ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Evaluation of Biotechnological Potential of Novel Mercury Tolerant Strain of Klebsiella Pneumonia
Himadri Gourav Behuria, Rashmi Shee, Sangam Gupta, Bijesh Kumar Biswal and Santosh Kumar Sahu*
Laboratory of Molecular Membrane Biology, Department of Biotechnology, North Orissa University, India
- *Corresponding Author:
- Santosh Kumar Sahu
Laboratory of Molecular Membrane Biology,
Department of Biotechnology, North Orissa University,
Odisha, India -757003
Tel: +91-9178939281
Fax: 06792-256906
E-mail: drsantoshnou@gmail.com
Received date: August 23, 2018; Accepyed date: September 03, 2018; Published date: September 10, 2018
Citation: Behuria HG, Shee R, Gupta S, Biswal BK, Sahu SK (2018) Evaluation of Biotechnological Potential of Novel Mercury Tolerant Strain of Klebsiella Pneumonia. J Appl Microbiol Biochem. Vol.2 No.3:11
Abstract
In order to investigate the effect of elevated mercury pollution on antibiotic tolerance and enzyme activities of human pathogens, we isolated a novel K. pneumoniae strain from the soil of a metallurgical dump yard that was capable of tolerating 250 μM HgCl2. Increasing concentration of Hg2+ in growth medium led to increased tolerance of the strain to ampicillin that was accompanied by increased secretion of extracellular polymeric substance (EPS). These results indicate that Hg2+-induced increased synthesis of EPS might be a key factor for increased tolerance of K. pneumoniae to ampicillin. The strain exhibited high catalase, alkaline phosphatase, mercuric reductase, amylase and lipase activity. Presence of 250 μM HgCl2 in growth medium increased mercuric reductase activity to 3 fold, however, no significant alteration in catalase, alkaline phosphatase and lipase was observed. The strain retained 86% amylase activity when grown in presence of 250 μM Hg2+. However, cell free lysate of the bacterium exhibited sensitivity towards Hg2+ leading to 30 to 40% decrease of these enzyme activities at 250 μM Hg2+. These results show that the bacterium possesses cellular mechanism to protect its enzymes from exogenous HgCl2. Our study reveals that increased heavy metal in the environment is a key factor leading to cross resistance of K. pneumoniae against membrane-targeted antibiotics ampicillin. Further, it’s a novel mercury-tolerant strain of K. pneumoniae isolate that exhibits high biotechnological potential for simultaneous bioremediation of mercury and production of industrially important enzymes.
Keywords
Mercury; Antibiotic; Mercuric reductase; Extracellular polymeric substance; Klebsiella; Alkaline phosphatase; Catalase
List of Abbreviations
ALKP: Alkaline Phosphatase; PSI-BLAST: Position Specific Iterated Basic Local Alignment Search Tool; BSA: Bovine Serum Albumin; CR: Congored; EPS: Extracellular Polymeric Substance; MerA: Mercuric Reductase; PMSF: Phenyl Methyl Sulfonyl Fluoride; PNP: Para-Nitrophenyl Phosphatate; MIC: Minimum Inhibitory Concentration.
Introduction
Anthropogenic activities such as mining, smelting, coal combustion, waste incineration and chlor-alkali plants have increased the load of mercury in water, soil and atmosphere by 3 folds in last 500 years [1]. Most inorganic Hg released into environment is converted into organic mercury by soil microbes. These organomercurials accumulate within the organisms that inhabit Hg-contaminated ecological niches and are subsequently biomagnified as they move along the food chain up to human [2,3]. In India, rapidly expanding industrialization and energy requirement have increased Hg emission at an alarming rate from 920 tons in 2001 to 1450 tons in 2010 [4]. Application of Hg-contaminated sewage sludge in agricultural lands as green manure, a common practice in India has increased Hg accumulation in crops and vegetables [5,6]. Consumption of Hgcontaminated water, aquatic animals (e.g. sea food), plants and their products constitutes the major route of mercury toxicity in human [7]. In human, mercury is known to affect renal, cardiovascular, reproductive, central nervous system (CNS) and interferes with fetal development [7,8].
Microbes growing in Hg2+ contaminated soil and water bodies exhibit resistance to Hg2+ by altering their biochemical and molecular characteristics. Microbial Hg2+ tolerance is proposed to be a bio-indicator of environmental Hg2+ toxicity [9]. Some important biochemical alteration in microbes exposed to elevated level of Hg2+ include over-expression of oxidative stress regulatory enzymes such as catalase and mercury reducing enzyme MerA [10,11]. However, Hg2+ is known to inhibit function of multiple proteins and enzymes. Binding of Hg2+ to sulfhydryl (-SH) groups leading to their covalent modification and displacement of metal ion cofactors (e.g. calcium or iron) from metalloenzymes are amongst important mechanisms of Hg2+-induced inhibition of enzymes [12]. However, mercury-tolerant bacteria exhibit multiple mechanisms to tolerate high concentration of Hg2+ in growth medium. Common Hg2+ resistance mechanisms involve over-expression of MerA, Hg2+-tolerant enzymes and oxidative stress regulatory enzymes (e.g. catalase). Modification of cell envelope in bacteria such as change in membrane lipid composition, increased secretion of EPS and formation of biofilm on solid surface contribute to their Hg2+ tolerance. Many heavy metal tolerant bacteria possess plasmids that harbor genetic information to tolerate high concentration of heavy metal in the growth medium [9].
K. pneumoniae is a common pathogen of human visceral epithelium such as gut, respiratory and urinary epithelium and the causative agent for acute pneumonia. This bacterium exhibits drug resistance against a number of antimicrobials including the cell wall targeted antibiotic ampicillin. Atomic force microscopy of antibiotic resistant K. pneumoniae strain shows that these bacteria exhibit morphological alteration in their cell wall [13]. Alteration of membrane lipid composition, increased the synthesis of extracellular polymeric substances (EPS) and biofilm formation are amongst the most common morphological changes that enables resistance of K. pneumoniae to Hg2+ and other antimicrobials [14,15]. Research by multiple researchers reveals that many bacterial species have coevolved antibiotic tolerance along with heavy metal tolerance [16]. However, the mechanism of heavy metal-induced antibiotic tolerance in K. pneumoniae remains unexplored.
Bacteria are otherwise termed as microbial cell factories that produce industrially important enzymes. However, most bacteria are sensitive to heavy metals in their growth medium that significantly inhibit production of enzymes at industrial scale. Heavy metal tolerant bacteria that produce industrially relevant enzymes or metabolites constitute useful bio-resources as they could be utilized for multiple purposes such as heavy metal detoxification as well as production of enzymes. To investigate the effect of heavy metal on antibiotic tolerance and enzyme activities of common human pathogens that inhabit soil, we isolated a novel Klebsiella pneumoniae strain from the soil of a metallurgical dump yard. This strain not only tolerates high concentration of HgCl2 in growth medium, but also exhibits resistance to ampicillin and produces high level of some common industrial enzymes. We evaluated the biotechnological potential of the strain for production of five common industrial enzymes: catalase, ALKP, MerA, amylase and lipase.
Materials and Methods
Microorganism
Soil sample collected from a dump yard of Rourkela steel plant, Odisha, India that was used for dumping industrial waste products for more than 50 years. The sample was screened for the presence of Hg2+ tolerant bacteria by growing them in LB liquid medium containing increasing concentration of HgCl2 up to 250 μM. A single strain was isolated that possessed a minimum inhibitory concentration (MIC) of 250 μM HgCl2 and was identified by 16S rDNA sequencing using forward and reverse primers AGAGTTTGATCCTGGCTCAG and TACGGTTACCTTGTTACGACTT respectively at AgriGenome Labs Pvt. Ltd, Kerala, India. The sequence was matched to the existing bacterial 16S rDNA sequences in NCBI data base using position specific iterated basic local alignment search tool (PSI-BLAST) programme [17].
Growth, collection and lysis of cells
The K. pneumoniae isolate was maintained in LB Agar (LBA) medium, cultured in LB containing different concentration of Hg2+ for 16 h at 25°C and 200 rpm and collected by centrifugation at 13000 × g for 10 min at 4°C. Cell pellets were re-suspended at ̴100 mg mL-1 in re-suspension buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM BHT and 1 mM PMSF) and used immediately for further experiments. Cells were lysed by sequential incubation with lysozyme (1 mg mL-1) and Triton-X-100 (1% (w/v)) at 37°C for 30min with intermittent mixing. Supernatant was collected after centrifugation of the lysate at 4°C for 20min at 17, 000 × g. Protein was quantitated by Lowry method using a Systronics double beam spectrophotometer (Model Visiscan 167, India) [18].
Evaluation of enzyme activities
Activity of catalase, mercury reductase (MerA), alkaline phosphatase (ALKP) and amylase were measured by spectrophotometric assay using a Systronics double beam spectrophotometer (Model 2202, Japan). Lipase activity was quantitated using plate assay method on freshly collected cell lysate. Data obtained from at least four sets of experiments were analyzed using GraphPad prism (version 6).
Catalase
Catalase activity was quantitated following the methods of Beers and Sizer [19].Briefly, Catalase activity in 0.3 ml lystae containing 1 mg protein was quantitated by monitoring the time dependent depletion of 6.6 mM H2O2 at 240 nm in 3 ml assay buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl).
MerA
MerA assay was performed following procedures of Fox and Walsh in cell lysate containing 1 mg protein in 3 ml assay buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM β-mercaptoethanol, 200 μM NAD(P)H and 50 μM HgCl2) [20]. Briefly, MerA activity was quantitated by measuring Hg2+-dependent oxidation of NAD(P) H to NADP+ resulting in depletion of absorbance of NADPH (A340). Initial rate of NAD(P)H oxidation was determined in the first 10s, when A340 decreased linearly with time. One unit MerA activity is defined as the activity that oxidized 1 μmol of NAD(P)H min-1 in the above specified condition.
ALKP
ALKP activity was quantitated by monitoring time-dependent hydrolysis of para-nitrophenyl phosphate (PNP) to PO4 and paranitrophenol (PN) that has absorption maxima at 405 nm [21]. Briefly, time-dependent increase in absorption of PN (A405) in the assay mix containing 1 mg protein in 3 ml assay buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM PNP was monitored for 3 min at 25°C. One unit ALKP activity was calculated as the enzyme activity that produced 1 μmol PN min-1 under above assay conditions.
Amylase
Amylase activity in the cell lysate containing 1 mg protein was monitored by time-dependent depletion of 1mg amylose in 3ml assay buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl) at 25°C [22]. One unit amylase activity was defined as the activity that degraded 1μg starch per min in one ml assay mix in the above assay condition.
Lipase
Lipase activity in the cell lysate was quantitated following the method of Kouker and Jaeger using rhodamine B agar plates [23]. Briefly, cell lysates containing 1 mg protein in 50 μl volume was placed in wells of 5mm diameter on agar plates containing 2.5% (w/v) olive oil and 0.001% (w/v) rhodamine B. The plate was incubated for 48 h at 37°C and the diameter of the orange fluorescent hallos produced around the well was visualized in UV (350 nm) was measured. Lipase activity was calculated from a standard curve obtained by placing known amount lipases in the agar wells in the same condition used for unknown samples.
Quantitation of EPS production
EPS produced by the isolate was quantitated using congo red (CR) binding assay as described previously with modification [24]. Briefly, stationary phase cultures were adjusted to OD600=10 in 3 ml resuspension buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM BHT) containing 40 mg L-1 CR and incubated for 1 h at 25°C and 200 rpm. Cells were removed by centrifugation at 25°C and 10,000 × g for 5 min and A490 of the supernatant was determined by measuring the absorbance of the residual CR in the resuspension buffer.
Determination of ampicillin resistance
Ampicillin resistance of the K. pneumoniae isolate was determined by agar-cup assay [25]. Briefly, K. pneumoniae grown in LB containing increasing concentration of Hg2+ were uniformly plated at OD600 of 0.1 on LB-agar plates. Increasing concentration of ampicillin (0, 250 μg/ml, 500 μg/ml, 750 μg/ml and 1000 μg/ml was applied the in wells of 6 mm diameter in 50 μl total volume and incubated at 37°C for 12 h. Diameter of the clear zone produced around each well shows the sensitivity of the bacterium to ampicillin.
Statistical analysis
Statistical analysis was performed using GraphPad prism (Version 6). Statistical significance of data was evaluated by Student’s “t” test for the biochemical studies and One-way ANOVA test was used to compare the results whenever more than two experimental groups were compared. All the data are expressed as Mean ± STDEV (standard deviation) (n=3). Differences were considered to be significant at a probability of P<0.05.
Results
Isolation and identification of K. pneumoniae industrial isolate
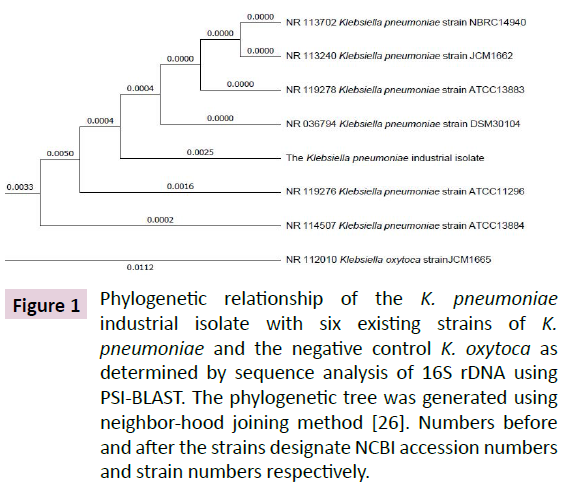
Gradual enrichment of the LB growth medium containing mixed culture of microbial strains collected from soil of industrial dumping area up to 300 μM led to isolation of a single Gram negative bacillus that produced mucoid colonies on LBA plate. Amplification of the 16S rDNA region of its genomic DNA produced a 1415 bp amplicon. Analysis of its sequence at NCBI genomic data base using PSI-BLAST exhibited 99% identity to six highestscoring 16S rDNA sequences from existing K. pneumoniae strains [26].The 16S rDNA from K. oxytoca used as a negative control exhibited 98% sequence identity (Figure 1). These results show that the isolate is a novel strain of K. pneumoniae.
Figure 1: Phylogenetic relationship of the K. pneumoniae industrial isolate with six existing strains of K. pneumoniae and the negative control K. oxytoca as determined by sequence analysis of 16S rDNA using PSI-BLAST. The phylogenetic tree was generated using neighbor-hood joining method [26]. Numbers before and after the strains designate NCBI accession numbers and strain numbers respectively.
Determination of mercury tolerance of the K. pneumoniae strain
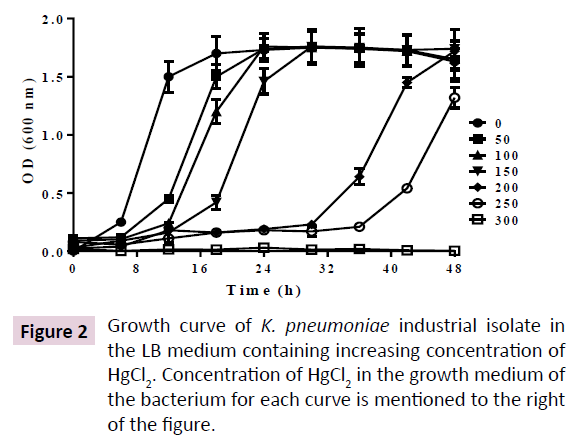
The K. pneumoniae was maintained in LBA and grown in LB at 25°C and 200 rpm. In above condition, the culture was saturated at 16h. In presence of increasing concentration of HgCl2, up to 150 μM, lag phase was increased up to 16h, leading to an increase in saturation time up to 30 h. However, further increase of HgCl2 up to 250 μM, prolonged the lag phase up to 32 h, leading to saturation phase of more than 48 h.However, no growth was observed at 300 μM HgCl2 (Figure 2). Incubating the cells till 72 h in 300 μM HgCl2 didn’t result in growth of cells, making the minimum inhibitory concentration (MIC)>250 μM and <300 μM.
Hg2+ increases ampicillin resistance of the K. pneumoniae isolate.
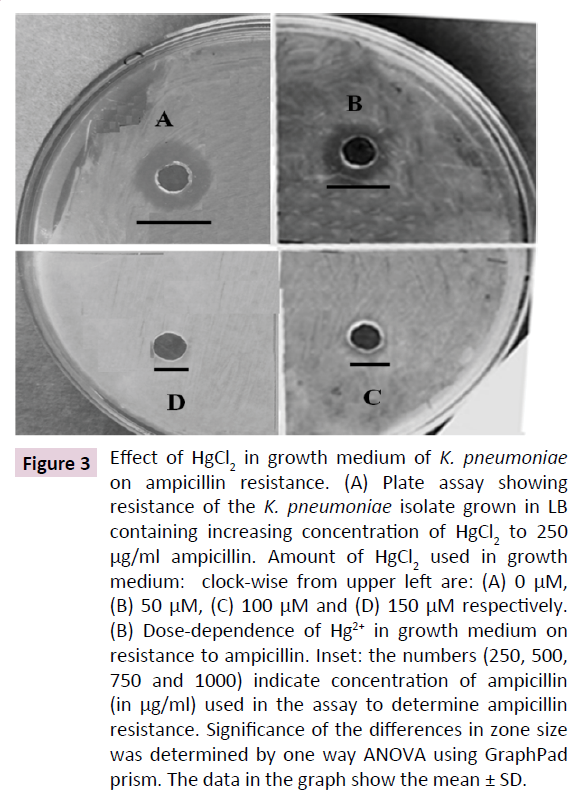
K. pneumoniae is known to resist cell wall-targeting antibiotics such as ampicillin. In many bacterial species, heavy metal resistance and antibiotic tolerance have been coevolved. Hence, we determined the effect of Hg2+ on the sensitivity of the K. pneumoniae to ampicillin, an antibiotic that targets the peptidoglycan layer in bacteria. Our results show that elevated concentration of Hg2+ in growth medium increases ampicillin resistance of the bacterium as indicated by a dose-dependent decrease in the size of clear zones around the agar wells. Figure 3A shows the result of a representative assay of the effect of 0, 50, 100 and 150 μM Hg2+ in growth medium of the K. pneumoniae isolate towards its sensitivity to 250 μg/ml ampicillin. The results show that increasing doses of HgCl2 in growth medium gradually decrease size of the zones, leading to its complete abolishment at 150 μM. A gradual dose-dependent decrease in diameter of clear zones was observed up to 1000 μg/ml ampicillin, upon subjecting the cells to increasing doses of HgCl2 in growth medium. These results indicate that HgCl2 in medium induces ampicillin resistance in the K. pneumoniae isolate (Figure 3B). Up to 40% decrease in zone size was observed at 500 to 1000 μg/ml ampicillin when the cells were subjected to 200 μM HgCl2. This concentration is 5-10 times the concentration normally used to inhibit wild type K. pneumoniae. These data show that HgCl2 increases ampicillin tolerance of K. pneumniae isolate.
Figure 3: Effect of HgCl2 in growth medium of K. pneumoniae on ampicillin resistance. (A) Plate assay showing resistance of the K. pneumoniae isolate grown in LB containing increasing concentration of HgCl2 to 250 μg/ml ampicillin. Amount of HgCl2 used in growth medium: clock-wise from upper left are: (A) 0 μM, (B) 50 μM, (C) 100 μM and (D) 150 μM respectively. (B) Dose-dependence of Hg2+ in growth medium on resistance to ampicillin. Inset: the numbers (250, 500, 750 and 1000) indicate concentration of ampicillin (in μg/ml) used in the assay to determine ampicillin resistance. Significance of the differences in zone size was determined by one way ANOVA using GraphPad prism. The data in the graph show the mean ± SD.
Hg2+ and ampicillin resistance in K. pneumoniae isolate is accompanied by enhanced secretion of EPS
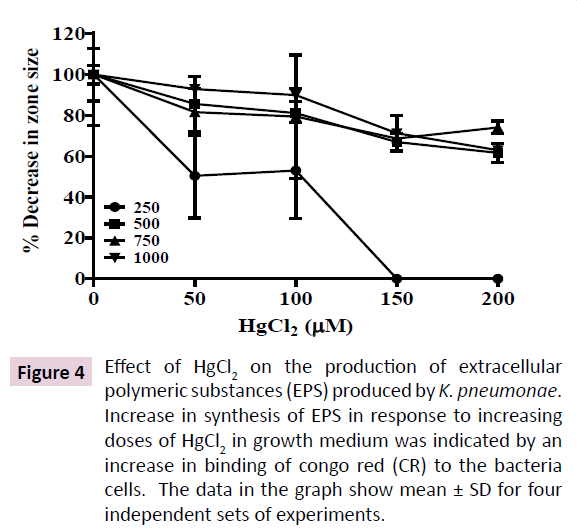
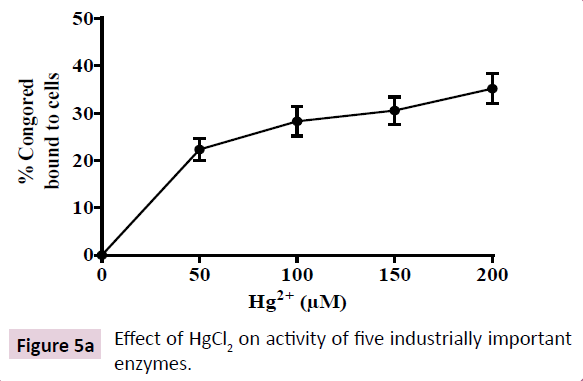
Soil bacteria are known to exhibit high biofilm forming abilities, a feat enabling them to survive harsh environmental conditions such as heavy metal toxicity that correlates with increased secretion of EPS. EPS is mostly a negatively charged lipo-polysaccharide secretion that serves as a protective barrier in bacteria inhabiting harsh ecological niches. Increased EPS secretion correlates with increased anti-biotic resistance, colonization on respiratory epithelium and pathogenicity in K. pneumoniae. Hence, we quantitated the effect of increasing HgCl2 in growth medium on EPS production by the K. pneumoniae isolate. Hg2+ at 200 μM enhanced EPS secretion in the K. pneumoniae isolate by 40% as indicated by increased binding to CR (Figure 4). However, EPS secretion was not enhanced further at 250 μM Hg2+, showing that EPS secretion is saturable around 200 μM HgCl2 in growth medium. These results indicate the probable involvement of an enzymatic mechanism that is activated by HgCl2 in growth medium.
Figure 4: Effect of HgCl2 on the production of extracellular polymeric substances (EPS) produced by K. pneumonae. Increase in synthesis of EPS in response to increasing doses of HgCl2 in growth medium was indicated by an increase in binding of congo red (CR) to the bacteria cells. The data in the graph show mean ± SD for four independent sets of experiments.
Production of industrially important enzymes from the mercury tolerant K. pneumoniae isolate
Evaluation of activities of common industrially relevant enzymes in the strain shows that the bacterium possesses high potential to produce catalase, MerA, ALKP, lipase and amylase (Table 1). Catalase is an important oxidative stress regulatory enzyme that is over expressed in response to the presence of heavy metals that enhances oxidative stress. The K. pneumoniae isolate produces 23 units/mg catalase that is not affected by the presence of HgCl2 in growth medium. This finding shows that the bacterium is adapted to secrete elevated level of catalase wooing to elevated concentration of Hg in its environment. However, MerA was increased to 3 folds in presence of 200 μM HgCl2. This inducibility of MerA activity shows that exogenous Hg2+ increases the cytosolic level of MerA activity in the K. pneumoniae isolate. MerA is the primary enzyme expressed in response to Hg toxicity that converts ionic mercury (Hg2+) to the less toxic form, metallic mercury (Hg0). Our results show that the strain exhibits high HgCl2 tolerance by over expressing MerA that accelerates the conversion of more toxic ionic mercury (Hg2+) in the growth medium to less toxic metallic mercury (Hg0) that vaporizes to the atmosphere. ALKP is essentially required in bacteria for phosphate assimilation. We observed 22 units of ALKP activity/mg of total protein in cytosol of the K. pneumoniae isolate that remained unaffected with increase of HgCl2 in the growth medium up to 200 μM. However, a 13% depletion of ALKP activity was observed at 250 μM HgCl2. Amylase and lipase are two industrially important enzymes that are produced by much soil bacterial for utilization of plant remains and oil in the environment. We evaluated the α-amylose and lipase activities of crude lysate from the K. pneumoniae isolate. We observed 17 and 12 units of activities for amylase and lipase in the K. pneumoniae isolate respectively when grown in LB. A gradual decrease of amylase activity up to 15% was observed when grown in presence of Hg2+ up to 250 μM. However, no significant alteration in lipid degrading activity was observed in presence of exogenous HgCl2. These results indicate that the Hg2+ tolerant K. pneumoniae industrial isolate possess high biotechnological potential for production of catalase, MerA, ALKP, amylase and lipase. Further, activity of the above enzymes was not depleted in the cytosol of the K. pneumoniae isolate when grown in presence of high concentration of mercury in the growth medium.
| HgCl2 (µM) | ALKP | MerA | Amylase | Lipase | Catalase |
|---|---|---|---|---|---|
| 0 | 22.56 ± 1.3 | 6.4 ± 0.45 | 17.25 ± 1.06 | 12.14 ± 0.87 | 22.43 ± 2.1 |
| 50 | 22.24 ± 2.1 | 7.5 ± 0.73 | 15.36 ± 0.98 | 12.34 ± 0.93 | 23.12 ± 2.34 |
| 100 | 21.21 ± 1.23 | 10.5 ± 0.98 | 15.56 ± 0.57 | 12.65 ± 1.4 | 24.12 ± 2.14 |
| 150 | 20.67 ± 1.12 | 15.3 ± 1.21 | 16.24 ± 1.12 | 12.92 ± 0.79 | 23.45 ± 1.98 |
| 200 | 20.45 ± 2.1 | 16.45 ± 1.32 | 14.89 ± 1.35 | 13.35 ± 1.2 | 23.13 ± 1.57 |
| 250 | 17.41 ± 1.22 | 20.13 ± 1.75 | 14.8 ± 1.43 | 13.05 ± 1.3 | 23.21 ± 2.15 |
Table 1 Effect of Hg2+ on activity of some industrially important enzymes units (average ± SD).
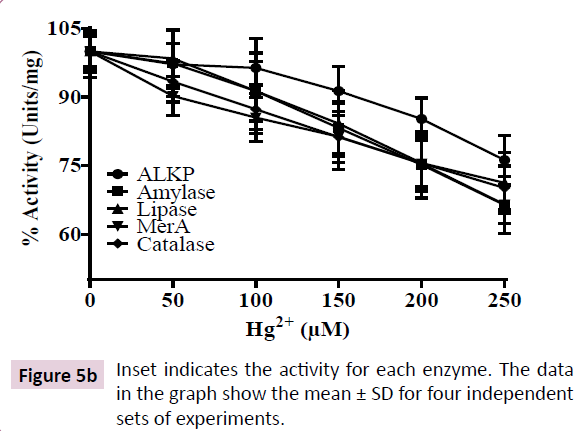
To determine the sensitivity of above enzymes to Hg2+, we measured their activity in cell-free lysate of the K. pneumoniae isolate grown in LB in presence of Hg2+ in their respective assay buffers. However, we observed 30-40% depletion in activities of the above enzymes when the cell free lysate was incubated with increasing HgCl2 up to 250 μM. These results show that the bacterium exhibits acute Hg2+ regulatory mechanism to reduce cytosolic Hg2+ thereby, preventing its interaction with cellular enzymes. However, the cell free lysate from the K. pneumoniae isolate lacks this regulatory mechanism, resulting in binding of Hg2+ to the enzymes leading to their inhibition.
Discussion
Indiscriminate use of antibiotics in the sectors of health, food and animal husbandry are the primary sources of antibiotics in the sewage, soil and water bodies. Similar widespread release of heavy metals and their derivatives from different anthropogenic sources lead to their accumulation in the environment that is a leading cause for heavy metal tolerance in soil bacteria. Heavy metal tolerance in many bacteria correlates with their high antibiotic tolerance [27]. However, the cross talk between their heavy metal tolerance and antibiotic tolerance remains elusive. In order to investigate the effect of elevated environmental Hg2+ on antibiotic resistance in human pathogenic bacteria, we isolated a K. pneumoniae strain from soil samples of an industrial dump yard that was used to dump industrial wastes for more than 50 years. Our analysis led to identification of a novel K. pneumoniae strain (Figure 1). We investigated Hg2+ induced biochemical adaptation of the K. pneumoniae isolate by determining its Hg2+-dependent antibiotic tolerance and production of common enzymes. Our study shows that the bacterium exhibits a minimum inhibitory concentration of >250 μM HgCl2 in its growth medium. However, at 300 μM HgCl2 no growth was observed till 72 h (Figure 2). It is highly probable that our K. pneumoniae isolate could also resist other heavy metals as observed for many mercury tolerant bacterial strains.
Interestingly, we observed that increase in HgCl2 in growth medium led to enhanced resistance of the K. pneumoniae isolate to the cell wall-targeted antibiotic ampicillin (Figure 3). K. pneumoniae is known to exhibit resistance to penicillin antibiotics including ampicillin that inhibits [14]. Ampicillin binds irreversibly to the cell wall cross-linking enzyme transpeptidase, resulting in inhibition of cell wall biosynthesis. Our study is the first report showing that increased Hg2+ in growth medium increases tolerance of K. pneumoniae to ampicillin. A few reports show that there is a high correlation between heavy metal tolerance and antibiotic resistance in bacteria. This correlation is proposed to arise from sharing of genes between the molecular pathways leading to heavy metal tolerance and antibiotic tolerance in these bacteria [15,27]. This finding shows that not only indiscriminate use of antibiotics but also wide spread use of heavy metals and their unrestricted disposal into the environment is an important determinant contributing to antibiotic resistance in K. pneumoniae. One possible mechanism of Hg2+-induced ampicillin resistance in K. pneumoniae is secretion of penicillin degrading enzymes that decreases the effective concentration of ampicillin in the medium [28]. Other probable explanation being Hg2+-mediated covalent modification of transpeptidases and transglycosylases that are the targets of ampicillin.Ampicillintargeting sites of above proteins are proposed to be periplasmic and hence are easily accessed by exogenous Hg2+ [29]. As Hg2+ is a covalent modifier of cysteines, it could inhibit the binding of ampicillin to these enzymes leading to the observed ampicillin tolerance in the K. pneumonae isolate [30].
Alternatively, formation of EPS in K. pneumoniae reduces diffusion of ampicillin into the cells, hence, reducing its effective concentration in bacterial cells [14]. Hg2+ in growth medium is known to stimulate secretion of EPS and biofilm formation in many mercury tolerant bacteria as a protective mechanism [24]. Hence, we quantitated the EPS formation in our K. pneumoniae isolate in response to increased Hg2+ in growth medium. Our results show that the K. pneumoniae isolate increases production of EPS by the bacterium. 40% increase in EPS content was observed when the bacterium was grown in presence of 200-250 μM HgCl2 (Figure 4). EPS is negatively charged and acts as a trap to immobilize cationic Hg2+ in outside the cell, hence, preventing its entry into the cytoplasm [25]. Increased EPS is proposed to be a key regulator of bacterial resistance against a multitude of antimicrobials.
To evaluate the biotechnological potential of the Hg2+-tolerant K. pneumoniae strain, we quantitated production of five common industrially significant enzymes in the bacterium and investigated the effect of exogenous Hg2+ in growth medium on the activity of these enzymes. Our results show that the bacterium exhibits high potential to produce catalase, MerA, ALKP, α-amylase and lipase (Table 1). Exogenously added Hg2+ had no effect on activities of catalase, ALKP and lipase, indicating that the bacterium is adapted to high concentration of Hg2+ in its natural habitat and elevated Hg2+ in the growth medium doesn’t interfere with production or activity of above enzymes. However, activity of MerA was increased to 3 fold when the strain were subjected to 250 μM HgCl2 in growth medium, indicating that the bacterium produces more MerA to accelerate reduction of more toxic Hg2+ to Hg0, that is much less toxic to the cell [31]. Hg0 being volatile, evaporates out from the cell without making further damage to the cellular components. α-amylase shows 16% reduction in activity at 250 μM compared to cells grown in LB, showing that Hg2+ only marginally affects its production or activity.
In order to evaluate the sensitivity of above enzymes to Hg2+, we measured the effect of Hg2+ on their activities using cell free lysates. Our results show that there was a 30-40% depletion in activity of all five enzymes in presence of 250 μM HgCl2 in the assay buffer (Figure 5A and 5B). These results indicate that Hg2+ inhibits the activities of above enzymes, possibly, by covalent modification of sulfhydryl (-SH) groups of enzymes [12]. Further, in metalloenzymes, Hg2+ is known to displace the metal cofactor, leading to their inhibition [32]. Our results show that the enzymes exhibit sensitivity towards Hg2+, however, the bacterium possesses biochemical adaptation for acute regulation of Hg2+ in its cytoplasm.
Conclusion
In conclusion, we have isolated and biochemically characterized a novel K. pneumoniae strain that tolerates 250 μM HgCl2. Hg2+ induces ampicillin resistance in the bacterium that correlates with increased production of EPS in the cell envelope. The strain is an important bio-resource that exhibits high activities of industrially important enzymes such as catalase, MerA, ALKP, α-amylase and lipase. Presence of Hg2+ in growth medium doesn’t inhibit the activity of these enzymes showing that the novel K. pneumoniae isolate could be utilized for efficient and simultaneous bioremediation of environmental Hg2+ as well as production of industrially important enzymes.
Acknowledgement
We gratefully acknowledge Department of Science and Technology, Govt. of Odisha for funding the present work. H.G. Behuria acknowledges DST, Govt of India for providing Inspire fellowship.
References
- Tazisong IA, Senwo ZN, Williams MI (2012) Mercury speciation and effects on soil microbial activities. J Environ Sci Health A Tox Hazard Subst Environ Eng 47: 854-862.
- Chakraborty LB, Qureshi A, Vadenbo C, Hellweg S (2013) Anthropogenic mercury flows in India and impacts of emission controls. Environ Sci Technol 47: 8105-8113.
- Zeyaullah Md, Islam B, Ali A (2010) Isolation, identification and PCR amplification of merA gene from highly mercury polluted Yamuna River. Afr J Biotechnol 9: 3510-3515.
- Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways and effects. Environ Sci Technol 47: 4967-4983.
- Davis A, Bloom NS, Hee SSQ (1997) The environmental geochemistry and bioaccessibility of mercury in soils and sediments. Risk Analysis 17: 557-569.
- Perezdemora A, Burgos, Madejon (2006) Microbial community structure and function in a soil contaminated by heavy metals: effects of plant growth and different amendments. Soil Biol Biochem 38: 327-341.
- Valko M, Morris H, Cronin MTD (2005) Metals, toxicity, and oxidative stress. Curr Med Chem 12: 1161-1208.
- Xu FF, Imlay JA (2012) Silver (I), mercury (II), cadmium (II), and zinc (II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl Environ Microbiol 78: 3614-3621.
- Branco V, Caito S, Farina M, Teixeira da RJ (2017) Biomarkers of mercury toxicity: past, present, and future trends. J Toxicol Environ Health B Crit Rev 20: 119-154.
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5: 9-19.
- Freedman Z, Zhu C, Barkay T (2012) Mercury Resistance and Mercuric Reductase Activities and Expression among Chemotrophic Thermophilic Aquificae. Appl Environ Microbiol 78: 6568-6575.
- Djaman O, Outten FW, Imlay JA (2004) Repair of oxidized iron-sulfur clusters in Escherichia coli. J Biol Chem 279: 44590-44599.
- Ierardi V, Domenichini P, Reali S, Chiappara GM, Devoto G, et al. (2017) Klebsiella pneumoniae antibiotic resistance identified by atomic force microscopy. J Biosci 42: 623-636.
- Ander JF, Frankli MJ, Stewart PS (2000) Role of Antibiotic Penetration Limitation in Klebsiella pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob Agents Chemother 44: 1818-1824.
- Singh S, Singh SS, Chowdhury I, Singh R (2017) Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol J 11: 53-6.
- Timoney JF, Port J, Giles J, Spanier J (1978) Heavy-metal and antibiotic resistance in the bacterial flora of sediments of New York Bight. Appl Environ Microbiol 36: 465-72.
- Altschul SF, Madden SL, Schaffer AA, Zhang J (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389-3402.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Beers RF, Sizer IWA (1952) Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 795: 133-140.
- Fox B, Walsh CT (1982) “Mercuric reductase” purification and characterization of a transposonencoded flavoprotein containing an oxidation-reduction-active disulfide. J Biol Chem 257: 2498-2503.
- Assali FB, Baratti J, Michel GPF (1993) Purification and properties of a phosphate-irrepressible membrane-bound alkaline phosphatase from Zymumonas mubilis. J Gen Microbiol 139: 229-235.
- McCready RM, Guggolz J, Silviera V, Owens HS (1950) Determination of starch and amylose in vegetables. Anal Chem 22: 1156-1158.
- Kouker G, Jaeger KE (1987) Specific and sensitive plate assays for bacterial lipases. J Appl Environ Microbiol 53: 211-213.
- Ma L, Jackson KD, Landry RM, Parsek MR (2006) Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure post attachment. J Bacteriol 188: 8213-8221.
- Bonev B, Hooper J, Parisot J (2008) Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J of Antimic Chem 61: 1295-1301.
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406-425.
- Chen S, Li X, Sun G, Zhang Y (2015) Heavy metal induced antibiotic resistance in bacterium LSJC7. Int J Mol Sci 16: 23390-23404.
- Garcia-Fernandez A, Carattoli A (2010) Plasmid double locus sequence typing for IncHI2 plasmids, a subtyping scheme for the characterization of IncHI2 plasmids carrying extended-spectrum beta-lactamase and quinolone resistance genes. J Antimicrob Chemother 65: 1155-1161.
- Scheffers DJ, Pinho MG (2005) Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev 69: 585-607.
- Kohanski MA, Dwyer DJ, Collins JJ (2010) How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 8: 423-435.
- Fatimawali, Kepel B, Tallei TE (2015) Overproduction of mercuric reductase from mercury-resistant bacteria Klebsiella pneumonia isolate A1.1.1. Indonesian J Pharm 26: 141-146.
- Lemire JA, Harrison JJ, Turner JR (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11: 371-384.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences