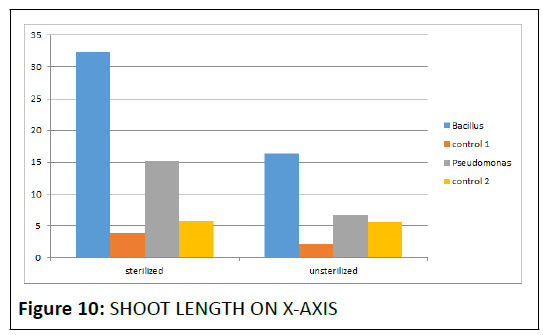ISSN : 2348-1927
Annals of Biological Sciences
Evaluation of Bacillus and Pseudomonas Isolates from Soil as Plant Probiotics
Ekta Sehgal*
Department of Food Science and Technology CCS HAU, Hisar, India
- *Corresponding Author:
- Ekta Sehgal
Department of Food Science and Technology, CCS HAUHisar,
India;
Email: aku7ekta@gmail.com
Received date: January 10, 2023, Manuscript No. ABS-23-11554; Editor assigned date: January 12, 2023, PreQC No. ABS-23-11554 (PQ); Reviewed date: January 26, 2023, QC No. ABS-23-11554; Revised date: February 09, 2023, Manuscript No. ABS-23-11554 (R); Published date: February 16, 2023, DOI: 10.36648/2348-1927.11.1.70
Citation: Sehgal E (2023) Evaluation of Bacillus and Pseudomonas Isolates from Soil. Ann Biol Sci Vol:11 No:1
Abstract
Preparations of live microorganisms (bacteria, fungi) utilized for improving plant growth and crop productivity are generally referred to as biofertilizers or microbial inoculants Plant Growth Promoting Rhizobacteria (PGPR) are free-living, soil-borne bacteria, isolated from the rhizosphere, which, when applied to seeds or crops, enhance the growth of the plant or reduce the damage from soil-borne plant pathogens.
Keywords
Rhizobacteria; Bacillus, Bacteria, Fungi
Introduction
Pathogenic microorganisms affecting plant health are major threat to food production, and traditional methods viz crop rotation, breeding for resistant plant cultivars, and application of chemical pesticides, seem to be insufficient to control root diseases to important crop plants. Further, it appears inevitable that fewer pesticides will be used in future and that greater reliance will be laid on biotechnological applications including use of microorganisms as antagonists. Therefore, there arises a need to find alternatives of chemicals used for disease control and improving plant growth. Bacillus spp. expresses antagonistic activities by suppressing the pathogens [1-5].
Sample collection, preparation and analysis
Soil sample was collected from a village named Amlapur, lying in the vicinity of Karnal, India. Soil samples were collected in L.D.P.E.and then brought for in vitro works.
Isolation of bacterial strains
Bacterial strains to be isolated were pre-decided on the basis of I.A.A. producing capacity of the microorganism’s i.e. Pseudomonas luorescens and Bacillus sub ilis. Isolation was achieved by serial dilution metho. For serial dilution 1 gm of soil was dissolved in 10 ml distilled water. Seven test tubes each with 9 ml distilled water were prepared. One ml from stock solution was aseptically transferredto first test tube making the dilution 10-1. Solution was then vortexed for proper mixing and 1 ml from this tube (10-1) was transferred aseptically to the next test tube making it as 10-2. Similar transfers were made till 10-9 dilution was achieved. From dilutions, 10-2 to 10-9, were spreaded to sterile Medium Agar plates,The plates were then kept for incubation at 37 degrees Celsius for 48-72 hrs. Streak plate method and spread plate technique was used for the isolation of bacterial colonies. Selective medias were used to recognize the desired microorganism.
Different media used for isolation of bacteria
King's B media: It is the selective media for the isolation of Pseudomonas fluorescens and durind pour plating it was used for the identification. After incubation, well separated individual colonies with green color pigments were marked and detected. The individual colonies were picked with sterile loop and transferred to fresh King B medium containing test tubes and the obtained pure cultures were stored in refrigeration at 4 degrees Celsius for further use.
Nutrient agar media: It was used for isolating Bcillus species using dilution. Rhizosphere soil sample was suspended in 99 ml of 0.85% normal saline (pH-7.0) and shaken vigorously at 150 r.p.m.at 18 degrees Celsius for 1 hr. The resulting slurry was serially diluted and then spread plating was performed on Nutrient Agar medium. The cultures obtained were incubated at 37 degrees Celsius for 2 days. For experimental use, isolates were transferred, when needed, to, Nutrient Agar medium stored at 4 degrees Celsius.
Morphological characterization of the obtained Isolates: Morphological study of the isolates was done on a solid media. To observe cellular morphology cells were observed under microscope by making smear of the purified colonies selected.
Colony characteristics: Colony characteristics of strain like Colony Size, Shape, Opticity, Margin and Motility were studied on nutrient agar plate [6-12].
Gram staining: A bacterial smear was prepared and heat fixed on slide, the smear was flooded with crystal violet for 30 sec. Followed by a gentle wash with tap water. Then flooded the smear with Gram’s iodine for 30 sec. And washed with tap water, kept the slide in slanting position and decolorized the stain with 90% ethyl alcohol by dropping the reagent drop-wise until the crystal violet fails to wash from the smear. Washed it with tap water and counter stain with safranin after 15 sec. And washed again with tap water and air-dried. The smear was examined under oil immersion in a light microscope. The Gram-positive bacterial cells appeared violet while Gram-negative cells turned pink tored [13-19].
Biochemical tests
Pseudomonas fluorescens
Catalase test: A bacterial colony was pickedfrom the plate and transferred on the glass slide in a drop of water. Few drops of H2O2 (dilute 30% commercial solution 1:10) was placed over the culture. Evolution of gas bubbles indicated the positive result.
Starch Hydrolysis: Nutrient gar medium was supplemented with 2% soluble starch. The isolated strains were streaked on the solidified starch agar plate and incubated at 37 degrees celsiusfor 24- 48 hrs. As growth appeared in the plates, iodine solution was flooded and clear zones against blue-black ground were observed [20-26].
Fluorescent pigment test: The test tubes containing sterilized Kings B medium were inoculated with the isolate of Pseudomonas species incubated for 5 days and observed. Yellowish green pigment observed under UV light (365 nm) indicated positive results.
Phosphate solubilisation: The plates were preparedwith Pikovskaya’s agar plate and phosphate solubilising activity was estimated after 4 days of incubation at 28 degrees Celsius. Phosphate solubilization activity was determined by the development of the clear zone around the bacterial colonies.
Methyl red and voges–proskauer test: Four MRVP broth test tubes were taken, three tubes (2 sets) were inoculated with P6,P7,P8 isolates and one as control respectively. All the tubes were incubated at 37 degrees Celsius for 48 hrs. And 5 drops of methyl red indicator were added into the inoculated tubes and observed for the color changes. To the control and second set of tubed added, 10 drops of VP-1 and 2-3 drops of VP-2 reagent. Observed for the color change.
Indole production: The Tryptone Broth was inoculated with bacterial strain and incubated at 28 degrees Celsius for 48 hrs. Kovac, s reagent (1 ml) was addedto each test tube including the control. Appearance of a cherry (deep red) coloured layer on the top of the tubes was taken positive test for the Indole production [27-32].
Biochemical tests
Bacillus subtilis
Catalase test: A bacterial colony was pickedfrom the plate and transferred on the glass slide in a drop of water. Few drops of H2O2 (dilute 30% commercial solution 1:10) was placed over the culture. Evolution of gas bubbles indicated the positive result.
Starch hydrolysis: Nutrient Agar medium was supplemented with 2% soluble starch. The isolated strains were streaked on the solidified starch agar plate and incubated at 37 degrees celsiusfor 24-48 hrs. As growth appeared in the plates, iodine solution was flooded and clear zones against blue-black ground were observed [33-39].
Phosphate solubilisation: The plates were preparedwith Pikovskaya’s agar plate and phosphate solubilising activity was estimated after 4 days of incubation at 28 degrees Celsius. Phosphate solubilization activity was determined by the development of the clear zone around the bacterial colonies.
Methyl red and Voges–Proskauer test: Four MRVP broth test tubes were taken, three tubes (2 sets) were inoculated with P6,P7,P8 isolates and one as control respectively. All the tubes were incubated at 37 degrees Celsius for 48 hrs. And 5 drops of methyl red indicator were added into the inoculated tubes and observed for the color changes. To the control and second set of tubed added, 10 drops of VP-1 and 2-3 drops of VP-2 reagent. Observed for the color change [40-48].
Indole production: The Tryptone Broth was inoculated with bacterial strain and incubated at 28 degrees Celsius for 48 hrs. Kovac,s reagent (1 ml) was addedto each test tube including the control. Appearance of a cherry (deep red) coloured layer on the top of the tubes was taken positive test for the Indole production.
Result and Discussions
6 strains were isolated from soil samole from U.P and they were identified by morphological and biochemical characteristics.
Morphological and biochemical characterization of isolates
The following morphological and biochemical tests were performed for the identification of the isolates.
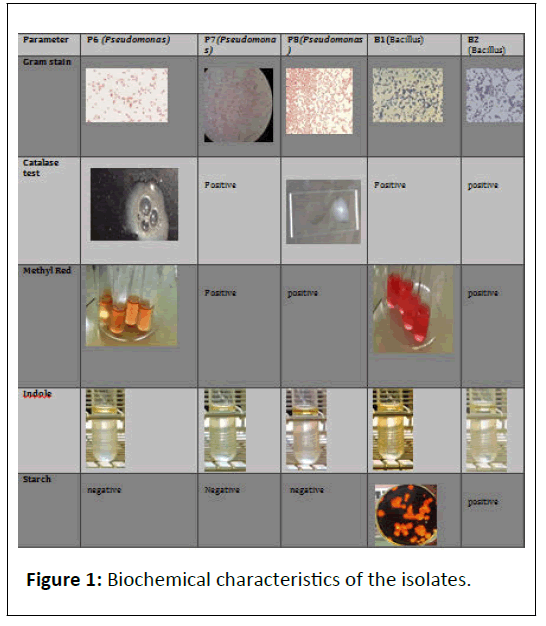
Gram staining: It is a method of differentiating the bacterial species into 2 large groups i.e. Gram positive (which retain the color of crystal violet) and Gram-negative (red or pink colored). Basis on the color obtained in the electron microscope, they are termed as Gram–negative or positive.
Catalase test: Catalase is an enzyme which prevents the cell from oxidative damage by reactive oxygen species like hydrogen peroxides. The presence of bubbles on reaction of bacterial species with hydrogen peroxide proves the test positive, otherwise negative [49-55].
Fluorescent pigment test: The test was done to check the fluoresce obtained under UV light study. The greenish yellow fluor was obtained in case of Pseudomonas fluorescens
Indole test: The test was performed to determine the ability of an organism to convert tryptophan (an amino acid) into indole. The test was negativefor both the strains.
MR-VP test: The test gives a good identification of microbial respiration and also confirms the differences between E.coli and Enterobacter. The positivity of test that organism is aerobic in nature [56-59].
Phosphate solubilisation: This test explains the ability of organisms to convert insoluble phosphates into soluble phosphates. More the phosphate solubilisation, more the ability of the organism towards higher yielding of growth promoting hormones for plants.
On the basis of Gram staining, 3 isolates (P1,P2,P3) were found to be Gram negative rods. Their cultural characteristics showed that the 3 isolates produced circular, convex and smooth colonies (Table 1).
| Isolates | Cell shape | Colony type | Colony colour | Type of growth |
|---|---|---|---|---|
| P1 | Rod | Round | Greenish yellow | Moderate |
| P2 | Rod | Round | Greenish yellow | Fast |
| P3 | Rod | Round | Greenish yellow | Moderate |
Table 1: Cultural characteristics of different isolates of Pseudomonas fluorescens.
The results of the biochemical tests (Table 2 and 3) performed for the identification of the isolates showed that P1,P2 and P3 isolates produced similar results with regard to gram staining (negative), indole (negative), MR (positive), VP ( positive), catalase test (positive), and fluorescent pigmentation (positive). The morphological and biochemical tests confirmed the isolates to be P. fluorescens as reported by earlier workers [61-64].
| Isolates | Cell shape | Colony type | Colony color | Type of growth |
|---|---|---|---|---|
| B1 | Rod | Round | Creamish white | Fast |
| B2 | Rod | Round | Creamish white | Moderate |
| B3 | Rod | Round | Creamish white | moderate |
Table 2: Cultural characteristics of different isolates of Bacillus subtilis.
| Parameters | P6 | P7 | P8 | B1 | B2 | B3 |
|---|---|---|---|---|---|---|
| Gram staining | Negative | negative | negative | Positive | positive | Positive |
| Catalase test | + | + | + | + | + | + |
| Fluorescent pigment | + | + | + | - | - | - |
| Indole | - | - | - | - | - | - |
| MR-VP | + | + | + | + | + | + |
| Phosphate solubilization | + | + | + | + | + | + |
Table 3: Biochemical characteristics of the isolates.
| Days | Bacillus control | Bacillus | Pseudomonas control | Pseudomonas |
|---|---|---|---|---|
| Nov 19, 2014 (day 1) | 0 cm | 1.7 cm | 1.0 cm | 2.5 cm |
| Nov 20, 2014 (day 2) | 0 cm | 2.8 cm | 1.3 cm | 3.2 cm |
| Nov 21, 2014 (day 3) | 0.5 cm | 6.5 cm | 1.5 cm | 4.5 cm |
| Nov 22, 2014 (day 4) | 1.0 cm | 9.0 cm | 3.2 cm | 4.7 cm |
| Nov 23, 2014 (day 5) | 1.5 cm | 11.0 cm | 4.3 cm | 7.0 cm |
| Nov 24, 2014 (day 6) | 2.0 cm | 12.1 cm | 4.6 cm | 7.8 cm |
| Nov 26, 2014 (day 7) | 2.5 cm | 13.0 cm | 4.9 cm | 9.1 cm |
| Nov 27, 2014 (day 8) | 3.0 cm | 13.2 cm | 5.2 cm | 10.2 cm |
| Nov 28, 2014 (day 9) | 3.2 cm | 14.8 cm | 5.3 cm | 10.5 cm |
| Nov 29, 2014 (day 10) | 3.5 cm | 14.9 cm | 5.3 cm | 10.7 cm |
| Nov 30, 2014 (day 11) | 3.6 cm | 15.7 cm | 5.4 cm | 10.8 cm |
| Dec 1, 2014 (day 12) | 3.7 cm | 20.7 cm | 5.5 cm | 10.9 cm |
| Dec 2, 2014 (day 13) | 3.8 cm | 25.7 cm | 5.6 cm | 12.2 cm |
| Dec 3, 2014 (day 14) | 3.8 cm | 27.2 cm | 5.7 cm | 13.0 cm |
| Dec 4, 2014 (day 15) | 3.9 cm | 32.3 cm | 5.8 cm | 15.2 cm |
Table 4: Shoot length (centimeters).
According to Todar (2004), more than half of the Pseudomonas bacteria produce pyocyanin which is a blue-green pigment, while the nonpathogenic saprophyte P. fluorescens produces fluorescent pigment that is soluble and greenish. In this study, all the three identified gram-negative Pseudomonas fluorescence isolates produced greenish yellow pigment on King’s B media. This further confirmed the isolates to be P. fluorescens. All the strains showed moderate as well as fast growth [65-68].
All the isolates showed positive results in phosphate solublization and IAA production.
The Pseudomonas spp. strains from rhizosphere of different crops were isolated and extensively studied by characterized Pseudomonas fluorescens on the basis of morphological, physiological and biochemical tests also isolated Pseudomonas strains from wheat rhizoshere and characterized by cultural and biochemical characteristics like production of IAA, catalase, phophatase enzymes etc [69,70].
The other 3 selected soil isolates B1,B2 and B3 were Gram positive bacilli arranged in chains. The colonies were round and creamish white showing fast to moderate growth Table 2). The results of the biochemical tests performed for the identification of the isolates showed that B1,B2 and B3 isolates produced similar results with regard to gram staining (positive), indole (negative), MR (positive), VP ( positive), catalase test (positive), and white pigmentation [71].
Pseudomonas: P.fluorescens is a common gram-negative, rodshaped bacterium. P.fluorescens has multiple flagella. It is an obligate aerobe, but certain strains are capable of using nitrate instead of oxygen as a final electron acceptor during cellular respiration. Optimal temperatures for growth of Pseudomonas fluorescens are 25-30 degrees Celsius.
Bacillus: Bacillus is a genus of gram-positive, rod-shaped, bacteria and a member of the phylum firmicutes. Bacillus species can be obligate aerobes, or facultative anaerobes. They will test positive for the enzyme catalase when there has been oxygen used or present.
Fluorescent Pseudomonas has been recognized as biocontrol agents against certain soil-borne plant pathogens. They produce yellow-green pigments (PYOVERDINES) which fluoresce under UV light and function as siderophores. They deprive pathogens of the iron required for their growth and pathogenesis (Figure 1).
It is not clear exactly how the plant growth-promoting properties of Pseudomonas fluorescens are achieved; theories include:
• The bacteria might include systematic resistance in the host plant, so it can better resist attack by a true pathogen.
• The bacteria might outcompete other (pathogenic) soil microbes, e.g., by siderophores, giving a competitive advantage at scavenging for iron.
• The bacteria might produce compounds antagonistic to other soil microbes, such as phenazine-type antibiotics or hydrogen cyanide.
• They produce the secondary metabolite 2,4- diacetylphloroglucinol(2,4-DAPG), the compound found to be responsible for anti-phytopathogenic and bio-control properties in these strains.
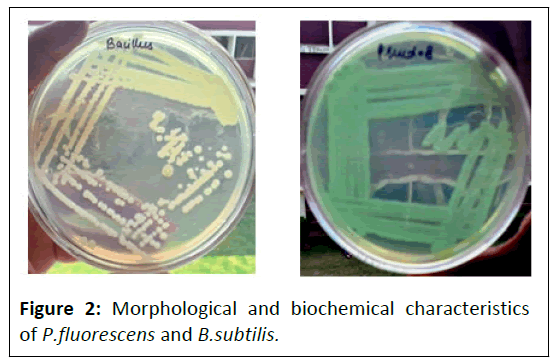
In the present investigation with PGPR such as P. fluorescence and B.subtilis and it was observed that the seed germination seedling vigor, protein and carbohydrate contentincreased significantly. (1999) reported that P.fluorescence was increasing seed germination, vigor index and field emergence (Figure 2).
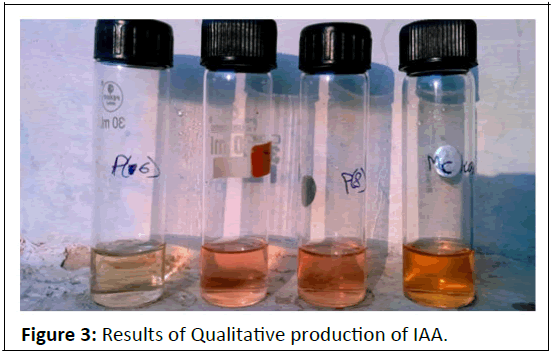
Qualitative analysis of Indole-acetic-acid (IAA production by the isolates: One of the principal mechanisms of promoting plant growth is related to the capability of the microorganisms to produce plant growth promoting substances. All the 6 isolates were tested for qualitative estimation of IAA. Among 6,4 isolates were found to be positive for IAA production. The results showed that Bacillus subtilis strains produced more pink color on addition of salkowski reagent than Pseudomonas fluorescens strains.
Effect o f B acillus subtilis an d Ps eudomonas fluorescens on chickpea plant growth
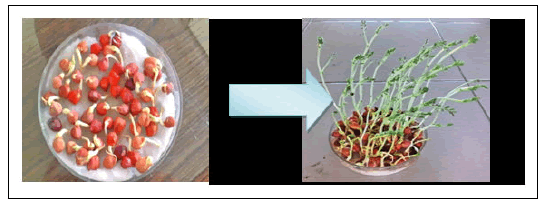
Among the 4 positive IAA strains were selected to study their PGPR properties. Chickpea plants (Cicer arientum L.) were inoculated with the bacterial cultures. After 15 days of planting, effect of Bacillus subtilis and Pseudomonas fluorescens culture supernatants on shoot length was determined. Both sterilized and unsterilized batches were used. The sterilized batch means that the soil was sterilized so as to show the impact of only the inoculated PGPR strains (Figure 3).
Shoot length (centimeters): The table gives the details of the growth obtained when the obtained strains were used to improve the shoot length of chickpea plant.Likewise; pictures have been attached from Day 0 to Day 15. After 15 days, it was seen that the plant where Bacillus was used as PGPR had reached nearly 32.3 cm whereas the plant where Pseudomonas was used as PGPR had growth about 15.2 cmwhile their control reached 3.9 and 5.8 cm, respectively, thus proving the significance and the necessity of PGPR.
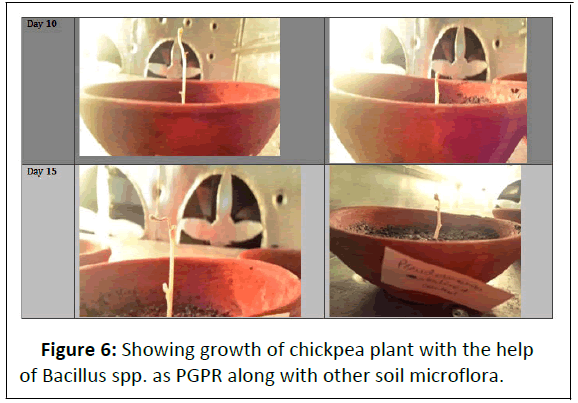
Pictorial representation of plant growth for 15 days. Figure 4 showing growth of chickpea plant with the help of bacillus spp. as PGPR

Unsterilized batch: This means that the soil was having whole lot of micro lora including the obtained strains (Figure 4).
Shoot length (cm): It proved that the soil in which Bacillus was added as PGPR again showed improved growth (16.4 cm) and the plant where Pseudomonas was added as PGPR also improved growth to nearly 6.6 cm which was applaudable on comparison with their controls i.e.2.2 cm and 5.5 cm, respectively (Figure 5 and 6).
In all the test plant species, both the inoculants enhanced growth that was comparatively greater than control, Among the inoculants, difference was not that much statistically significant, although the highest mean shoot length for chickpea plant treated with Bacillus spp. was highly greater than that of Pseudomonas fluorescens as inferred from the obtained graph which is as follows.
Graphical representation of shoot length growth over 15 days.
Discussion
The objective of this project was to isolate bacterial strains of bacillus species and P.fluorescens to show their PGPR activity on chickpea plant’s shoot length and finally on the root length. The two isolated strains possessed multiple PGP traits such as catalase production, phosphate solubilization, production of IAA like compounds and also antagonism to soil borne pathogens. (Figure 7)
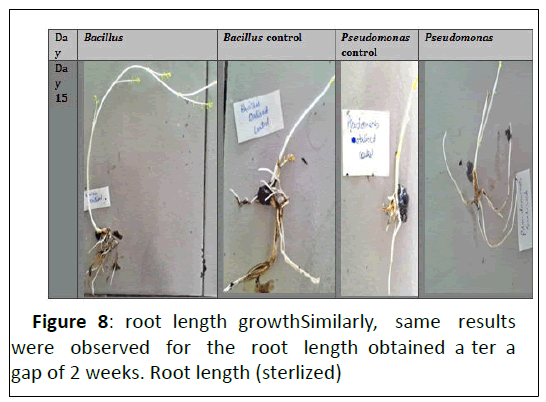
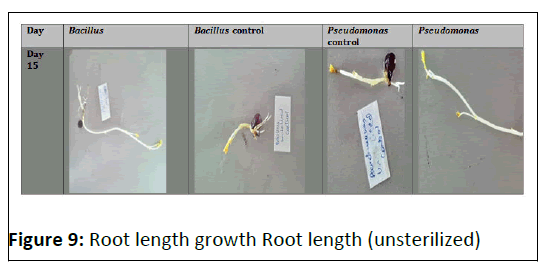
Coming to bacillus species identification which was obtained as flat, dull, dry, creamish-white lobate colonies with optimal growth temperature range of 27-35 degrees Celsius (T1). While P.fluorescens was identified as round, rod- shaped, greenish yellow colonies with optimal growth temperatures ranging from 25-30 degree Celsius (T2). (Figure 8),(Figure 9)
Biochemical tests included:
• Gram staining: out of the isolated bacteria P.fluorescens was gram negative and bacillus was gram positive.
• Catalase test where bubble formation occurred in case of P.fluorescens while null was observed in case of bacillus species. This indicates that P.fluorescens is capable of breaking down H2O2 into CO2 and O2. This is done by it because of its property to produce catalse. Whereas bacillus spp. did not produce catalase enzyme.
• P.fluorescens as per its name gave a greenish yellow fluor under UV light study.
• Indole test is a biochemical test performed on bscterial species to determine the ability of the organism to convert tryptophan into indole. This division is performed by a achain of a number of different intracellular enzymes, a system gemerally referred as “tryptophanase”. Indole is generated by reductive deamination form tyrptophan the intermediate molecule indolepyruvic acid. Tryptophanase catalyzes the deamination reaction, during which the amine (–NH2 ) group of the tryptophan molecule is removed. Final products of the reaction are indole, pyruvic acid, ammonium and energy. Pyridoxal phosphate is required as acoenzyme. the test was negative for both the strains indicating that they did not produce indole.
• MRVP was positive for both the strains proving that they are aerobic in nature. The methyl red test detects production of acid formed during metabolism using mixed acid fermentation pathway using pyruvate as asubstrate. The pH indicator methyl red is added to one tube and a red color appears at pH’s lower than 4.2 indicating a positive test. An dthe solution remaining yellow (pH=6.2 or above) indicates a negative test. The VP test uses alpha-naphthol and potassium hydroxide to test for the presence of acetymethylcarbinol (acetoin), an intermediate of the 2,3-butanediol fermentation pathway, afteradding both reagents, the tube was shaken vigorously then allowed to sit for 5-10 mins. A pinkish –red color indicates oa positive test meaning the 2, 3-butanediol fermentation pathway is used.
•Phosphate solubilization indicated that t he organisms were able to convert insoluble phosphates into soluble phosphates (T4) gives the practical view of these in-vitro tests.
• The graphical representation indicates that the sterilized batch showed better s hoot a nd root growth t han t he unsterlised batch. Therefore sterilized batch was taken into consideration in this bacillus spp. provided growth upto 2.5 cm and pseudomonas spp. upto 15.2 cm (Figure 6).
Conclusion
Our results confirm growth promotion by one representative each of both the species of bacteria.(Bacillus spp. and Pseudomonas fluorescens)but only, a little variations were observed in the bacterial effectiveness among the parameters (like shoot length and root length ) and plant type.Considering, shoot length, majorly, there were significant differences, between the performances of P. fluorescens and Bacillus spp. Also, based on the results, among the representative organisms of the two genera in this study Bacillus is a better PGPR Pseudomonas. Keeping in view, our work we recommend further comparative studies of the PGPR properties between many representatives of the two genera. (Figure 10).
References
- AAltschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. (1997) "GAPPED BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs". Nucleic Acids Res 25:3389– 3402
- Baakza A, Vala AK, Dave BP, Dube HC (2004) "A Comparative Study of Siderophores by Fungi from Marine and Terrestrial Habitats". J Exp Mar Biol Ecol 311: 1-93
- Bakker AW, Schipper B (1987)"Microbial Cyanide Production in the Rhizosphere in Relation to Potato Yield Reduction and Pseudomonas Spp. Mediated Plant Growth Stimulation". Soil Biol Biochem 19: 451–457
- Barea JM, Pozo M J, Azcon R, Azcon Aguilar C (2005) "Microbial Co-Operation in the Rhizosphere". JExp Bot 56: 1761–1778
[Crossresff] [Googlescholar] [Pubmed]
- Baruah TC, Barthakur HP, Basak BB, Biswas DR (2010) A Textbook of Soil Analysis, Vikas Publishing House Pvt. Ltd., New Delhi. "Co- Inoculation of Potassium Solubilizing and Nitrogen Fixing Bacteria on Solubilization of Waste Mica and Their Effect on Growth Promotion and Nutrient Acquisition by a Forage Crop," Biol Fertil Soils 46: 641-648
- Bashan Y, Holguin G (1998) "Proposal for the Division of Plant Growth-Promoting Rhizobacteria into Two Classifications: Biocontrol-PGPB (Plant Growth-Promoting Other
- Berg G (2009) "Plant–Microbe Interactions Promoting Plant Growth and Health: Rhizobacteria) and PGPB," Soil Biol Biochem 30:1225-1228
[Crossresff] [Googlescholar] [Pubmed]
- Bazzicalupo M, Fani R (1995) "The Use of RAPD for Generating Specific DNA Probes for Microorganisms," In: Clap JP (Eds) Methods in Molecular Biology, Species Diagonistic Protocols: PCR and Perspectives for Controlled Use of Microorganisms in Agriculture," Appl Microbiol Biotechnol 84:11–18
[Crossresff] [Googlescholar] [Pubmed]
- Bevivino A, Sarrocco S, Daimastri C,Tabacchioni S, Cantale C, et al. (1998)"Characterization of a Free-Living Maize-Rhizosphere Population of Burkholderia Cepacia: Effect of SeedTreatment on Disease Suppression and Growth Promotion of Maize,"FEMS Microbiol Ecol 27:225–237
- Bhatia VS, Singh P, Wani SP, Chauhan GS, Kesava Rao, et al. (2008) "Analysis of Potential Yields and Yield Gaps of Rainfed Soybean in India Using CROPGRO-Soybean Model". Agric For Meteorol 148:1252–1265
- Bradford MA (1976) "A Rapid andSensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding". Ann Clin Biochem 72:248-254
[Crossresff] [Pubmed]
- Caravaca F, Figueroa D, Roldan A, Azcon-Aguilar C (2003) "Alteration in the Rhizosphere Soil Properties of Afforested Rhamnus Lycioides Seedlings in Short-Term Response to Inoculation with Glomus Intraradices and Organic Amendment". Environ Manage 31: 412–420
- Correa OS, Montecchia MS, Berti MF, Fernandez Ferrari MC, Pucheu NL, et al. (2009) "Bacillus amyloliquefaciens BNM122, a Potential Microbial Biocontrol Agent Applied on Seeds, Cause a Minor Impact onRhizosphere and Soil Microbial Communities". Applied Soil Ecology 41:185-194
- Dastager SG, Deepa CK, Pandey A (2011) "Plant Growth Promoting Potentialof Pontibacter Niistensis in Cowpea (VignaUnguiculata (L.) Walp.)". Appl Soil Ecol 49:250-255
- Defago G, Berling CH, Burger U, Haas D, Kahr G (1990) 'Suppression of BlackRoot of Tobacco by a Pseudomonas Strain:Potential Application and Mechanisms,'(Eds) Biocontrol of Soil-Borne Plant Pathogens.CAB International, Oxon, UK 93-108
- Dupare B U, Billore S D, Joshi OP (2010) "Farmers' Problems Associated with Cultivation of Soybean in Madhya Pradesh, India". J Agric Sci Technol 4:71-78
- Dworkin M, Foster JW (1958) "Experiment with Some Microorganisms Which Utilize Ethane and Hydrogen". J Bacteriol 75: 529-601
[Crossresff] [Googlescholar] [Pubmed]
- Erturk Y, Ercisli S, Haznedar A, Cakmakci R (2010) "Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Rooting and Root Growth of Kiwifruit (Actinidia Deliciosa) Stem Cutting". Biol Res 43: 91-98
[Crossresff] [Googlescholar] [Pubmed]
- Fasim F, Ahmed N, Parsons R, Gadd GM (2002) "Solubilization of Zinc Salts by a Bacterium Isolated from the Air Environment of a Tannery". FEMS Microbiol Lett 213:1-6
[Crossresff] [Googlescholar] [Pubmed]
- Fernandez L, Agaras B, Zalba P, Wall L G, Valverde C, et al. (2012) "Pseudomonas Spp Isolates with High Phosphate-Mobilizing Potential and Root Colonization Properties from Agricultural Bulk Soils under No-Till Management". Biol Fertil Soils 48:763-773
- Glick B R (1995) "The Enhancement of Plant Growth by Free Living Bacteria". Can J Microbiol 41: 109- 117
- Gordon SA, Weber R P (1951) "Colorimetric Estimation of Indole Acetic Acid," Plant Physiol 26:192-195
[Crossresff] [Googlescholar] [Pubmed]
- Gulati HK, Chadha BS, Saini HS (2007) "Production and Characterization of Thermostable Alkaline Phytase and Bacillus Laevolacticus Isolated from Rhizosphere Soil". World J Microbiol Biotechnol 34:91-98
- Hameeda B, Rupela OP, Reddy G, Satyavani K (2006) "Application of Plant Growth-Promoting Bacteria Associated with Composts and Macrofauna for Growth Promotion of Pearl Millet (Pennisetum Glaucum L)". Biol Fertil Soils 43:221-227
- Hayat R, Ali S, Amara U, Khalid R, Ahmed I, et al. (2010) "Soil Beneficial Bacteria And Their Role in Plant Growth Promotion: A Review". Ann Microbio 60: 579–598
- Helman Y, Burdman S, Okon Y, Rosenberg E, Gophna U, et al. (2011)"Plant Growth Promotion by Rhizosphere Bacteria through Direct Effects," In: (Eds) Beneficial Microorganisms in Multicellular Life Form. Heidelberg: Springer 89–103
- Holt JG, Kreig NR, Sneath PHA, Stanley JT, Williams ST, et al. (1994) 'Bergey's Manual of Determinative Bacteriology,' Williams,Wilkins, Baltimore, MD
- Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Growth promotion of Soil Science Society. Soil Sci Soc Am J 63: 1670-1680
- Chuang CC, Kuo YL, Chao CC, Chao WL (2007)"Solubilization of InorganicPhosphates and Plant Growth by Aspergillus Niger". Biol Fertil 43: 575-584
- Borriss R (2002) "Extracellular Phytase Activity of Bacillus amyloliquefaciens FZB45 Contributes to Its Plant Growth-Promoting Effect". Micro biol 148: 2097–2109
[Crossresff] [Googlescholar] [Pubmed]
- Idriss EE, Iglesias DJ, Talon M, Borriss R (2007) "Tryptophan Dependent Production of Indole-3-Acetic Acid (IAA) Affects Level of Plant Growth Promotion by Bacillus amyloliquefaciens FZB42". Mol Plant-Microbe Interact 19:619–626
[Crossresff] [Googlescholar] [Pubmed]
- Jacobson CB, Pasternak JJ, Glick B R (1994) "Partial Purification and Characterization of 1-Aminocyclopropane- 1-Carboxylate Deaminase from the Plant Growth Promoting Rhizobacterium Pseudomonas Putida GR12-2". Can J Microbiol 40:1019–1025
- Jakobsen I, Leggett ME, Richardson AE (2005) Rhizosphere microorganisms and plant phosphorus uptake. Phosphorus: agriculture and the environment 46:437-94
- Joergensen RG, Emmerling C (2006) "Methods for Evaluating Human Impact on Soil Microorganisms Based on Their Activity, Biomass, and Diversity in Agricultural Soils". J Plant Nutr Soil Sci 169: 295–309
- Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg BJJ (2006) "Effects of the Tomato Pathogen Fusarium Oxysporum F. Sp. Radicis-Lycopersici and of the Biocontrol Bacterium Pseudomonas Fluorescens WCS365 on the Composition of Organic Acids and Sugars in Tomato Root Exudates". Mol Plant-Microbe Interact19:1121–1126
[Crossresff] [Googlescholar] [Pubmed]
- Kerovuo J, Lauraeus M, Nurminen P, Kallckinen N, Apajalahti J (1998) "Isolation, Characterization, Molecular Gene Cloning, and Sequencing of a Novel Phytase from Bacillus Subtilis". Appl Environ Microbiol 64: 2079–2086
[Crossresff] [Googlescholar] [Pubmed]
- Khalid A, Arshad M, Zahir ZA (2004) "Screening Plant Growth-Promoting Rhizobacteria for Improving Growth and Yield of Wheat". J Appl Microbiol 96:473–480
[Crossresff] [Googlescholar] [Pubmed]
- Kloepper J W, Liftshitz R, Zablotowitcz R M (1989) "Free-Living Bacterial Inocula for Enhancing Crop Productivity". Trends in Biotechnology 7: 39–43
- Kohler J, Caravaca F, Carrasco L, Roldan A (2007) "Interactions between a Plant Growth-Promoting Rhizobacterium, an AM Fungus and a Phosphate-Solubilising Fungus in the Rhizosphere of Lactuca Sativa". App Soil Ecol 35: 480–487
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC, et al. (1992) 'Color Atlas and Textbook of Diagnostic Microbiology,' 4th Edn. J.B. Lippincott Company, Philadelphia
- Koumoutsi A, Chen XH, Henne A,Liesegang H, Hitzeroth GF,et al (2004)"Structural and Functional Characterization of Gene Clusters Directing Nonribosomal Synthesis of Bioactive Cyclic Lipopeptides in Bacillus amyloliquefaciens Strain FZB42," J Bacteriol, 186:1084–1096
- Krebs B, Hoding B, Kubart S, Workie M A, Junge H, et al. (1998) "Use of Bacillus Subtilis as Biocontrol Agent, I. Activities and Characterization of Bacillus Subtilis Strains". J Plant Dis Prot, 105: 181–197
- Kunitsky C, Osterhout G, Sasser M (2006) "Identification of Microorganisms Using Fatty Acid Methyl Ester (FAME) Analysis and MIDI Sherlock Microbial Identification System". Encyclopedia of Rapid Microbiological Methods, 3:1-18
- Mader P, Kiser F, Adholeya A, Singh R, Uppal HS, et al (2010) "Inoculation of Root Microorganisms for Sustainable Wheat-Rice and Wheat-Blackgram Rotations in India," Soil Biology and Biochemistry. J Virol 43:609-619
- Marschner P, Crowley D, Rengel Z (2011)"Rhizosphere Interactions between Microorganisms and Plants Govern Iron and Phosphorus Acquisition along the Root Axis E Model and Research Methods". Soil Biol. Bioche 43:883-894
- Mullis KB, Pollack JR, Neilands JB(1971) Structure of schizokinen, an iron-transport compound from Bacillus megaterium. Biochemistry10:4894-4898
[Crossresff] [Googlescholar] [Pubmed]
- Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, et al. (2009) "Phosphate Solubilizing Microorganisms Isolated from Rhizosphere of Maize Cultivated in an Oxisol of the Brazilian Cerrado Biome". Soil Biol Biochem 41:1782-1787
- Payne S M (1994). "Detection, Isolation and Characterization of Siderophores". Method Enzymol 235:329-344
[Crossresff] [Googlescholar] [Pubmed]
- Perez-Miranda S, Cabirol N, George-Tellez R, Zamudio-Rivera L S Fernandez FJ,et al.(2007) "O-CAS: A Fast and Universal Method for Siderophore Detection," J Microbiol Methods, 70:127-131
[Crossresff] [Googlescholar] [Pubmed]
- Prathuangwong S, Buensanteai N (2007) "Bacillus amyloliquefaciens Induced Systemic Resistance against Bacterial Pustule Pathogen with Increased Phenols, Peroxides, and 1, 3-s-Glucanase in Soybean Plant," Acta Phytopathol Entomol Hung 42:321-330
- Puente M E, Li C Y, Bashan Y (2004) "Microbial Populations and Activities in the Rhizoplane of Rock-Weathering Desert Plants. II. Growth Promotion of Cactus Seedlings". Plant Biology 6:643-650
[Crossresff] [Googlescholar] [Pubmed]
- Ramesh A, Sharma SK, Joshi OP, Khan IR (2011) "Phytase, Phosphatase Activity and P-Nutrition of Soybean as Influenced by Inoculation of Bacillus". Indian J Microbiol 51: 94-99
[Crossresff] [Googlescholar] [Pubmed]
- Ramirez CA, Kloepper JW (2010) "Plant Growth Promotion by Bacillus amyloliquefaciens FZB45 Depends on Inoculam Rate and P-Related Soil Properties". Biol Fertil 46:835-844
- Rana A, Joshi M, Prasanna R, Shivay Y S, Nain L, et al.(2012) "Biofortification of Wheat through Inoculation of Plant Growth Promoting Rhizobacteria and Cyanobacteria". Eur J Soil Biol 50:118-126
- Reddy GSN, Uttam A, Shivaji S (2008)"Bacillus Cecembensis Sp. Nov, Isolated from the Pindari Glacier of the Indian Himalayas". Int J Syst Evol 58: 2330-2335
[Crossresff] [Googlescholar] [Pubmed]
- Reva ON, Dixelius C, Meijer J, Priest FG (2004) "Taxonomic Characterization and Plant Coloninzing Abilities of Some Bacteria Related to Bacillus amyloliquefaciens and Bacillus Subtilis". FEMS Microbiol Ecol 48: 249-259
[Crossresff] [Googlescholar] [Pubmed]
- Riberio CM, Cardoso EJBN (2012) "Isolation and Characterization of Root-Associated Growth Promoting Bactertia in Brazil Pine (Araucaria Angustifolia)". Microbiological Research 167: 69-78
[Crossresff] [Googlescholar] [Pubmed]
- Rosas SB, Avanzini G, Carlier E, Pasluosta C, Pastor N, et al. (2009) "Root Colonization and Growth Promotion of Wheat and Maize by Pseudomonas Aurantiaca SR1". Soil Biol Biochem 41: 1802-1806
- Saini VK, Bhandari SC, Tarafdar JC (2004) "Comparison of Crop Yield, Soil Microbial C, N and P, N-Fixation, Nodulation and Mycorrhizal Infection in Inoculated andNon-Inoculated Sorghum and ChickpeaCrops". Field Crops Research 89: 39-47
- Saravanan VS, Madhaiyan M, Thangaraju M (2007) "Solubilization of Zinc Compounds by the Diazotrophic, Plant Growth Promoting bacterium Gluconacetobacter Diazotrophicus”. Chemosphere 66:1794-1798
[Crossresff] [Googlescholar] [Pubmed]
- Sarwar M, Arshad M, Martens D A, Frankenberger W T Jr (1992) "Tryptophan-Dependent Biosynthesis of Auxins in Soil". Plant and Soil 147:207-215
- Schwyn, B, Neilands J B (1987). "Universal Chemical Assay for the Detection and Determination of Siderophores". Ann Clin Biochem 160:47-56
[Crossresff] [Googlescholar] [Pubmed]
- Sharma SK, Johri BN, Ramesh A, Joshi OP,Sai Prasad SV (2011) "Selection of Plant Growth-Promoting Pseudomonas Spp. That Enhanced Productivity of Soybean- Wheat Cropping System in Central India". J Microbiol Biotechnol 21:1127-1142
[Crossresff] [Googlescholar] [Pubmed]
- Sharma SK, Sharma MP, Ramesh A, Joshi OP (2012) "Characterization of Zinc-Solubilizing Bacillus Isolates and Their Potential to Influence Zinc Assimilation in Soybean Seeds". J Microbiol Biotechnol 22: 352-359
[Crossresff] [Googlescholar] [Pubmed]
- Sharma SK, Ramesh A, Sharma MP, Joshi OP, Govaerts B (2010) "Microbial Community Structure and Diversity as Indicators for Evaluating Soil Quality," In: Eric Lichtfouse (Eds) Biodiversity, Biofuel, Agroforestry and Conservation Agriculture. Sustainable Agriculture Review 5: 317-358
- Sneath PHA, Mair NS, Sharphe ME, Holt JG (1986) Bergey's Manual of Systematic Bacteriology, 2
- Sturz AV, Christie B R, Novak J (2000)"Bacterial Endophytes: Potential Role in Developing Sustainable System of Crop Production". CRC Crit Rev Plant Sci 19:1–30
- Suresh K, Sharma R, Verma D (2011) "Characterization and Phylogenetic Diversity of Carboxymethyl Cellulase Producing Bacillus Species from a Landfill Ecosystem," Indian J Microbiol 51: 531–535
[Crossresff] [Googlescholar] [Pubmed]
- Tabatabai MA (1994) "Soil Enzymes," In: Weaver R W, Angle J S, Bottomley PS (Eds),Methods of Soil Analysis: Microbiological and Biochemical Properties. Soil Sci Soc Am J 775-833
- Tarafdar JC, Marschner H (1994) "Efficiency of VAM in Utilization of Organic Phosphorus by Wheat Plants". Soil Sci Plant Nutr 40: 593-600
- Teather RM, Wood PJ (1982) "Use of Congo Red Polysaccharide Interaction in Enumeration and Characterization of Cellulolytic Bacteria from Bovine Rumen". Appl Environ Microbiol 43: 777-780
[Crossresff] [Googlescholar] [Pubmed]
- Tilak KVBR, Reddy BS (2006) 'Bacillus Cereus and B. Circulans-Novel Inoculants for Crops. curr Sci 90:642-644
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences