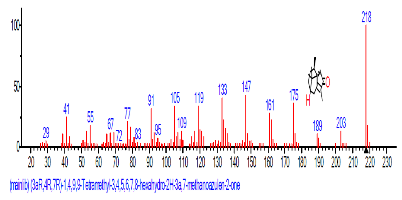 |
| 3 | Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3.beta.)- |
33.498 | 23.151 | C30H48O3 | 456.700 |  |
Table 1: Bioactive components of methanol extract of sclerotia of P. tuberregium.
| S/N | Compound | Retention Time (min) | Percentage of the total | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|---|---|
| 1 | Cyclododecane | 11.964 | 0.300 | C12H2 | 168.3190 | 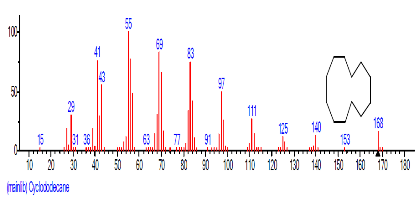 |
| 2 | Nonane, 2-methyl- | 12.537 | 0.401 | C10H22 | 142.2817 | 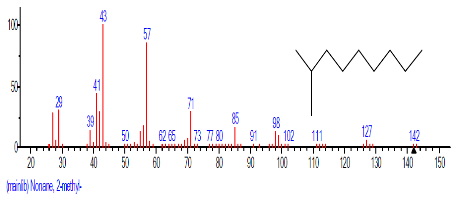 |
| 3 | Undecane | 13.133 | 0.363 | C11H24 | 156.3083 |  |
| 4 | Dodecane, 2,6,10-trimethyl- | 13.196 | 1.234 | C15H32 | 212.4146 |  |
| 5 | Octadecane, 1-chloro- | 14.246 | 0.435 | C18H37Cl | 288.939 |  |
| 6 | Tetratetracontane | 14.351 | 0.764 | C44H90 | 619.1854 |  |
| 7 | 1,1,1,5,7,7,7-Heptamethyl-3,3-bis(trimet hylsiloxy)tetrasiloxane | 14.641 | 0.549 | C13H40O5Si6 | 444.967 | 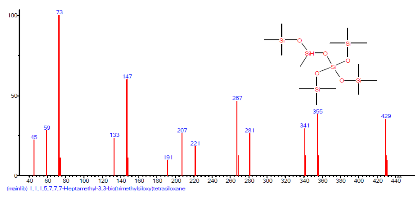 |
| 8 | Hexadecanoic acid, methyl ester | 15.784 | 0.688 | C17H34O2 | 270.4507 | 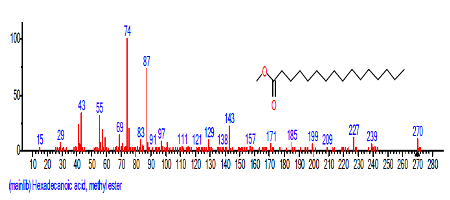 |
| 9 | n-Hexadecanoic acid | 16.501 | 7.223 | C16H32O2 | 256.4241 | 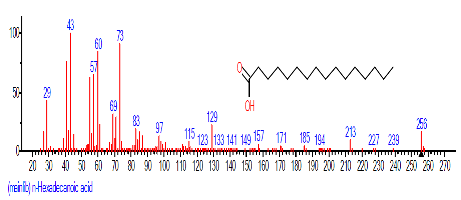 |
| 10 | Cycloheptasiloxane, tetradeca methyl- | 16.562 | 1.909 | C14H42O7Si7 | 519.0776 | 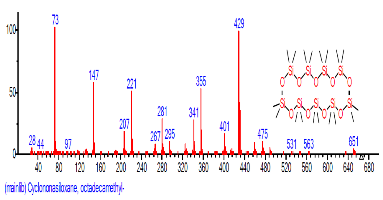 |
| 11 | Hexadecanoic acid, ethyl ester | 16.667 | 1.913 | C18H36O2 | 284.4772 | 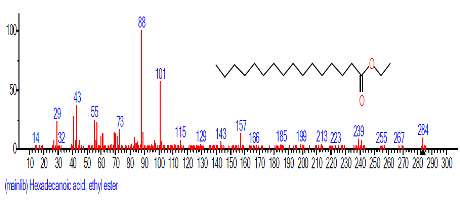 |
| 12 | 9,12-Octadecadienoic acid,methyl ester, (E,E)- | 18.031 | 0.765 | C19H34O2 | 294.4721 | 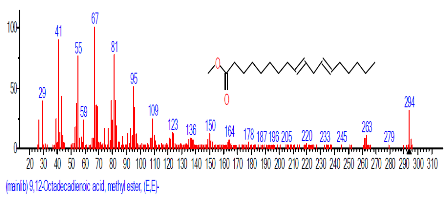 |
| 13 | 9-Octadecenoic acid, methyl ester (E)- | 18.116 | 0.643 | C19H36O2 | 296.4879 | 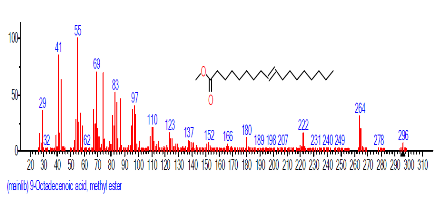 |
| 14 | Methyl stearate | 18.501 | 0.600 | C19H38O2 | 298.511 | 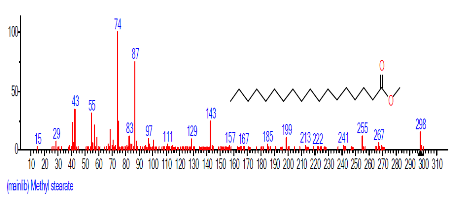 |
| 15 | Heptasiloxane, hexadeca methyl- | 18.672 | 1.149 | C16H48O6Si7 | 533.1472 | 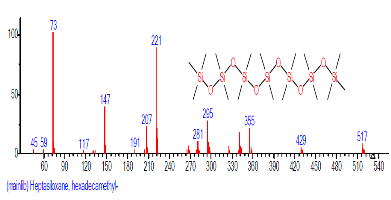 |
| 16 | Linoelaidic acid | 18.877 | 8.730 | C18H32O2 | 280.4455 | 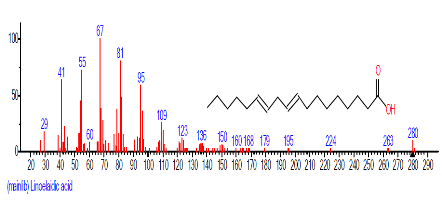 |
| 17 | 9,17-Octadecadienal, (Z)- | 18.955 | 3.233 | C18H32O | 264.4461 | 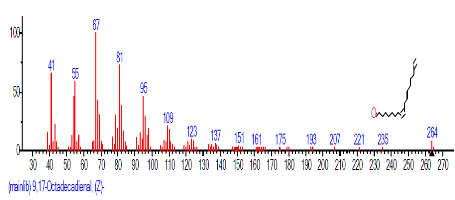 |
| 18 | Linoleic acid ethyl ester | 18.981 | 4.337 | C20H36O2 | 308.4986 | 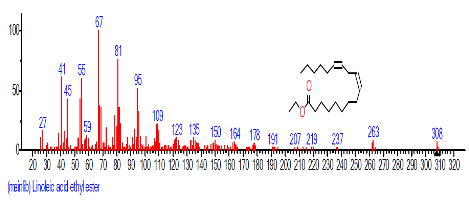 |
| 19 | 9,12-Octadecadienoic acid (Z,Z)- | 19.081 | 3.668 | C18H32O2 | 280.4455 | 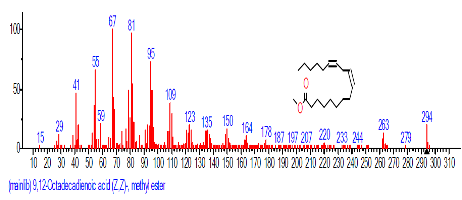 |
| 20 | Octadecanoic acid | 19.234 | 1.839 | C18H36O2 | 284.4772 | 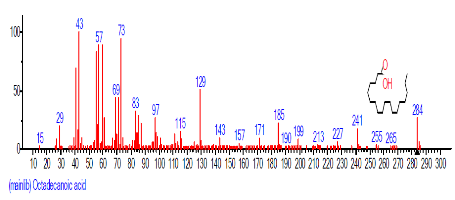 |
| 21 | Hexadecanoic acid, 2-methyl propyl ester | 19.346 | 1.622 | C20H40O2 | 312.5304 | 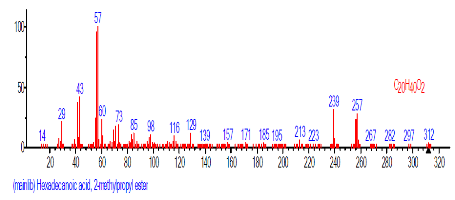 |
| 22 | Cycloeicosane | 19.522 | 2.099 | C20H40 | 280.5316 | 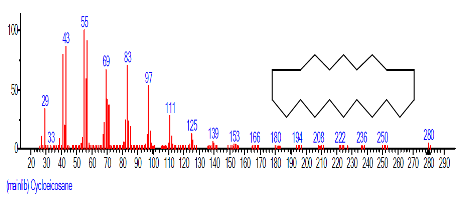 |
| 23 | Cyclononasiloxane, octadeca methyl- | 20.865 | 1.227 | C18H54O9Si9 | 667.3855 |  |
| 24 | Tricosane | 21.009 | 2.206 | C23H48 | 324.6272 |  |
| 25 | Tetracosane | 22.515 | 4.205 | C24H50 | 338.6538 |  |
| 26 | 3-Isopropoxy-1,1,1,5,7,7,7-Heptamethyl-3,3-bis(trimet hylsiloxy)tetrasiloxane | 23.052 | 1.645 | C18H52O7Si7 | 577.200 |  |
| 27 | Pentacosane | 24.016 | 5.347 | C25H52 | 352.6804 |  |
| 28 | 2,5-Dihydroxy benzoic acid, 3TMS derivative | 25.115 | 2.085 | C16H30O4Si3 | 370.6635 |  |
| 29 | Hexacosane | 25.342 | 4.261 | C26H54 | 366.7070 |  |
| 30 | Heptacosane | 26.463 | 2.728 | C27H56 | 380.7335 |  |
| 31 | Cyclononasiloxane, octadeca methyl- | 26.720 | 2.297 | C18H54O9Si9 | 667.3855 | 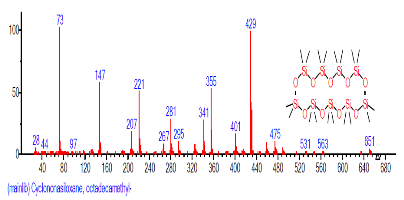 |
| 32 | Docosane, 7-hexyl- | 27.448 | 1.261 | C28H58 | 394.7601 | 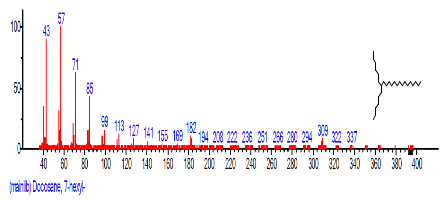 |
| 33 | 2,6,10-Trimethyltridecane | 27.700 | 1.004 | C16H26 | 218.3776 | 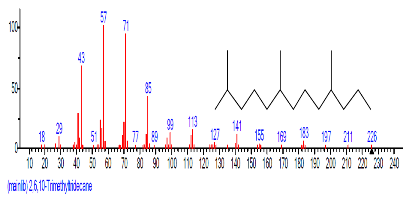 |
| 34 | Cyclononasiloxane, octadeca methyl- | 28.042 | 0.904 | C18H54O9Si9 | 667.3855 | 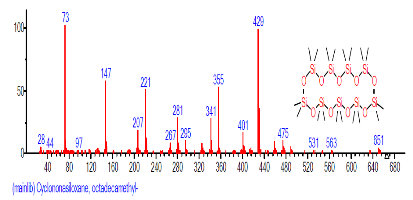 |
| 35 | Hexacosane, 13-dodecyl- | 0.737 | 28.339 | C38H78 | 535.0259 | 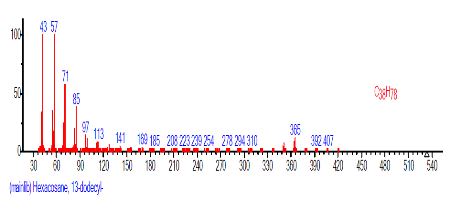 |
| 36 | Ergost-5-en-3-ol, acetate, (3.beta.,24R)- | 29.096 | 0.718 | C30H50O2 | 442.7168 | 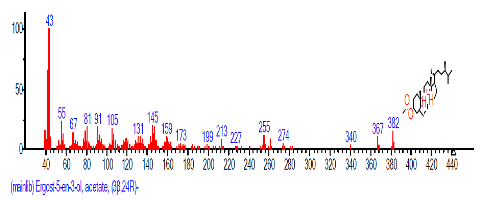 |
| 37 | Hexasiloxane, tetradecamethyl- | 1.095 | 29.170 | C14H42O5Si6 | 458.9933 | 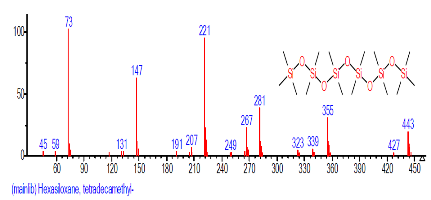 |
| 38 | (R)-6-Methoxy-2,8-dimethyl-2-((4R, 8R)-4,8, 12-trimethyltri decyl)chroman | 29.594 | 1.316 | C28H48O2 | 416.6795 | 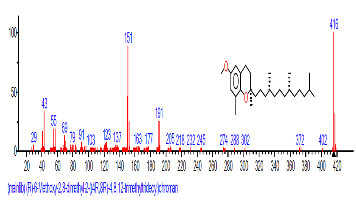 |
| 39 | Ergosta-4,7,22- trien-3-one | 29.652 | 0.885 | C28H42O | 394.633 | 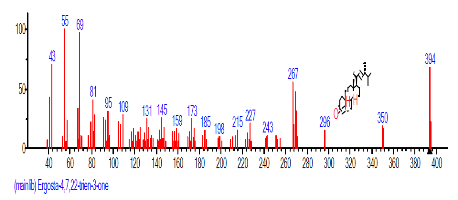 |
| 40 | Stigmastan-3,5-diene | 29.825 | 3.742 | C29H48 | 396.6914 | 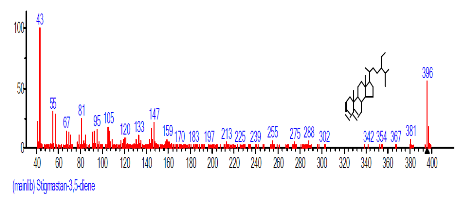 |
| 41 | Vitamin E | 30.196 | 4.094 | C29H50O2 | 430.71 | 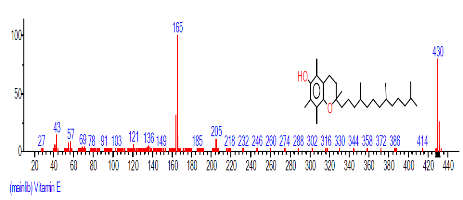 |
| 42 | Ergost-5-en-3-ol, (3.beta.)- | 30.823 | 1.070 | C28H48O | 400.6801 | 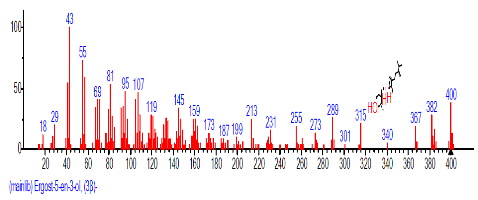 |
| 43 | Stigmasterol | 31.058 | 1.514 | C29H48O | 412.6908 | 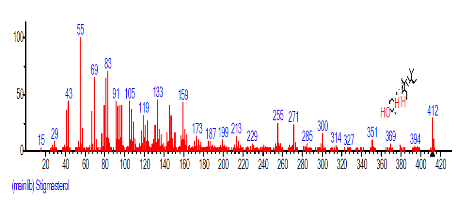 |
| 44 | (1-Cyclohexyl methyl-3-methylbut-2- enylthio) benzene | 31.396 | 0.374 | C13H18 | 174.282 | 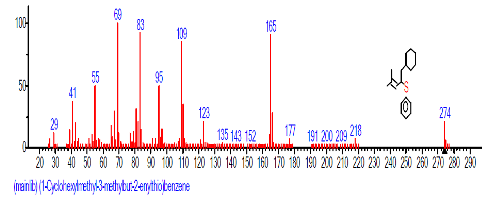 |
| 45 | gamma.-Sitosterol | 31.459 | 5.677 | C29H50O | 414.7067 | 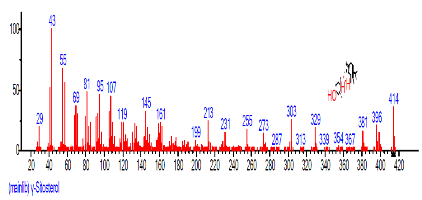 |
| 46 | 2(1H)Naphthalenone, 3,5,6,7, 8,8a-hexa hydro-4,8a-dimethyl-6-(1-methyl ethenyl)- | 31.958 | 2.504 | C15H22O | 218.3346 | 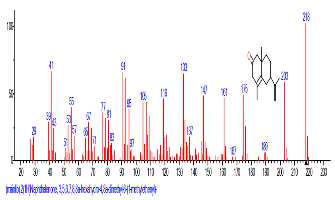 |
| 47 | 3-(2-Thienyl)-4,5,dihydro-5-isoxazo lemethanol | 35.955 | 0.295 | C8H9NO2S | 183.2276 | 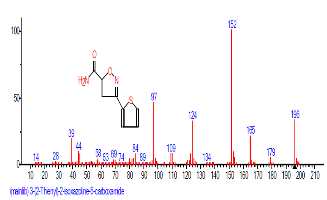 |
| 48 | (3aR,4R,7R)-1,4,9,9-Tetra methyl-3, 4,5,6, 7,8-hexahydro -2H-3a,7-meth anoazulen-2-one | 32.620 | 1.010 | C8H15N3 | 153.22 | 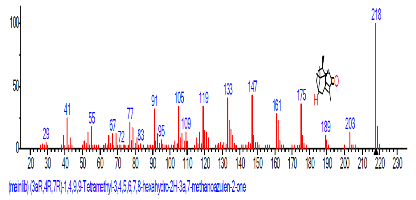 |
| 49 | Tert-Butyl dimethylsilyl 2,3-dimethyl | 33.447 | 0.675 | C14H22O2Si | 250.4088 | 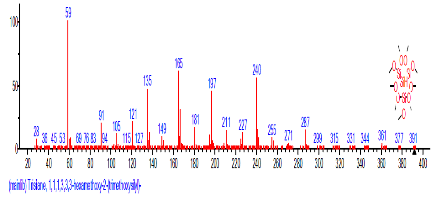 |
Table 2: Bioactive components of hexane extract of sclerotia of P. tuberregium
| S/N | Compound | Retention Time (min) | Percentage of the total | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|---|---|
| 1. | Carbonic acid, hexadecyl prop-1-en -2-yl ester | 8.085 | 0.173 | C9H10O3 | 166.1739 | 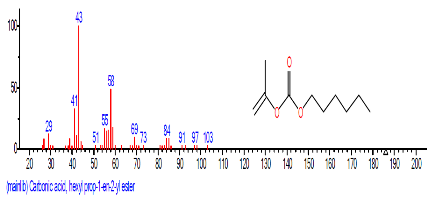 |
| 2. | Carbonic acid, nonyl vinyl ester | 9.440 | 0.792 | C12H22O3 | 214.3013 | 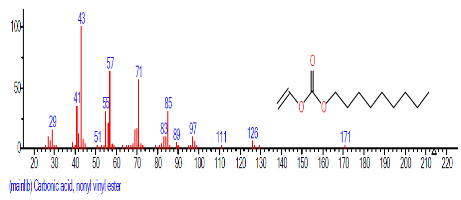 |
| 3. | 5-Acetoxy methyl-2-furaldehyde | 10.634 | 0.982 | C8H8O4 | 168.1467 | 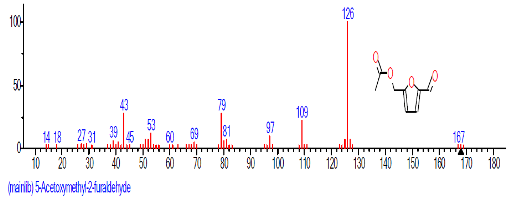 |
| 4. | Hexadecane | 11.962 | 1.672 | C16H34 | 226.4412 | 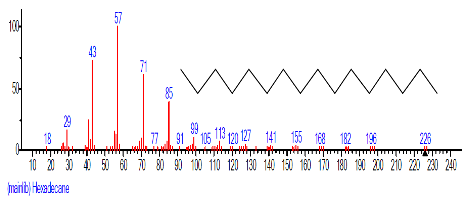 |
| 5. | [1,2,3,4]Tetrazolo[1,5-b][1,2, 4]triazine, 5,6, 7,8-tetrahydro- | 13.482 | 0.663 | C3H6N6 | 126.12 | 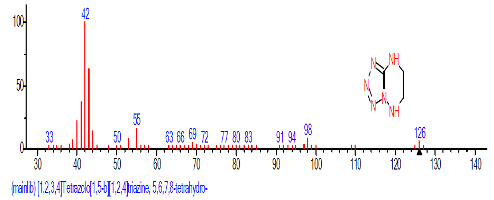 |
| 6. | 1-Nonadecene | 14.173 | 1.811 | C19H38 | 266.5050 | 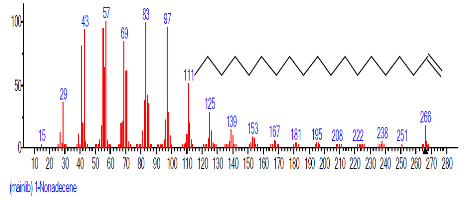 |
| 7. | Octadecane | 14.254 | 4.162 | C18H38 | 254.4943 | 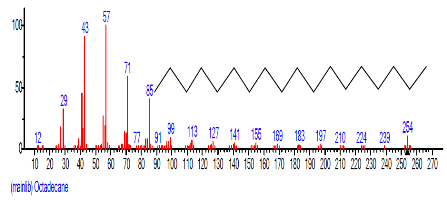 |
| 8. | Bicyclo[6.1.0]non-1-ene | 14.431 | 0.793 | C9H14 | 122.211 | 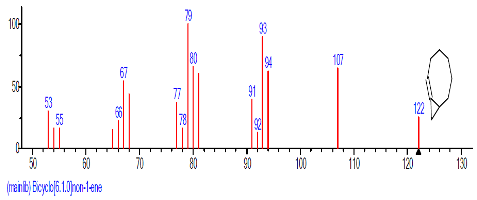 |
| 9. | 1-Methylene-2-vinylcyclo pentane | 15.193 | 0.875 | C8H12 | 108.181 | 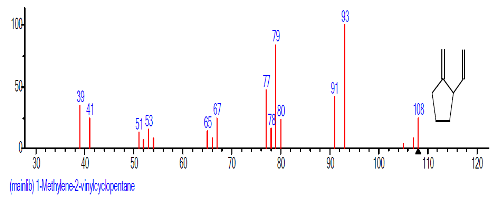 |
| 10. | Nonadecane | 15.423 | 0.774 | C19H40 | 268.5209 | 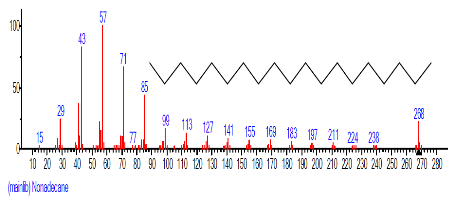 |
| 11. | Hexadecanoic acid, methyl ester | 15.780 | 0.950 | C17H34O2 | 270.4507 | 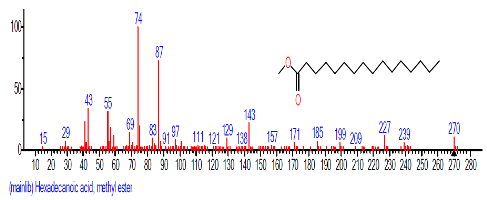 |
| 12. | Dibutyl phthalate | 16.283 | 3.100 | C16H22O4 | 278.3435 | 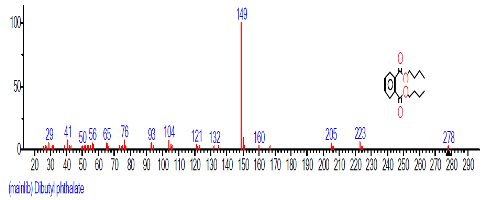 |
| 13. | n-Hexadecanoic acid | 16.425 | 3.167 | C16H32O2 | 256.4241 | 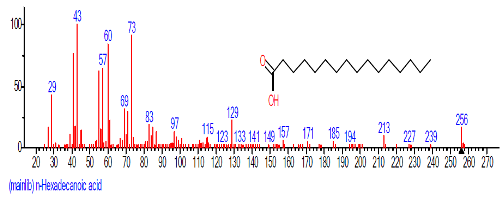 |
| 14. | 1-Docosene | 16.625 | 3.364 | C22H44 | 308.5848 | 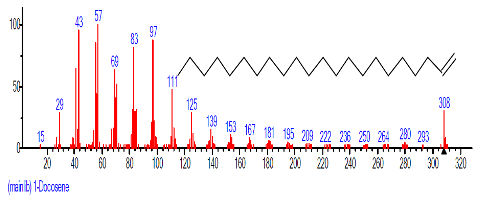 |
| 15. | Eicosane | 16.717 | 4.721 | C20H42 | 282.5475 | 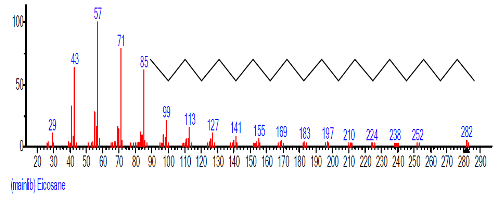 |
| 16 | 9,12-Octadeca dienoic acid (Z,Z)-, methyl ester | 18.029 | 0.689 | C19H34O2 | 294.4721 | 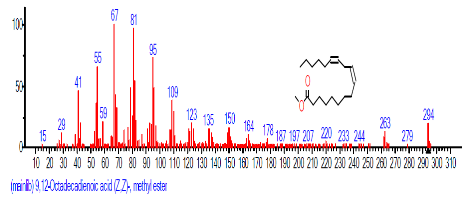 |
| 17 | Heneicosane | 18.070 | 1.175 | C21H44 | 296.5741 | 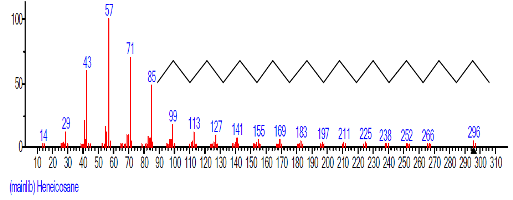 |
| 18 | 9,12-Octadeca dienoic acid (Z,Z)- | 18.808 | 2.689 | C18H32O2 | 280.4455 | 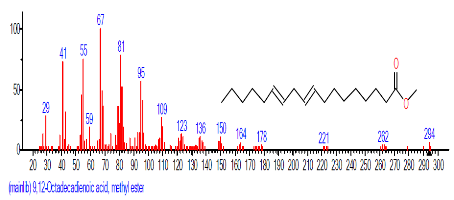 |
| 19 | 1-Docosene | 19.438 | 3.145 | C22H44 | 308.5848 | 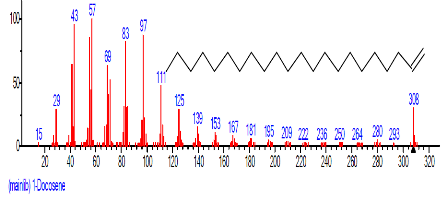 |
| 20 | Docosane | 19.533 | 4.304 | C22H46 | 310.6006 | 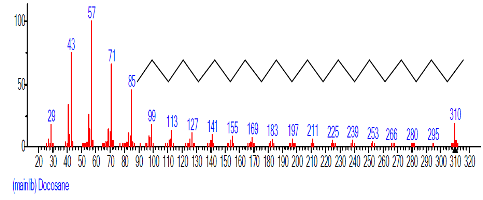 |
| 21 | Methoxyacetic acid, 2-tridecyl est er | 20.995 | 1.043 | C16H32O3 | 272.429 | 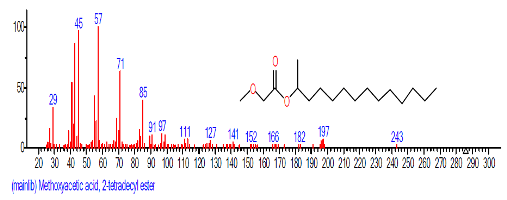 |
| 22 | Heptadecyl trifluoroacetate | 22.424 | 3.008 | C19H35F3O2 | 352.4752 | 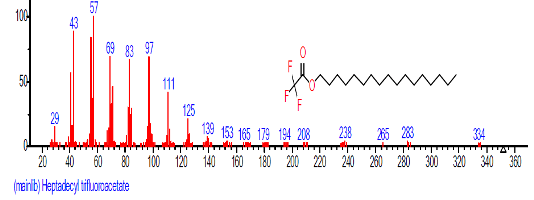 |
| 23 | Tricosane, 2-methyl- | 22.509 | 3.745 | C24H50 | 338.6538 | 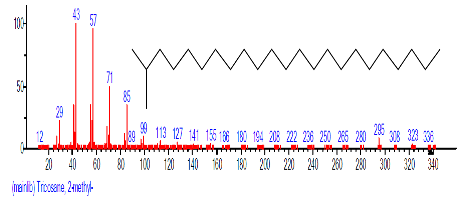 |
| 24 | Pentacosane | 23.987 | 1.344 | C25H52 | 352.6804 | 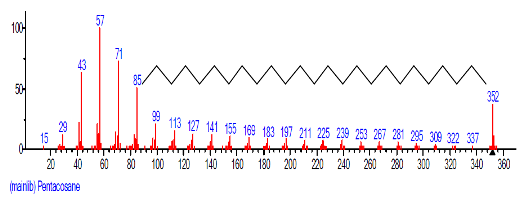 |
| 25 | Bis(2-ethylhexyl) phthalate | 24.674 | 5.092 | C24H38O4 | 390.5561 | 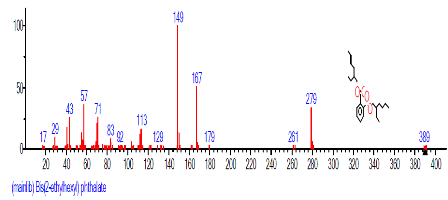 |
| 26 | Trichloroacetic acid, pentade cyl ester | 25.275 | 2.827 | C17H31Cl3O2 | 373.786 | 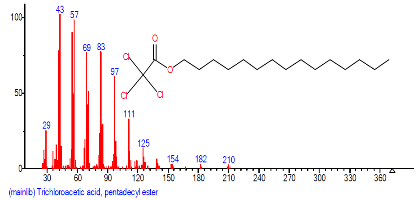 |
| 27 | Heptadecane, 2-methyl- | 25.340 | 3.866 | C18H38 | 254.4943 | 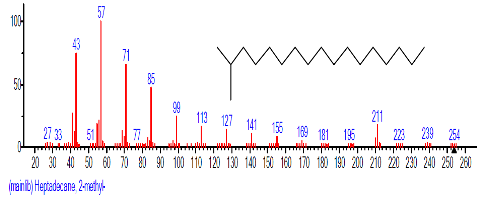 |
| 28 | Heptacosane | 26.460 | 2.475 | C27H56 | 380.7335 | 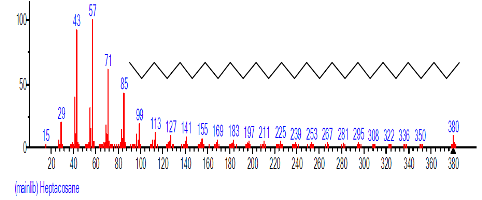 |
| 29 | 17-Pentatria contene | 27.417 | 2.249 | C35H72 | 492.9462 | 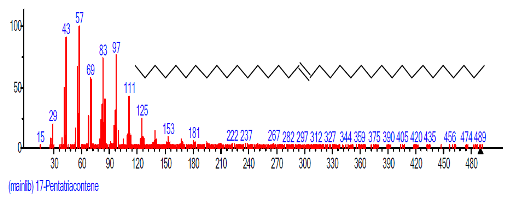 |
| 30 | Octacosane | 27.458 | 3.463 | C28H58 | 394.7601 |  |
| 31 | Squalene | 27.711 | 0.905 | C30H50 | 410.718 |  |
| 32 | Tricosane | 28.348 | 4.378 | C24H50 | 338.6538 |  |
| 33 | Docosane, 9-octyl- | 29.161 | 4.520 | C30H62 | 422.8133 |  |
| 34 | Stigmastan-6, 22-dien, 3,5-de dihydro- | 29.629 | 0.770 | C28H46O | 398.68 | 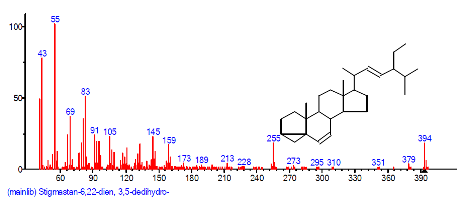 |
| 35 | 1-Octadecane sulphonyl chloride | 29.923 | 1.161 | C18H37ClO2S | 353.003 | 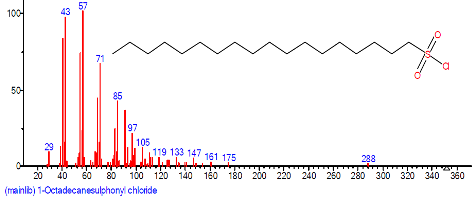 |
| 36 | 26-Nor-5-chole sten-3.beta. -ol-25-one | 30.017 | 1.215 | C29H48O2 | 428.6902 | 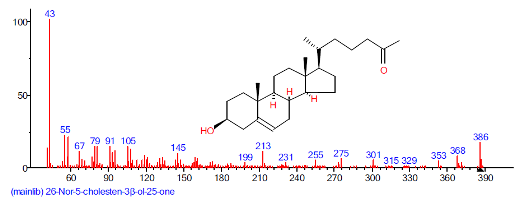 |
| 37 | Tetracontane, 3,5,24-trimethyl- | 30.639 | 2.995 | C43H88 | 605.1770 | 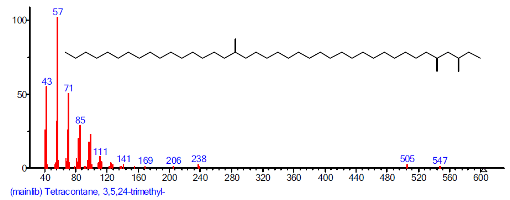 |
| 38 | Ergost-5-en-3-ol, (3.beta.)- | 30.829 | 1.637 | C28H48O | 400.691 | 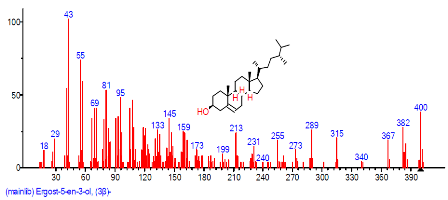 |
| 39 | Stigmasterol | 31.061 | 4.407 | C29H48O | 412.6908 | 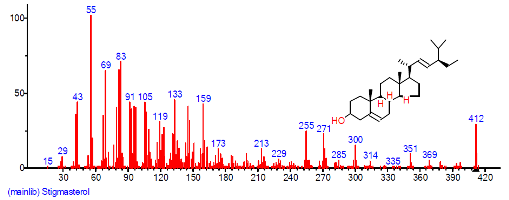 |
| 40 | Octacosane | 31.312 | 0.834 | C28H58 | 394.7601 | 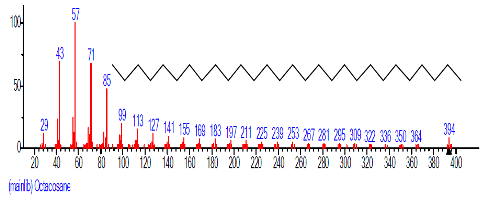 |
| 41 | beta.-Sitosterol | 31.464 | 2.247 | C29H50O | 414.7067 | 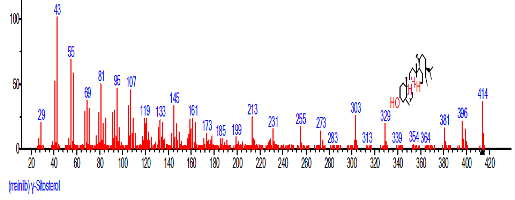 |
| 42 | 1H-1,2,3-Triazole-4-carboxylic acid, 5-amino-1-(phenylmethyl)-, hydrazide | 31.516 | 1.001 | C11H11N3O2 | 217.22 | 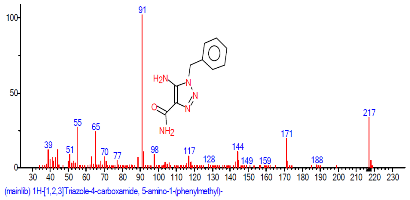 |
| 43 | Octacosane | 31.960 | 1.864 | C28H58 | 394.7601 | 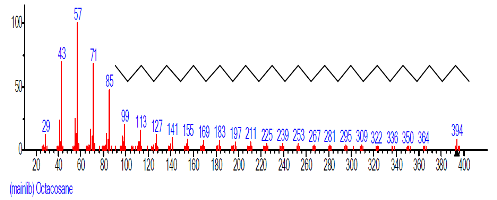 |
| 44 | 2H-Cyclopropa[a]naphthalen-2-one,1,1a,4,5,6, 7,7a,7b-octa hydro-1,1,7,7a-tetramethyl-, (1a.alpha.,7. alpha.,7a.alpha.,7b.alpha.)- | 32.354 | 0.601 | C15H24 | 204.3511 | 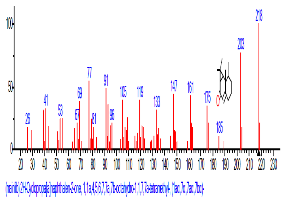 |
| 45 | Urs-12-en-3-ol, acetate, (3.beta.) | 32.632 | 2.351 | C32H52O2 | 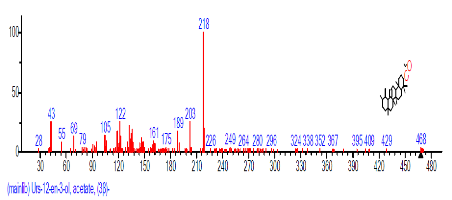 |
Table 3: Bioactive components of dichloromethane extract of sclerotia of P. tuberregium
| S/N | Compound | Retention Time (min) | Percentage of the total | Molecular formula | Molecular weight | Structure |
|---|---|---|---|---|---|---|
| 1 | Tricyclo[2.2.1.0(2,6)]heptan-3-one, oxime | 14.025 | 2.331 | C7H10 | 94.1543 | 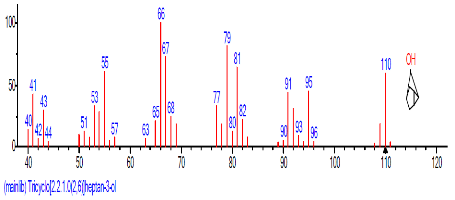 |
| 2 | Undec-10-ynoic acid, but-3-yn-2-yl ester | 14.095 | 5.056 | C11H18O2 | 182.2594 | 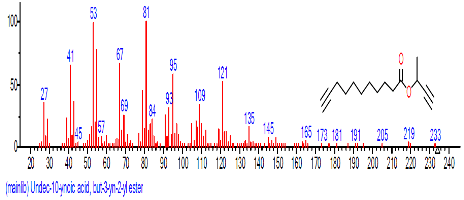 |
| 3 | 2,5-Cyclohexa diene-1,4-dione, 2,5-dihydroxy-3-methyl-6-(1-methylethyl)- | 15.610 | 6.192 | C10H12O2 | 164.2000 | 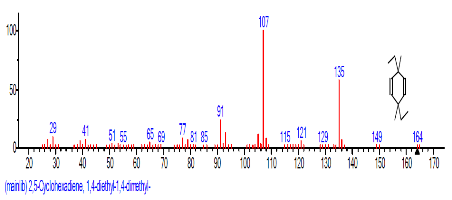 |
| 4 | 3-Cyclopropen oic acid,1butyl, methyl ester | 15.727 | 0.993 | C17H24O4 | 292.3701 | 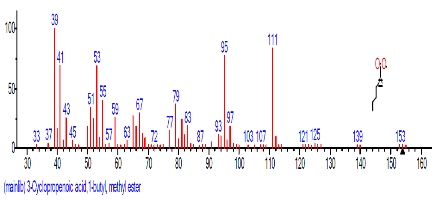 |
| 5 | 4-Pyridinol | 16.096 | 3.539 | C5H5NO | 95.1000 | 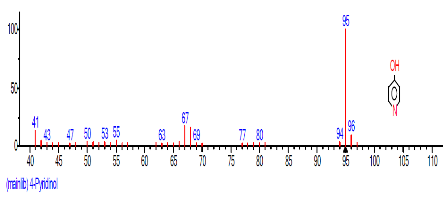 |
| 6 | Phthalic acid, 3-chlorobenzyl butyl ester | 16.303 | 25.490 | 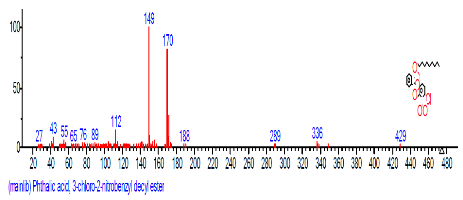 |
||
| 7 | Hexadecanoic acid, methyl ester | 17.798 | 2.602 | C17H34O2 | 270.4507 | 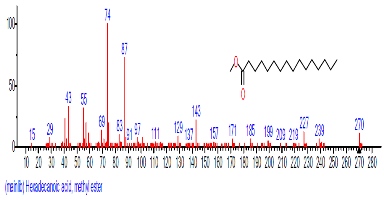 |
| 8 | 2-Methyl-3-(phenylsulfonyl)methyl-2-cyclopenten-1-one | 17.892 | 8.177 | C6H8sO | 96.1290 | 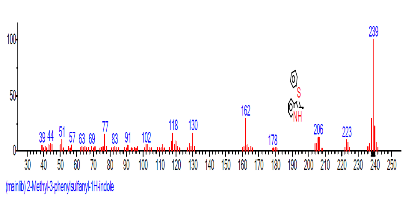 |
| 9 | 1,4-Naphtha lenedione, 5,8-dihydroxy-2,7-dimethoxy- | 18.060 | 23.513 | C10H6O4 | 190.15 | 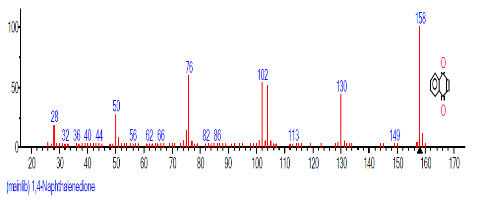 |
| 10 | 2-Cyclopenten-1-one, 3-methyl- | 18.663 | 19.395 | CH3C5H5O | 96.1300 | 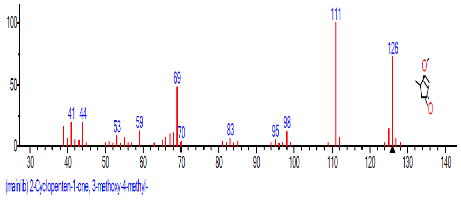 |
| 11 | n-Hexadecanoic acid | 28.131 | 2.713 | C16H32O2 | 256.4241 | 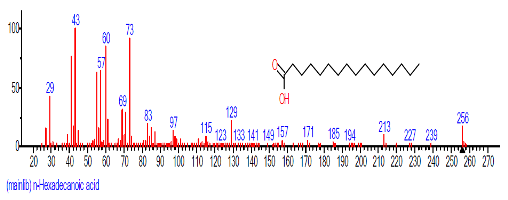 |
Table 4: Bioactive components of soxhlet extract of sclerotia of P. tuberregium.
Discussion
The disparity in both compound quantity and concentration observed in the methanol, hexane, dichloromethane and soxhlet extracts of the sclerotia of P. tuberregium shows varying capabilities of different solvents to dissolve and liberate different compounds from a substrate. The ability of methanol to extract only three uncommon polycyclic compounds (2H-Cyclopropa[a]naphthalen-2-one, (3aR,4R,7R)-1,4,9,9-Tetramethyl-3,4,5,6,7,8-hexahydro-2H-3a,7- methane and Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3.beta.)-) from the sclerotia of this mushroom. Table 1 indicates that the use of methanol as an extraction solvent may favour the extraction of polycyclic compounds especially in a lignin based substrate like mushroom. As a very polar compound, the yield and concentration of extracts in methanol extraction may be lower than in a non-polar solvent. This indicates that methanol can dissolve a larger proportion of polar compounds. However, its solubility may reduce when used as solvent for the extraction nonpolar compounds. The result of this study is an indication that methanol may not be a solvent of choice for extraction where both polar and non-polar compounds are needed from a substrate.
Although different solvents has been used for extraction, hexane has been considered ideal for extraction processes because of its non-polar properties, low boiling point (67°C), and its high solubility of lipid compounds. Moreover, Clough and Mulholland [10] also reported that the low reactivity exhibited by hexane also makes it a suitable solvent for the extraction of reactive compounds. The hexane extract of the sclerotia of this mushroom shows the presence of 49 different bioactive components made up of linear, branched, monocyclic and polycyclic compounds (Table 2). The large number of compounds observed in the hexane extract shows the ability of hexane to penetrate, solubilize and release these compounds from the substrate.
The 45 components observed from the dichloromethane extract (Table 4) shows that the volatility and ability of dichloromethane to dissolve a wide range of organic compounds makes it a useful solvent for many chemical processes [11]. The presence of both linear and cyclic compounds in the extract is also an indication that dichloromethane has the potentials to penetrate and extract most constituent compounds from a lignin based sample. Though dichloromethane is the least toxic amongst the simple chlorohydrocarbons it low boiling point also makes it a choice solvent for extraction [11]. As an extraction process, soxhlet extraction is mainly used in the extraction of compounds that are either insoluble or sparingly soluble in water. Though soxhlet extraction is a discontinuous extraction process, the repeated application of hot solvent (ethanol in this case) to the mushroom sample allows for a continuous penetration of the solvent into the sample. However, the yield of the extraction process is dependent on the extraction solvent. This may be responsible for the low yield (11 compounds) observed in the soxhlet extract of the sclerotia of this mushroom.
Conclusion
Hexane and dichloromethane extracts yielded more bioactive components with better nutriceutical and medicinal properties than methanol and soxhlet extracts. Sequel to these findings, hexane and dichloromethane are better solvents for extraction from a lignin based substrate such as mushrooms and other fungi.
References
- Dadi DW, Emire SA, Hagos AD, Eun JB (2018) Improvement of yield of bioactive compounds and antioxidant activity of moringa stenopetala leaves by optimizing ultrasonic-assisted extraction res & rev. J Food Sci Technol 7: 1-11.
- Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME (2008) The immunobiology of mushrooms. Exp Biol Med (Maywood) 233: 259-276.
- Lull C, Wichers HJ, Savelkoul HF (2005) Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm 2: 63-80.
- Ohiri RC, Amadi BA, Isoje FE (2018) Phytochemical confinement in sections of pleurotus tuber-regium (king tuber mushroom) basidocarp. Am J Biomed Sci 10: 195-201.
- Chen AW, Huang N (2004) Production of tuber-like sclerotia of medicinal value by Pleurotus tuberregium (Fr.) Sing (Agaricomycetideae). Int J Med Mushrooms 5: 313-319.
- Isikhuemhen SO, LeBauer DS (2004) Growing Pleurotus tuberregium. Mushworld Pub 1: 264-274.
- Oso BA (1997) Pleurotus tuber-regium from Nigeria. Mycologia 69: 271-279
- Gregori A, Svagelj M, Pohleven J (2007) Cultivation techniques and medicinal properties of pleurotus spp. Food Tech Biotech 45: 238- 249.
- Patel Y, Naraian R, Singh VK (2012) Medicinal properties of pleurotus species (oyster mushroom) a review. World J Fungal Plant Biol 3: 1-12.
- Clough SR, Mulholland L (2005) "Hexane". Encyclopedia of toxicology 2 (2nd edn). Elsevier, USA.
- Tlili A, Schranck J (2017) The application of dichloromethane and chloroform as reagents in organic synthesis; in: solvents as reagents in organic synthesis: reactions and applications. Wiley, USA.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences