ISSN : 2347-5447
British Biomedical Bulletin
Nature's Apothecary: Unveiling the Healing Power of Biomimicry in Modern Medicine
O.K. Tharushi Nethmini Silva*
Department of Technology, University of Sri Jayewardhenepura, Nugegoda, Sri Lanka
- *Corresponding Author:
- O.K. Tharushi Nethmini Silva
- Department of Technology, University of Sri Jayewardhenepura, Nugegoda, Sri Lanka
- E-mail: tharushinethmini1217@gmail.com
Received date: July 16, 2024, Manuscript No. IPBBB-24-19379; Editor assigned date: July 18, 2024, PreQC No. IPBBB-24-19379 (PQ); Reviewed date: August 01, 2024, QC No. IPBBB-24-19379; Revised date: April 07, 2025, Manuscript No. IPBBB-24-19379 (R); Published date: April 15, 2025, DOI: 10.36648/2347-5447.13.2.89
Citation: Silva OKTN (2025) Nature's Apothecary: Unveiling the Healing Power of Biomimicry in Modern Medicine. Br Biomed Bull Vol.13 No.2
Abstract
For millennia, nature has served as a silent healer, offering a vast repository of solutions for human ailments. Modern medicine, however, is now actively unlocking this potential through the lens of biomimicry. This review delves into the exciting world of biomimetic medicine, exploring how scientists are drawing inspiration from nature's ingenious designs to revolutionize medical treatments. We examine how biomimicry is shaping advancements in areas like drug delivery, tissue engineering and surgical tools, all to mimic nature's remarkable healing power. By unveiling the secrets hidden within nature's apothecary, biomimicry promises to usher in a new era of healthcare, offering us innovative solutions for a healthier future.
Keywords
Biomimicry; Nature’s inspiration; Drug delivery system; Tissue engineering; Biocompatible materials; Surgical tools; Regenerative medicine; Biomedical engineering; Biomedical ethics; SDG 3: Good health and well-being
Introduction
Nature has been a constant source of inspiration for human innovation. Biomimicry takes this a step further by not just copying what we see, but by deeply understanding the how and why behind nature's genius. Scientists study the structures, functions and processes of living organisms and ecosystems, uncovering the intricate relationships between form and function. This knowledge becomes the springboard for bioinspired solutions across various fields [1]. The concept of biomimicry has gained traction in various fields, including medicine, where researchers and practitioners look to nature for inspiration in developing innovative medical technologies and treatments. In medicine, biomimicry involves drawing inspiration from biological systems and organisms to create new medical devices, drugs and treatment approaches. For example, researchers have looked at the structure of spider silk to develop stronger and more flexible materials for sutures and wound healing. Additionally, the study of how certain plants or animals have evolved to combat diseases or injuries can provide insights for developing new therapies or treatment strategies [2].
The history of biomimicry in medicine dates back to ancient times when healers and physicians observed and learned from nature to develop treatments and remedies. The concept of using natural substances and processes to heal and cure ailments has been a fundamental aspect of traditional medicine practices in various cultures around the world. For example, the use of plants and herbs for medicinal purposes, inspired by observations of animals consuming specific plants for healing, reflects an early form of biomimicry in medicine. In more recent history, the formalization of biomimicry as a scientific discipline can be traced back to the work of researchers and innovators who recognized the potential of nature-inspired solutions in healthcare. The term "biomimicry" was popularized by Janine Benyus in her book "Biomimicry: Innovation Inspired by Nature" published in 1997. Benyus advocated for learning from nature's designs, processes and systems to address human challenges and create sustainable solutions across various fields, including medicine and healthcare. In the context of medicine, biomimicry has influenced the development of innovative medical technologies, materials and treatments by emulating biological structures and functions. For example, the design of biomimetic materials for tissue engineering, inspired by the extracellular matrix of living tissues, has revolutionized regenerative medicine and organ transplantation. Similarly, the development of bioinspired drug delivery systems that mimic biological mechanisms for targeted and controlled release of therapeutics has improved the efficacy and safety of pharmaceutical treatments.
Biomimicry offers a revolutionary approach to healthcare innovation by drawing inspiration from nature's time-tested solutions. This science goes beyond mere imitation; it delves into the intricate relationships between an organism's structure and its function. By studying how biological systems are meticulously designed to perform specific tasks, researchers gain valuable insights for creating biomimetic technologies. Imagine mimicking the hierarchical structure of bone or cartilage to create biomaterials that promote tissue regeneration, or replicating the selective permeability of cell membranes to design targeted drug delivery systems. Biomimicry allows us to harness the efficiency and precision honed by millions of years of evolution, paving the way for groundbreaking advancements in healthcare.
The power of biomimicry lies in its ability to translate nature's design principles into tangible solutions for various healthcare needs. For instance, the hierarchical structure of natural tissues can inspire the development of biomaterials with similar organization, promoting cell growth and regeneration. Biomimicry can also guide the creation of drug delivery systems that mimic biological mechanisms for the controlled release of medication directly to targeted areas. By replicating the self-assembly processes found in nature, researchers can create biomaterials with specific properties like biocompatibility, biodegradability and tailored mechanical strength, making them ideal for various healthcare applications. From regenerative medicine to medical device fabrication, biomimicry offers a valuable framework for innovating biomaterials that address specific healthcare challenges. By embracing nature's wisdom, we can create more effective treatments, devices and therapies, ultimately leading to improved healthcare outcomes for patients [3].
Biomimicry is revolutionizing medicine by offering entirely new solutions inspired by nature's brilliance. A prime example is the innovative medical adhesive derived from the Dusky Arion slug. This biomimetic approach not only yielded a completely new way to adhere tissues but also outperformed existing options. The slug-inspired glue excels in wet environments, conforms to moving tissues like a beating heart and avoids the toxicity risks of current adhesives. This paves the way for safer and more effective surgical procedures. The success story of the slug adhesive highlights the broader potential of biomimicry in medicine. Research on a bone adhesive inspired by the Sandcastle worm exemplifies the vast possibilities for mimicking nature's ingenious solutions to develop groundbreaking medical advancements. By drawing inspiration from the natural world, biomimicry holds immense promise for shaping a brighter future of medicine [4].
Materials and Methods
Biomimetic materials for medical applications
Bioinspired materials are synthetic materials designed to mimic the structures and properties of natural materials found in living organisms. Imagine materials as strong as spider silk, as self-healing as a lizard's tail or as adhesive as a gecko's foot. That's the potential of biomimicry. Scientists achieve this by studying how natural materials work at various levels, from their microscopic structure to their overall function. This knowledge is then used to create new materials with similar properties using synthetic processes. These bioinspired materials have a wide range of potential applications, particularly in medicine. They could be used to create implants that better integrate with the body, drug delivery systems that target specific cells or even tissues engineered to regenerate damaged organs [5].
Tissue engineering and regenerative medicine: Tissue engineering tackles a major challenge in medicine regenerating damaged organs and tissues. This field holds immense promise for treating a vast array of injuries, diseases and degenerative conditions (think anything from burns and sports injuries to heart disease and organ failure). The ultimate goal is to create functional tissue equivalents that seamlessly integrate with the body and restore lost function [6].
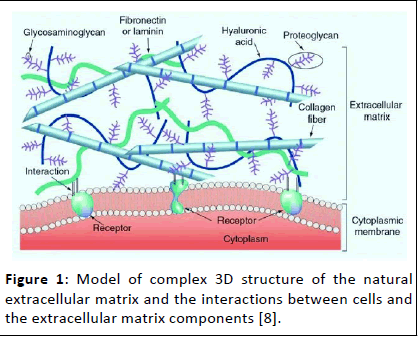
One of the most crucial elements to mimic is the Extracellular Matrix (ECM). This intricate 3D network within living tissues provides a supportive scaffold for cells. It's not just a passive structure; the ECM actively communicates with cells, dictating their growth, differentiation and organization. In essence, the ECM acts as a blueprint for tissue architecture and function. Traditional approaches in tissue engineering often relied on creating porous scaffolds for cell growth. However, these methods had limitations. Imagine building a house without a proper foundation-The structure might lack stability and support for its inhabitants (cells). Similarly, scaffolds without mimicking the ECM's complexity often faced challenges like uneven cell distribution and limited vascularization, leading to cell death within the scaffold. This is where biomimicry comes in. Researchers are employing various techniques to create biomimetic scaffolds that resemble the ECM's structure and functionalities. Some methods involve creating intricate pores using techniques like salt leaching or electrospinning nanofibers that mimic collagen structures. Others utilize rapid prototyping for precise control over the scaffold's architecture, mimicking natural shapes and offering better mechanical compatibility with the surrounding tissue. Beyond structure, biomimicry focuses on mimicking the biological cues within the ECM. Techniques like surface modification allow scientists to incorporate bioactive molecules like fibronectin and growth factors onto the scaffolds. These molecules act as signals, guiding cell behavior and promoting their differentiation into the desired tissue type (Figure 1) [7].
Figure 1: Model of complex 3D structure of the natural extracellular matrix and the interactions between cells and the extracellular matrix components [8].
Beyond mimicking the Extracellular Matrix (ECM), biomimicry has opened doors to developing novel biomaterials with superior properties for diverse tissue engineering applications. One well-researched example is the use of Oligomeric Proanthocyanidins (OPCs) derived from grape seeds and polydopamine, inspired by the adhesive properties of mussels. By combining these biomolecules, scientists have created biomaterials with exceptional photothermal effects. When exposed to light, these materials generate heat, promoting targeted tissue healing and potentially eliminating unwanted conventional biomaterials, inspiring researchers to design multifunctional materials with therapeutic functionalities beyond simple structural support.
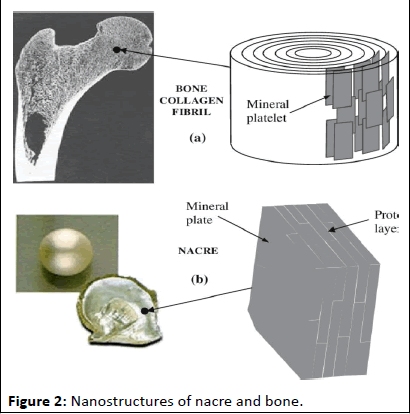
Another captivating example of biomimicry in action lies in mimicking the structure of natural materials. Nacre, (Figure 2) the iridescent layer of mollusks' shells, possesses a remarkable strength-to-weight ratio due to its intricate brick-and-mortar-like architecture. By mimicking this lamellar structure, researchers have fabricated biocompatible scaffolds that can withstand significant loads, making them ideal for bone repair applications. Similarly, tooth enamel, the hardest substance in the human body, serves as inspiration for the development of enamelinspired nanocomposites. These composites mimic the enamel's hierarchical structure, combining strength with biocompatibility, offering promising solutions for dental tissue engineering.
The overarching goal of biomimicry in tissue engineering is to replicate the ingenious design principles found in nature to create biomaterials that not only resemble the structure of natural tissues but also capture their remarkable functionalities. However, synthesizing these bioinspired materials remains a significant challenge. Scientists are constantly innovating to overcome these hurdles and advancements in techniques like self-assembly and bioinspired mineralization offer hope for the future. Self-assembly mimics the natural organization of molecules in biological systems, allowing for the creation of intricate structures with tailored properties. Bioinspired mineralization, on the other hand, focuses on replicating the processes by which organisms form mineralized tissues like bone. By incorporating these strategies, researchers aim to create a new generation of biomaterials with properties specifically designed to optimize tissue regeneration [9].
Figure 2: Nanostructures of nacre and bone.
Drug delivery systems: In the realm of medicine, a revolution is brewing. Biomimicry, the art of learning from and imitating nature's ingenious designs, is transforming drug delivery research. Pioneered by Otto Schmitt in the mid-20th century, biomimicry transcends being a mere subject; it's a whole new way of approaching challenges. Instead of solely relying on human ingenuity, biomimicry encourages us to draw inspiration from the intricate processes and structures found within living organisms. This approach holds immense promise for overcoming the limitations that have plagued traditional drug delivery methods for decades.
Imagine a future where therapeutic agents can be delivered directly to diseased cells with pinpoint accuracy, minimizing harm to healthy tissues. This is the vision that biomimicry brings to life. By studying the workings of cells at the subcellular level, scientists are developing revolutionary drug carriers that mimic natural mechanisms. These carriers act like Trojan horses, cleverly disguised to evade the body's defenses while precisely delivering their therapeutic payloads. Biomimicry offers powerful solutions to longstanding problems. Poor drug solubility, unintended side effects on healthy cells and inefficient drug release from carriers-These limitations are being tackled head-on through biomimetic strategies. These systems offer a range of carriers, each mimicking biological entities to enhance drug delivery efficiency and effectiveness.
Biomimetic polymeric drug carriers offer a compelling solution for drug delivery due to several advantages. Firstly, these polymers can be designed for controlled drug release, ensuring sustained medication delivery and potentially reducing dosing frequency. They also boast high drug loading capacities, maximizing the therapeutic payload delivered with each carrier. Furthermore, these biocompatible polymers can be readily processed into nanoscale structures, a crucial size range for effective drug delivery. Finally, the incorporation of specific functional groups into the polymer design allows for further customization. These groups can interact with biological molecules, potentially enhancing drug delivery to target cells or tissues. Researchers have created biopolymeric materials that mimic natural cell behavior, such as cell attachment and cytokine signaling. Additionally, the physiologically active bodies of these polymers can facilitate the transfer of medications across cell barriers within targeted sick cells. For instance, a biopolymeric material based on poly (carboxybetaine methacrylate) can be grafted onto a gold surface, allowing it to combine with biological ligands for targeted delivery. Scientists have also explored immobilizing specific proteins to enhance adsorption and drug delivery across cell barriers. In another example, a biomimetic biodegradable polymer resembling starch was coated with a calcium phosphate surface. This design, combined with a medicinal drug, helped regulate osteoblastic/ osteoclastic cell line behavior at the interface with injured bone, promoting bone repair.
Biomimetic nanostructures, sized between 10-100 nanometers, offer a revolutionary approach to drug delivery by mimicking nature's building blocks. These structures, comparable in size to DNA and proteins, can be crafted from various biomaterials. Some examples include self-assembled structures like graphene or rolled inorganic polymers forming hollow nanotubes. Additionally, three methods are employed to generate nanofibers: Electrospinning, phase separation and selfassembly, all utilizing natural or synthetic polymeric materials. Notably, these biomimetic nanostructures boast high biocompatibility, biodegradability and a strong drug-loading capacity. Let's delve deeper into specific types of biomimetic nanotubes: Polyelectrolyte multilayer nanotubes act as nanoreactors, encapsulating enzymes like urease to remove harmful calcium carbonate deposits. Their layered structure allows for controlled drug release while maintaining the stability of the encapsulated material. Another example is self-assembled Rosette Nanotubes (RNTs), which can be loaded with medications like dexamethasone for sustained release, promoting bone formation during orthopedic treatments. These are just a few examples of how biomimetic nanostructures are being designed to revolutionize drug delivery with targeted and controlled release mechanisms.
Biomimetic nanofibers offer a versatile platform for drug delivery, mimicking the natural extracellular matrix to promote tissue regeneration. Scientists have developed various nanofibers using techniques like electrospinning. For instance, Smith- Freshwater et al. created nanofibers from gelatin and PAMAM dendrimer, mimicking the natural environment for cells and allowing for a high concentration of antibiotics like doxycycline to be loaded within the fibers. This design facilitates a continuous release of medication to the wound site, making it ideal for wound dressings. Another example utilizes CDDP, an anti-cancer drug, combined with a Peptide Amphiphile (PA) to form nanofibers. This combination allows for self-assembly into a gel matrix, while the incorporation of specific functional groups like RGD and MMP-2 sensitive sequences facilitates the controlled and targeted release of CDDP into cancer cells. Beyond wound healing and cancer treatment, researchers have explored applications in bone regeneration. Li et al., used electrospinning to create a dual drug-loaded nanofiber containing BMP-2 and dexamethasone, mimicking the bone's extracellular matrix. This design promotes bone growth and improves bone tissue regeneration. These are just a few examples of how biomimetic nanofibers are being tailored for various therapeutic applications.
Biomimetic micelles offer a promising strategy for targeted drug delivery, drawing inspiration from nature's self-assembly processes. In an aqueous environment, these micelles form with a hydrophilic head and a hydrophobic core. This core acts as a reservoir for hydrophobic drugs, while the hydrophilic shell shields the drug and facilitates interaction with the biological environment. Researchers have developed various biomimetic micelles for targeted therapy. For instance, polymeric micelles with tailored structures allow for controlled drug release and enhanced drug encapsulation. Additionally, biomimetic block copolymers or biological molecules can be incorporated to create micelles that target specific cells. An example is the use of folic acid to target cancer cells, which overexpress folate receptors. By incorporating folic acid onto the micelle surface, researchers can achieve selective delivery of the encapsulated drug to these cancer cells. These advancements in biomimetic micelles hold significant promise for improving drug delivery efficacy and reducing side effects.
Biomimetic liposomes represent a groundbreaking convergence of nanotechnology and biomimicry, offering a powerful platform for targeted drug delivery and various therapeutic interventions. These microscopic spheres are crafted from lipid bilayers, mimicking the structure and function of natural cell membranes. This biocompatible design creates a versatile environment for encapsulating a wide range of therapeutic agents, from hydrophobic drugs to hydrophilic molecules. Biomimetic liposomes come in various forms to optimize drug delivery. Stealth liposomes, coated with Polyethylene Glycol (PEG), cloak themselves like natural cells, extending their circulation time in the body and reducing unwanted immune system interactions, ultimately enhancing drug delivery efficiency. Targeted liposomes take this a step further by incorporating specific molecules like antibodies or peptides on their surface. These ligands mimic the targeting specificity of cell surface receptors, allowing for precise delivery of the encapsulated drugs to specific cells or tissues. Immunoliposomes designed for targeted cancer therapy are a prime example of this approach. Finally, stimuli-responsive liposomes are engineered to respond to environmental cues like changes in pH, temperature, or enzymatic activity within the body. This allows for controlled release of the therapeutic payload, mimicking the dynamic behavior of biological membranes. Through this biomimetic design, liposome carriers hold immense potential for revolutionizing drug delivery strategies, enabling personalized medicine approaches and offering new avenues for therapeutic interventions across a broad spectrum of diseases.
Results and Discussion
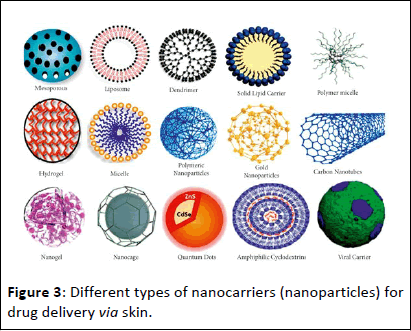
Biomimetic hydrogels are revolutionizing biomaterials with their ability to mimic the intricate structure and function of natural Extracellular Matrices (ECM). These versatile platforms hold immense potential for drug delivery, tissue engineering, and regenerative medicine. One key feature is self-healing hydrogels, inspired by wound healing. These hydrogels possess dynamic cross-linking mechanisms that maintain their structure even after being disrupted, mimicking the body's natural repair processes. Another innovation is nanocomposite hydrogels, which incorporate nanomaterials like graphene oxide for enhanced mechanical strength and conductivity, mirroring the hierarchical structure of tissues like bone. Additionally, cell-instructive hydrogels are enriched with growth factors or peptides mimicking natural ECM proteins. These factors regulate cellular behaviors crucial for tissue regeneration. Finally, responsive hydrogels are triggered by environmental cues like pH or temperature changes, enabling controlled drug release. An example is temperatureresponsive hydrogels based on PNIPAAm, which release drugs when the temperature rises. These diverse biomimetic hydrogels exemplify the power of biomimicry in advancing healthcare by harnessing nature's design principles. Figure 3 further illustrates the concept of biomimicry in drug delivery by showcasing additional examples of nanocarriers inspired by nature.
Figure 3: Different types of nanocarriers (nanoparticles) for drug delivery via skin.
Biomimicry in medical devices: The world of medical devices encompasses a vast array of tools, from the ubiquitous dental floss to life-saving pacemakers. These devices can be as simple or complex as their intended purpose dictates, serving functions like drug delivery, diagnostics, surgery and implantation. The design of these devices is constantly evolving, with a focus on innovative materials, methodologies, and manufacturing processes. One particularly promising avenue for advancement lies in bridging the gap between biomimetics, the study of nature's designs and medical device design.
Traditionally, medical device design revolves around understanding the anatomy and physiology of the human body to create solutions that "imitate" and provide an efficient intervention. However, biomimetics takes this a step further. It delves deeper into the fundamental aspects of any design: Form, fit and function. Biomimicry explores how these aspects are intricately linked to the intended use and the environment in which the design will operate. In the context of medical devices, this translates to a deeper understanding of how biological systems achieve their remarkable feats. Depending on the application, biomimetic-inspired devices can resemble the physical design of human physiology for seamless integration or they can actively interact with the body's natural processes to achieve a desired outcome. This marriage of biomimicry and medical device design has already begun to yield exciting results. Biocompatible materials, inspired by nature's solutions, are being developed to create implants that are better tolerated by the body. Surgical instruments are being designed to mimic the dexterity and precision of the human hand. And even the surfaces of medical devices are being engineered to take advantage of nature's anti-bacterial properties or self-cleaning mechanisms. By harnessing the power of biomimetics, the future of medical device design promises to be one of groundbreaking innovations, leading to improved patient care and a new era of medical advancements.
The type of material used is another crucial bridge connecting biomimetics and medical device design. Medical devices require materials that are both strong and lightweight, but also possess the critical quality of being "biocompatible." Biocompatible essentially means a material can perform its intended function within the body without causing any adverse reactions. The challenge with implantable materials lies in their need to not only offer mechanical support but also provide a haven for surrounding tissues, avoiding any harmful immune responses.
Commonly used materials in medical devices include titanium alloys, stainless steel and various polymers like Polyether Ether Ketone (PEEK), polyethylene and Polylactic-co-Glycolic Acid (PLGA). Additionally, composite materials like carbon fiberreinforced plastic and ceramics such as alumina, zirconia and bioglass find applications in device design. Polymers like PLGA exemplify a fascinating bridge between biomimicry and medical devices. These materials degrade and are absorbed by the body's enzymes over time, earning them the designation of "resorbable materials." This characteristic makes them ideal for sutures, orthopedic bone pins, plates and screws. Another prime example is the development of porous bone grafts for use in orthopedic surgeries. Inspired by the microscopic structure of natural bone, metals and polymers are used to create bone grafts with built-in porosity. These pores mimic the natural environment for new bone cells or osteoblasts, to infiltrate and promote bone healing through a process known as ossification or osteogenesis. Continued exploration into biomimetic and bioactive materials holds immense promise for advancements in various fields, including orthopedics, tissue engineering, implantable devices and even robotics.
Bone's remarkable combination of strength and flexibility is being mimicked in scaffold materials for bone regeneration. These scaffolds, with their intricate internal structures resembling natural bone, show great promise in promoting bone growth. Similarly, the protective shells and exoskeletons of arthropods and turtles are inspiring the development of innovative helmets and exoskeletons with exceptional impact resistance and durability. Mimicking the function of muscles and tendons is leading to the creation of artificial muscles and tendon replacements, offering new hope for patients with musculoskeletal disorders. Finally, biomimetic materials inspired by skin and membranes are being used to create advanced wound dressings, grafts and membranes that promote healing, reduce scarring, and provide effective protection against infections. By learning from nature's ingenious designs, biomimetic materials are transforming the landscape of medical devices and offering significant improvements in patient care across a wide range of applications.
PreciHealth, a Swiss medical device startup, drew inspiration from an unlikely source: The mosquito. The company was fascinated by the mosquito's ability to use its proboscis to simultaneously draw blood and deliver saliva (a powerful anticoagulant) through tiny tubes. This biomimicry sparked ideas for developing new drug delivery and blood collection platforms, particularly wearable and injectable devices. Existing injectable platforms typically deliver medication in milliliters, far exceeding the microliter scale of a mosquito's actions. The first hurdle was developing reliable and highly precise microneedle technology. Not surprisingly, given their Swiss heritage, PreciHealth's initial demonstration of this precision involved a new type of wristwatch. The successful commercialization of these watches by their parent company validated the microneedle technology and paved the way for its application in medical devices. The company's first medical device application, inspired by nature's mastery of microneedles, was a combination epinephrine delivery device. Existing epinephrine injectors faced two major drawbacks: Size (EpiPens can be quite bulky, requiring patients to carry two) and ease of misuse (accidental self-injection into the thumb was a concern). PreciHealth aimed to address these limitations with its innovative design.
In orthopedic surgery, plates and screws play a vital role in fixing fractures and fusing joints. These implants achieve this function by compressing the bone fragments or the joint together at the fracture/joint line. The design of the plate itself is critical for successful bone fixation and minimizing complications after surgery. Key features that can be tailored to different bone structures and injury types include the plate's shape, thickness, overall form (contour) and the design of the holes for screws. Biomimicry plays a significant role in designing these plates to match the bone's anatomy. For instance, straight plates are commonly used for long bone fractures in the limbs. This simple design works well for non-comminuted fractures, where the bone is broken into clean pieces. However, a different approach is needed for complex fractures, such as those in the calcaneus (heel bone). The calcaneus bears a significant amount of body weight, and high-impact events can cause it to shatter into multiple fragments. To capture and hold all these fragments securely, plates are designed to mimic the calcaneus's natural shape. This is where biomimicry comes into action. By analyzing CT scans from a broad patient population, engineers can design plates with hole locations that correspond to the bone's profile and typical fracture patterns. These computer-aided designs determine the plate's dimensions, contour, and overall profile to ensure it effectively covers the entire bone. Screw hole locations can be strategically placed based on existing classifications of calcaneal fractures. Additionally, offering plates in various sizes and with the option for intraoperative contouring allows surgeons to tailor the implant to the specific needs of each patient.
Biomimetic heart valves have become a game-changer in cardiovascular care. These innovative devices are designed to closely mimic the natural movements and functions of human heart valves. This approach leads to several key benefits for patients. Firstly, biomimetic valves offer superior hemodynamics. By replicating the natural valve's dynamic behavior, they optimize blood flow within the heart. This reduces turbulence, which can otherwise create complications. Secondly, the design of these valves minimizes the risk of blood clot formation. This is a significant improvement over some traditional heart valve replacements, where clotting can be a concern. Finally, biomimetic valves demonstrate exceptional durability. Their design, inspired by nature's efficiency, translates to longer-lasting implants for patients. Overall, the use of biomimetic heart valves has significantly improved the quality of life for those suffering from heart valve disorders. This breakthrough serves as a powerful example of how biomimicry, the practice of learning from nature's designs, can revolutionize the field of medical interventions.
Biomimetic materials are playing a revolutionary role in the development of neuroprosthetic devices. These devices hold immense promise for individuals struggling with paralysis, Parkinson's disease and other neurological conditions. By mimicking the intricate workings of the brain and nervous system, neuroprosthetics can bridge the gap between the brain and external systems, restoring lost communication and functionality. The key to neuroprosthetics lies in bioelectronics, which allows for direct communication between the brain and external devices. This technology offers renewed hope for those suffering from neurological impairments. By emulating the structure and function of neuronal networks, neuroprosthetic interfaces can help patients regain control over their bodies and restore lost functions. This translates to significant improvements in quality of life, allowing individuals to regain independence and perform daily activities. Biomimetic materials are crucial in creating these interfaces, ensuring biocompatibility and seamless integration with the human body. This exciting field of biomimetic neuroprosthetics represents a significant leap forward in neurological care, offering life-changing possibilities for patients.
The field of surgery is embracing biomimicry to create innovative surgical tools that mimic the remarkable dexterity and precision found in nature. This has led to the development of biomimetic surgical tools that are revolutionizing minimally invasive procedures. One prime example is the emergence of robotic instruments. These instruments are designed to mimic the intricate movements of a surgeon's hand, but with a level of precision and control that surpasses human capabilities. This translates to several benefits for both patients and surgeons. For patients, biomimetic robotic surgery minimizes trauma and discomfort associated with larger incisions. Additionally, the superior precision of these robotic tools allows surgeons to perform complex procedures with greater accuracy, potentially leading to improved surgical outcomes. Biomimicry is transforming the landscape of minimally invasive surgery, offering a future filled with less invasive procedures, faster recovery times and potentially better patient outcomes.
Challenges and future directions
While biomimetic materials offer remarkable benefits for medical devices, ensuring their safe and effective use requires careful consideration. Biocompatibility remains a critical challenge, demanding rigorous assessment for potential issues like immune system response (immunogenicity), toxicity and long-term effects within the body.
Furthermore, integrating biomimetic concepts into the product development process itself presents hurdles. The gap between a seemingly perfect design concept and its real-world application can be significant. In the field of medical devices, this is especially true. A design that functions flawlessly in a lab setting might require extensive real-world testing and user feedback to confirm its effectiveness and identify any unforeseen risks or failures. Cost is another crucial factor. Shifting from conventional manufacturing to biomimetic techniques, like 3D printing, can significantly increase initial investment. Additionally, these new processes come with their challenges, such as developing appropriate mechanical testing methods for non-standard designs. For instance, testing a biomimetic orthopedic plate requires replicating real-world physiological loading conditions, as traditional beam-based testing methods wouldn't provide a complete picture of its performance under stress.
Beyond these challenges, biomimicry faces additional hurdles specific to the complexities of the human body. Mimicking nature's intricate structures and functions can be difficult. For example, replicating the dynamic flexibility and self-healing properties of soft tissues like tendons and ligaments remains a significant feat. Additionally, mimicking the intricate signaling pathways and cellular interactions that occur within biological systems poses a complex engineering challenge. Some biomimetic designs may raise concerns about unintended consequences, such as the potential for biomimetic materials to introduce invasive species or disrupt ecological balances if not carefully managed. These factors, along with the advantages of biomimicry, need to be carefully weighed throughout the product development cycle to ensure the successful creation of innovative and safe medical devices. Biomimicry offers a powerful approach to advancing medical technology, but navigating these challenges is crucial for its responsible and successful implementation.
Conclusion
Biomimicry has woven itself into the fabric of medical device innovation, offering a treasure trove of inspiration for creating solutions that are more effective, less invasive, and better integrated with the human body. From biocompatible materials that mimic nature's elegance to surgical tools that rival a surgeon's dexterity, biomimicry is transforming healthcare, aligning perfectly with Sustainable Development Goal 3: Good health and well-being. Despite the challenges, the future of biomimicry in medicine is brimming with promise. As we delve deeper into nature's design genius and bridge the gap between biological inspiration and real-world applications, we can expect even more groundbreaking advancements. Biomimicry has the potential to revolutionize how we diagnose, treat and prevent diseases, ultimately leading to a healthier and more empowered future for patients. By embracing the power of biomimicry and navigating its challenges responsibly, we can unlock a new era of medical marvels inspired by nature's brilliance, contributing significantly to achieving SDG 3: Good health and well-being.
References
- Ilieva L, Ursano I, Traista L, Hoffmann B, Dahy H (2022) Biomimicry as a sustainable design methodology-Introducing the ‘biomimicry for sustainability’ framework. Biomimetics 7:37
[Crossref] [Google Scholar] [PubMed]
- Berthiaume F, Maguire TJ, Yarmush ML (2011) Tissue engineering and regenerative medicine: History, progress, and challenges. Annu Rev Chem Biomol Eng 2:403-430
[Crossref] [Google Scholar] [PubMed]
- Kim TG, Shin H, Lim DW (2012) Biomimetic scaffolds for tissue engineering. Adv Funct Mater 22:2446-268
- Aghmiuni AI, Khiavi AA (2017) Medicinal plants to calm and treat psoriasis disease. Aromatic and Medicinal Plants Back to Nature 2016:1-28
- Li T, Chang J, Zhu Y, Wu C (2020) 3D printing of bioinspired biomaterials for tissue regeneration. Adv Healthc Mater 9:2000208
[Crossref] [Google Scholar] [PubMed]
- Gao H (2006) Application of fracture mechanics concepts to hierarchical biomechanics of bone and bone-like materials. Int J Fract 138:101-137
- Sheikhpour M, Barani L, Kasaeian A (2017) Biomimetics in drug delivery systems: A critical review. J Control Release 253:97-109
[Crossref] [Google Scholar] [PubMed]
- Jamal A (2023) Embracing nature's therapeutic potential: Herbal medicine. Intern J Multidiscip Sci Arts 2:117-26
- Paniagua SA, Menezes DB, Murillo MF, Henriquez LC, Baudrit JR (2024) Nature-inspired innovations: Unlocking the potential of biomimicry in bionanotechnology and beyond. Discov Nano 19:186
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences