ISSN : 2348-9502
American Journal of Ethnomedicine
Ethnobotany and Ethnopharmacognosy of Lamiaceae Species from Central Burkina Faso: Leucas martinicensis (Jacquin) R. Brown, Hoslundia opposita Vahl and Orthosiphon pallidus Royle Ex Benth
1University of Ouagadougou, Laboratory of Biochemistry and Applied Chemistry (LABIOCA), Burkina Faso
2University of Ouagadougou, Laboratory of Biology and Plants Ecology (LEBEV), Burkina Faso
- *Corresponding Author:
- Kansole Michelline Marie Regina
University of Ouagadougou
Laboratory of Biochemistry and Applied Chemistry (LABIOCA)
Burkina Faso
E-mail: dieutrinity@hotmail.fr
Abstract
Objective: The main objective of the current work is to assess the ethnobotany and ethnopharmacognosy of Lamiaceae species from central Burkina Faso, and especially to focuss on the biological activities of Leucas martinicensis (Jacquin) R. Brown, Hoslundia opposita Vahl and Orthosiphon pallidus Royle Ex Benth.
Methods: Different methods have been used to reach the above objective such as the Ethnobotanical survey that concerned 13 Lamiaceae species of the study area, the phytochemicals screening, the antiradical activities via FRAP, DPPH and ABTS methods. The Thin Layer Chromatography (TLC) was also performed.
Results: The three species play an important role in the Burkinabe ethnomedicine as antibacterial since historical times. After some pharmacognostical surveys, the ethnobotanical investigation showed that these species are used in traditional medicine for different diseases treatment. All parts (stems, leaves, roots, flowers, fruits) of these species are used as antipyretic, anti-parasitic, anti-inflammatory, antiseptic, anti -diarrheic, eyes cleanser, and to heal rheumatic diseases, skin diseases, etc. Phytochemical investigation of these species showed the presence of compounds such as flavonoids (mainly for Orthosiphon pallidus Royle ex Benth), polyphenols, saponins and coumarins. Thin Layer Chromatography (TLC) analysis allowed to get more insight into the polyphenolic compounds and led to the isolation of cafeic acid and two undetermined flavonoids. The in vitro biological activity showed high bioactivity of the extracts as free radical scavenger and anti-gout.
Conclusion: The current study is a boost up to recognize and to valorize scientifically the use of these three Lamiaceae species in the traditional medicines, particularly in Burkina Faso via the results obtained.
Keywords
Ethnobotany, Lamiaceae, Phenolics, Flavonoids, Antioxidants, Gout, Burkina Faso.
Introduction
The use of plants for medicinal purpose has a long history in many indigenous societies and they are continuing to play important roles in the treatment of various diseases, mainly in rural areas in Africa. The practices of traditional medicine are based on a long period of beliefs and empirical observations [1]. Lamiaceae or Labiatae are an important family of plants which includes about 6000 species and about 210 genera. This family is an important source of essential oils, infusion and natural antibiotics for aromatherapy, perfumery although synthetic fragrances tend to replace these species. Cosmetical firms use Lamiaceae species usually for their moisturizing and antiseptic properties. Leucas martinicensis (Jacquin) R. Brown, Hoslundia opposita Vahl and Orthosiphon pallidus Royle ex Benth are three species of the Lamiaceae family growing in many regions of Burkina faso. In Ouagadougou, these plants are sold in medicinal drug markets as ingredients of recipes for treatment of infectious or inflammatory diseases.
The overall objective of the current study is to document the ethnobotanical knowledges on medicinal lamiaceae species in the region surrounding Ouagadougou. This region called Kadiogo Province comprises many ethnical groups of the country, because Ouagadougou is the capital city of Burkina Faso. From the most used species, we aim to screen the phytoconstituents and biological activities of some extracts.
MATERIAL AND METHODS
Study zone and inhabitants
Burkina Faso, usually known as the land of upright men is a landlocked country situated in the middle of West Africa and covers a surface of approximately 274 000 km². It is located inside the loop of the River Niger between 10° and 15° north latitude and between 2° east and 5° 30' west longitude. The climate is a dry season (October to May) and a non regular rainy season (June to September). The homogenous and seasonal dependent vegetal landscape is made of Parkia biglobosa (Néré), Vitellaria paradoxa (Karité), Cassia sp and Andasonia digitata ecosystems. Species such as Azadirachta indica (Neem) and Eucalyptus sp., have been introduced to slow down desertification. The Mossi plateau also called central plateau comprises the central part of the country where is located the Capital city, Ouagadougou. Mossi are the dominant ethnic group even though other people with different ethnic backgrounds have settled in the area. Cultivation of crops like sorghum and pastoralism are the major economic activities of the local people [2]. Because of the weakness of the modern health system and services, but mainly due to the cultural beliefs the majority of the population use traditional medicine and particularly medicinal plants as primary health care.
Sites and informants selection
Based on information from the Direction Générale de la Pharmacie, du Médicament et des Laboratoires (DGPML), the national administration in charge of traditional medicine, and the mayor office of Ouagadougou, sampling sites for ethnobotanical data collection were selected from seven sites that are located in the administrative department of Ouagadougou (Koubri, patte-d’oie, Gounghin, Pissy, Cissin, Tanghin dassouri, yagma). A total of 39 traditional medicine practitioners and 67 users of the traditional medicine products were systematically chosen according to the method of Martin [3].
Semi-structured interview
The survey was based on a questionnaire model. A confidence relationship was established with the traditional medicine practitioners. No money was given to informers. Interviews were conducted in French or in moore (the language spoken by Mossi people) sometimes with the help of translators who were conversant with the local language. The survey focused on following Lamiaceae species: leucas martinicensis (J.) R. Br, Hoslundia opposita Vahl., Orthosiphon pallidus R. ex B., Mentha viridis L., Mentha piperita L., Mentha aquatica L., Ocimum americanum L., Ocimum basilicum L., Solenostemon rotundifolius (Poir.) J. K. Morton, Solenostemon monostashyus (P.Beauv.) Brig, Hyptis spicigera Lam., Hyptis suaveolens (L.) Poit.) and Tinnea barteri Gürke.
The mentioned plants were harvested according to the interviewed persons and identified by Prof. Millogo J., from University of Ouagadougou biodiversity center where voucher herbarium specimens were deposited. The correspondences between the Moore names and scientific names have been verified from the literature [4].
Laboratory material and methods
Stems with leaves of the three most cited species were collected: Leucas martinicensis Jacquin) R. (Collected at University of Ouagadougou and deposited as LM 01.), Hoslundia opposita Vahl (collected at the Bangr - Weoogo urban park of Ouagadougou and deposited as HO 01.) and Orthosiphon pallidus Royle ex Benth (harvested at the University of Ouagadougou and deposited as OP 01.). The plant material was washed and dried in the laboratory away from sunlight and dust. They are then finely grinded and stored in clean plastic bags. In addition, some fresh plants were kept for botanical investigations (histochemistry).
Histochemical characterization
In order to characterize biochemically the different tissues of the plant (parenchyma, collenchyma, phloem, xylem, sclerenchyma), histochemical sections were performed on freshly harvested leaves, stems and roots of Leucas martinicensis (Jacquin) R. Brown, Hoslundia opposita Vahl and Orthosiphon pallidus Royle ex Benth. The sections were treated with green Carmino after successive passages in bleach and acetic acid 1%. Tannins and alkaloids have been characterized using aqueous solution of FeCl3 1% and lugol 3%, respectively. Specific reagents are then used to identify other secondary metabolites in the tissues5. Sections have then been observed and imaged under a light microscope at X 10.
Extraction
The plant powder (25 g) was extracted using a Soxhlet system with 250 ml chloroform and then 250 ml methanol (until clear supernatant; 3 to 8 h cycling).
The extracts were then dried using a Rotavapor system.
Phytochemical screening
The phytochemical screening consisted of a qualitative analysis of secondary metabolites present in the plant samples using classical color or precipitation reactions. Reactions were carried out (i) in chloroform extracts, for sterols, triterpenes, flavonoid and coumarinaglycone; and (ii) in methanol extracts, for flavonoids, catechic and gallic tannins, saponins, anthraquinones, alkaloids and coumarins.
Thin-layer chromatography profiling of polyphenols
Methanol extract of Orthosiphon pallidus (10mg/ml) was evaporated until dryness, dissolved in methanol then applied (10 μl) on silica gel plates (FLUKA, TLCPET foils). The plate (5cmx 10 cm) was eluted up to 8.5 cm with glacial acetic acid - ethyl acetate - formic acid - distilled water [0.9: 1.1: 2: 9.5], dried, treated with a 10 g/L solution of diphenylboric acid aminoethyl ester in methanol and then with a 50 g/L macrogol 400 solution diluted in methanol and examined in ultraviolet light at 254 and 365 nm. Different reference compounds (rhamnetin, rutin, caffeic acid, myricetin) have been co-eluted.
Determination of total polyphenols, flavonoids, flavonols and tannins
The determinations were made on methanol extracts (10 mg/ml).
The concentrations of total polyphenols were estimated via the measurement of the phosphomolybdotungstic reagent (Folin-Ciocalteu reagent) reduction, then the concentrations were expressed in mg gallic acid equivalents per 100 mg of extract [mg GA/100 mg] [6].
Flavonoids were determined using the method of Dowd that has been adapted by Arvouet-Grand et al. [7], based on the colorimetry of aluminium complexes. The results are expressed in mg quercetin equivalents per 100 mg of extract (mg EQ/100 mg).
Flavonols were assayed using the metal-chelation colometry method by Abarca et al. [8]. The results are expressed as Quercetin Equivalent for 100 mg of dry extract.
Tannins were measured by colorimetry of ferric complexes, calibrating with a tannic acid solution7. Results are expressed as mg of tannic acid equivalents per 100 mg of extract (mg TA/100 mg).
DPPH radical scavenging assay
Radical scavenging properties of the plant extracts against stable DPPH• (2, 2’- diphenyl-1- picrylhydrazyl, Fluka) were determined spectrophotometrically at 517 nm using the method of Vélazquez et al. [9]. Extract solutions were prepared by dissolving dry extract (10 mg) in methanol (10 ml). The samples were homogenized using an ultrasonic bath. For different concentrations, aliquots of 0.5 ml from all samples were added to methanolic DPPH• solution (1 ml, 20 mg/ml). After 15 min in the dark at room temperature, the decrease in absorption was measured. The blank sample was composed of the same amount of methanol and DPPH• solution. All experiments were performed in triplicate. Radical scavenging activities were calculated by the following formula: Inhibition (%) = (1 − A/A0) × 100 where A0 is the absorption of blank sample and A is the absorption of tested extract solution. The extract concentration allowing a 50% scavenging activity (IC50) was determined and expressed in μg/ml.
FRAP assay
FRAP (Ferric Reducing Antioxidant Power) assay was carried out as described by Hinneburg et al. [10]. A volume of an extract solution (0.5 mL, 0.1 mg/ml) was added and mixed together with phosphate buffer (1.25 mL, 0.2 M, pH 6.6) and aqueous potassium hexacyanoferrate (1.25 mL, K3Fe (CN)6, 1%). After 30 min incubation at 50°C, 10% trichloroacetic acid (1.25 mL) was added. The mixture was centrifuged at 2000 rpm for 10 min. The upper layer (0.625 mL) was mixed with 1% aqueous FeCl3 (0.125 mL). A blank without extract was prepared under the same conditions. The absorbance was recorded at 700 nm. All determinations were carried out in triplicate. Ascorbic acid was used as a standard and the Iron (III) reducing capacity was expressed as mmol ascorbic acid equivalents (AAE)/g of extract (dry weight).
By ABTS method
The method of Re et al. [11] was used. It is based on the decolorization of a stable radical cation ABTS•+ (2, 2 '- azynobis-[3- ethylbenzothiazoline -6-sulfonic acid]) in the presence of scavenging ABTS radical compound. The ABTS•+ (blue-green color) produced by direct reaction between ABTS and potassium persulfate has a maximum absorption at 734 nm.
Preparation of the solution of ABTS•+
A mass of 10 mg ABTS was dissolved in 2.6 ml of distilled water and 1.7212 mg of potassium persulfate was added and the mixture kept in the dark at at room temperature for 12 hours. The mixture was then diluted with ethanol until an absorbance of 0.70 ± 0.02 at 734 nm.
Samples’ test
In three Eppendorf tubes containing 15 μl of sample solution (1mg /ml) were added 985 μl of ABTS•+ solution (used immediately after preparation). The same operation is performed for the ascorbic acid used as a reference. The tubes are then protected from light for 15 minutes and the absorbance read at 734 nm in a spectrophotometer against a standard curve of ascorbic acid (264.15 mg/l). The concentration of compounds having a reducing effect on the radical cation ABTS•+ (antiradical compounds) is expressed in equivalent mmol of Ascorbic Acid (EAA)/g of dry extract according to the formula:
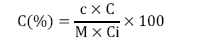
C: Concentration of antioxidant compounds in EAA mmol/g of dry extract.
c: Concentration of the sample been read.
D: Dilution factor of the sample.
Ci: Concentration of the extract.
M: Molecular weight of ascorbic acid (176.1 g/mol).
Determination of Xanthine Oxidase (XO) Inhibition
The XO activity, using xanthine as a substrate, was measured via spectrophotometry according to the method of Ferraz Filha et al. [12] with some modifications. The extracts were investigated for their XO inhibitory activity at a final concentration of 100 μg/mL. For the assay, the mixture contained phosphate buffer (150 μL, 1/15 M, pH 7.5), extract solution (50 μL) and enzyme solution (50 μL, 0.28 U/mL in phosphate buffer). The reaction was initiated by adding substrate solution (250 mL, 0.15 mM in water). The absorbance stability at 295 nm was verified for 1 min. Enzymatic kinetics were recorded at 295 nm for 2 min. A negative control (0% XO inhibition activity) was prepared with 1% methanol solution instead of the extract solution. All experiments were performed in triplicate. XO inhibitory activity was expressed as the percentage inhibition of XO, calculated as: % inhibition = (1− V/V0) × 100.
Where V0 is the linear change (blank inclination) in absorbance per minute of negative control, and V as the reaction kinetic’s rate.
Statistical analysis
Data are expressed as mean ± SD from three separate observations. For in vitro antioxidant assays one way ANOVA test followed by Tukey’s test (P < 0.05) was used to analyze the differences among EC50 of various fractions for different antioxidant assays.
RESULTS
Ethnobotanic knowledge
During the present ethnobotanical survey thirteen (13) plants species were reported by the informants for their medicinal usages (table 1).
Table 1. Ethnobotanical survey results
| scientific name | Mooré name | Parts of the plant used | applications | Informants consensus |
|---|---|---|---|---|
| Ocimum basilicum L. | Yuling- gnuuga | Dried seeds | Foreign body in the eye (insects, dust) |
100% |
| Leaves or whole plant | Nightmares; psychological trauma | 100% | ||
| Leaves to drink as a tea |
Deworming (mouth, stomach); antidiarrheal |
50% | ||
| Tinnea barteri Gürke | Kinkiris- kaandé | Leaves; stems; seeds | Complicated angina | 10% |
| Hemorrhoids | 10% | |||
| Toothache | 50% | |||
| Old wounds | 100% | |||
| Hoslundia opposita Vahl | Waamb- kiparé | Leaves | Constipation | 50% |
| Whole plant | Convulsion in children | 10% | ||
| Leaves + flowers | Skin diseases | 100 % | ||
| Leaves +leaves of Ocimum basilicum L |
Antidote against snake bite. | 10% | ||
| Roots decoction | Fever, stomach and lips aches |
50% | ||
| Leaves; stems; flowers | Muscles aches; rib aches; lung aches; stomachaches; difficulties to pee; skin cosmetic |
100 % | ||
| Hyptis spicigera L. | Rung- rungui | Leaves; stems; flowers; seeds | Teeth pain; malaria; fever; headaches, to conserve cereal grains in the attics |
100% |
| Solenostemon rotundifolius (Poir.) J. K. Morton |
Pèesa | Leaves | Dysentery | 100 % |
| Leaves+stems | Ocular disorders | 50 % | ||
| Stems | Animal feed | 100 % | ||
| Tubers (cooked) | Eaten (food) | 100 % | ||
| Solenostemon monostachyus (P. Beauv.) Brig. | Kiinga | Whole plant (tea) | Toilet to dead bodies | 50% |
| leaves; stems; flowers; seeds | Dental pain; colic; convulsions; fever; headache; infant cough; visual disturbances |
50% | ||
| Purifies and disinfects people attending patients until their death. |
100% | |||
| Hyptis suaveolens (L)Poit. | Ruung- rung- gnaaga |
Leaves; stems | Itchy skin; insect repellent (especially mosquitoes) | 100 % |
| Orthosiphon pallidus Royle ex Benth. | Nin- kaansega | Dried seeds | Foreign objects fallen into the eye (insect, dust) | 100 % |
| leaves; stems | Toilet to dead bodies; breast pain and poor quality of milk of women who newly delivered; gout |
75 % | ||
| Ocimum americanum L. | Yulin-gnu- raaga | Dried seeds | Foreign objects fallen into the eye (insect, dust) |
100 % |
| Whole plant | Nightmares; deworming; insect repellent. |
100 % | ||
| Leucas martinicensis (Jacq.) R. Br. | Bin-wubdo | Whole plant | Painful menstruation; wounds; fever; stomach pain; agalactia; hypogalactia; insect repellent (especially mosquitoes); asthma; insect bites |
100 % |
| Mentha aquatica L. Mentha piperita L. Mentha viridis L. |
Menthe | Leaves; stems | Used primarily for the aroma in drinks Facilitates digestion (tea) |
100 % |
Efforts have been made to find equivalent medical terms in English for each of the local disease names. Analysis of data revealed that Leucas martinicensis (Jacquin) R. Brown, Orthosiphon pallidus Royle ex Benth and Hoslundia opposita Vahl are applied to a wide range of diseases and are found everywhere in the region. Ocimum basilicum L., Hyptis suaveolens (L), and Ocimum americanum L. are mainly used against psychological or parasitisis diseases and for body cleaning. Tinnea barteri Gürke, Hyptis spicigera L. and Solenostemon monostachyus are used as antimicrobial and antalgic. Solenostemon rotundifolius and Mentha aquatica are used as nutraceuticals.
Plant parts used and methods of preparation
For most of these species leaves and/or stems are the most frequent parts used. Other parts used are respectively seeds, whole plant, leaves with flowers, roots and tubers. The main preparation methods are respectively in decreasing order: decoction, infusion (as tea), raw (dried seed) and cooking (tubers).
Route of administration and dosage
The recipes are used orally or by application on skin.
Informants’ consensus
From the 106 Lamiaceae users of these eleven species, fifty nine (59) have a 100% informant’s consensus, and four (04) have 75%, twenty seven (27) have 50% and sixteen (16) have 10%.
Pharmacognostic results
Knowledge of the chemical composition of the plant cells or tissues is useful for the selection of the best suited organ for extraction. It can help for threatened species protection by using leaves instead of stem bark or root when enough phytoconstituents could be recovered using quickly renewable organs like leaves. Histochemical cuttings made showed that only the roots of Leucas martinicensis (J.) R. Br are rich in alkaloids, and tannins as compared to the two other species investigated. Leaves and stems of the three species have unicellular hairs, which are essential characteristics of Lamiaceae plants. Screening of the main phytoconstituents of the tree species gave the results summarized in the table 2.
Table 2. Pharmacognostic screening of the three species
| Leucas martinicensis (Jacquin) R. Brown |
Orthosiphon pallidus Royle ex Benth |
Hoslundia opposita Vahl |
||||
|---|---|---|---|---|---|---|
| ME | CE | ME | CE | ME | CE | |
| Flavonoids | + | - | - | - | + | - |
| Tannins/polyphenols | + | - | + | - | + | - |
| Saponins | - | - | - | - | + | - |
| Coumarins | + | - | + | - | + | - |
| Alkaloids | - | - | - | - | - | - |
ME: Methanol Extacts / CE: Chloroform Extacts.
The results show a better presence of phytochemical substances in methanol extracts rather than chloroform ones. Methanolic extract of Hoslundia opposita is the richest of the six extracts. Phenolic compounds are the most frequents as compared to saponins and alkaloids. Leucas martinicensis seems more closed to Hoslundia opposita as the tree species are compared for their phytoconstituents composition.
Main phytoconstituents dosage
Among the three plants investigated, only Orthosiphon pallidus Royle ex Benth showed the best results in all assays: 33.25 ± 0.65 mg EQ/100mg of extract for total of extract for total tannins and 5.37 ± 0.39 mg EQ/100mg of extract for total flavonoids. The total polyphenols are also important in the other two plants: 14.90 ± 0.66 mg of EQ/100mg for Leucas martinicensis (Jacquin) R. Brown and 15.02 ± 0.030 mg EQ/100mg in Hoslundia opposita Vahl. Total flavonoids are quite important in Hoslundia opposita Vahl (2.41 ± 0.26 mg EQ/100mg extract). All the three plants had a very low amount in total flavonols.
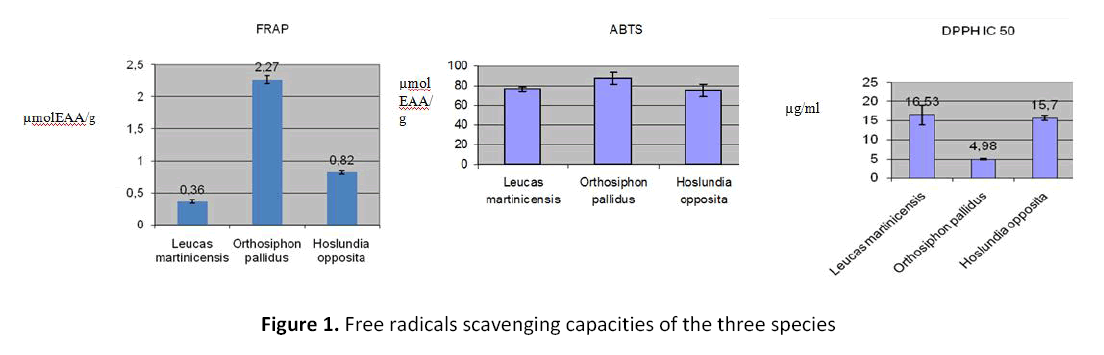
Free radicals scavenging capacity results
The following results summarized in figure 1 have been obtained through testing with three methods (FRAP, ABTS and DPPH), after graphic determinations: Orthosiphon pallidus Royle ex Benth species showed the best results with the DPPH method with an IC50 of 4.98 ± 0.20 mg/ml. A reduction of ferric ions to ferrous ions in the range of 2.27 ± 0.06 μmol EAA/g has been observed for the same species. Using ABTS methods, Orthosiphon pallidus Royle ex Benth species still showed the best results by reducing ascorbic acid at 88.65 ± 5.20 μmol EAA/g.
The Xanthine Oxidase Inhibition efficiency
We got the best inhibition efficiency (24.97 ± 3.47% for 1 μg/ml of extract) with Orthosiphon pallidus methanol extracts. Least significant results have been obtained with the two other species.
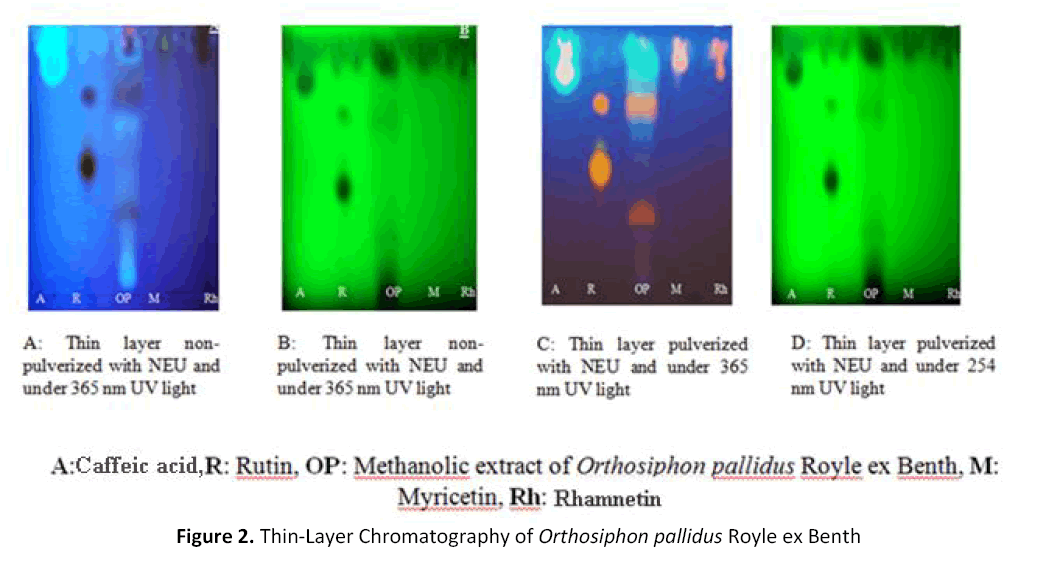
The Thin-Layer Chromatography (TLC)
The elution system: Formic acid/ Water/Ethyl Acetate Acid/Glacial Acetic Acid/distilled water (9.5 / 0.9 / 1.1 / 2, v / v / v/v) gave the separation of some compounds from the methanol extract of Orthosiphon pallidus Royle ex Benth and we isolated from the figure 2 one phenolic acid (caffeic acid) and two non-determined flavonoids compounds.
DISCUSSION
Ethnobotany review from table 1 shows that the whole plant of Hoslundia opposita or various parts of it are used. In traditional medicine the plant is used as a purgative, diuretic, febrifuge, antiepileptic and antiseptic in west and southern Africa13. Such usages are likewise to those obtained from our survey.
Leucas martinicensis is known as “Mat-burisa” in Yabellozone of Ethiopia and is well used for its medicinal properties. It is taken mainly to prevent diarrhea [14]. Our results however show the use of this species to treat fever, pain, hypogalactia and asthma. From pharmacology data on Labiatea, it is kown that: menthol which is used in medicine and as a flavoring material is one of the most important constituents of the oils. Thymol, extracted from thyme, is used medicinally as a very powerful antiseptic [15]. Usually, extracts obtained via methanol or ethyl acetate fractions show higher antifungal activities [16].
The nature of the main chemicals groups highlighted by the phytochemical screening foreshadows interesting pharmacological activities of the methanol extracts. It is basically:
Coumarins
They are beneficial in cases of skin diseases, as they are responsible for wound healing [17]. They could explain the traditional use of these three plants as anti-infective agent against stomach ache, dead bodies’ toilet, fever, convulsions, and diseases of the skin.
Saponins
Their main property is to be antifungal, anti-inflammatory and anthelmintic [1] . Using Hoslundia opposita Vahl in treating dermatoses, as a cosmetic for skin and as an anti- inflammatory agent may be due to the presence of saponins in its extracts.
Tannins and polyphenols
They are identified in all the three species. Their presence could explain the species use in the treatment of skin diseases, for the release of insects’ bites’ pain, in diarrhea treatment and the dead bodies’ toilet.
Flavonoids
These are strong sources of phytoestrogen, insecticides and antiinflammatory, they can be antibacterial and antiviral18; they could justify the use of Leucas martinicensis (Jacquin ) R. Brown as insect repellents and for the treatment of inflammatory diseases (painful menstruations, fever, ...).
From literature review, phytochemical analysis of Hoslundia opposita19 allowed the isolation of tree compounds: 5, 7-dimethoxy -6- methylflavone, Hoslunddiol and euscaphic acid (jaracandic acid ). The acaricidal active principle of H. opposita was characterized as ursolic acid, a triterpene mainly from the leaves. The overall acaricidal effect of H. opposita may have been due to synergy with other principles having acaricidal properties because ursolic acid itself is not as efficient as the methanolic and ethyl acetate fractions where its isolation has been made [19]. The essential oil from the leaves of Hoslundia opposita was extracted by hydrodistillation. GC/MS analysis of the volatile oil showed that it contains mainly sesquiterpenes and sesquiterpene alcohols [19]. The essential oil was tested against bacterial and fungal species. The results showed that the essential oil from Hoslundia opposita has a significant activity against Aspergillus niger, Acinetobacter calcoacetica, Brochothrix thermosphacta and Flavobacterium suaveolens. The chloroform extract of the root of Hoslundia opposita has been investigated for their potential effects on the central nervous system (CNS). The neuropharmacological screening revealed the presence of CNS depression. abietanetype esters, 3-O-benzoylhosloppone, 3-Ocinnamoylhosloppone, 3-O-benzoylhinokiol and 3-O-benzoylhosloquinone were isolated from the root bark of Hoslundia opposita which could justify the plant’s antimalarial properties. 3-O-Benzoylhosloppone inhibits the growth of the multidrug resistant strain K1 of Plasmodium falciparum in vitro with an IC50-value of 0.4 μg ml−120. Essential oil extract from leaves of H. opposita normalized Hyperglycemia-induced dyslipidemia associated with diabetic complications on sub-chronic treatment with the oil. This activity may be of benefits in the management of type 2 diabetes and its associated vascular complications. The hepatoprotective potentials of the stem extract of Hoslundia opposita Vahl have also been investigated. Some treatments conducted with methanol extract of Hoslundia opposita (100 mg/kg) ameliorated effects of hepatoxins and reduced significantly (P>0.05) the high levels of the utilized biochemical markers (enzymes). The extracts showed good toxicity profile with an LD50 value above 5000 mg/kg for the methanol extract. Phytochemical screening of the plant showed the presence of some resins, flavonoids, sterols/triterpenes, tannins, saponins, alkaloids, reducing sugars, cardiac glycosides and proteins in the extracts. These results confirmed that the stem of Hoslundia opposita contains some bioactive properties leading to some hepatoprotective effects of the plant. Chemically, the major compounds obtained from the GC/MS analysis of ethanolic extract of Orthosiphon pallidus were Trihydroxy-16a, 17apropylmethylenedioxypregna- 1,4-diene-3,20 dione, 9, 12, 15 Octadecatrienoic acid, 1, 6, 10-Dodecatrien-3-ol, 3, 7, 11-trimethyl, Octadecanoic acid and Docosanoic acid [21].
From the unsaponifiable molecules isolated via the petroleum ether extraction of Orthosiphon pallidus, an alkaloid, the orthosiphonol (C30H50O2) has been isolated. Of the 150 species of Leucas, about seven species have so far been chemically investigated. A number of secondary metabolites have been isolated and characterized. From a number of Leucas species compounds like diterpenes, triterpenes, flavones, alkaloids, glycosides, sitosterols, chromon, sterol, oleanolic acid, ursolic acid, leucolacton, stigmasterol, campesterol, isopimarane, rhamnoglucoside, etc. have been isolated and characterized. For Leucas martinicensis, essential oils from leaves and flowers were found to have 1- hepten -3- ol and germacrene [22]. The volatile oil obtained is suitable for numerous usages in pharmaceutics, cosmetics and alimentation products (for example in lotions, creams, soaps, shampoos, rinses, gargles, candies, etc.) [23]. Its antibacterial activities and antifungal properties have been reported [24]. Chloroform extracts of the leaves contain triacontane (E2’), tetracosane (E2’-C) and lutein (E45) and the methanol extracts showed the presence of cholesterol (E24) [14]. The phytochemical composition closeness of Leucas martinicensis and Hoslundia opposita could explain why both are used for pain and infection treatment whereas Orthosiphon pallidus has more metabolic and functional indications, as indicated by our results. Our biological test on xanthine Oxidase Inhibition efficiency revealed that Methanol extract of Orthosiphon pallidus Royle ex has the best potential. In humans, inhibition of xanthine oxidase reduces the production of uric acid. Thus xanthine oxidase (XO) inhibitors can be used in the treatment of hyperuricemia (abnormally high level of uric acid in the blood) and in the treatment of cardiovascular disease [25]. Other studies showed that ethanol extracts of some Lamiaceae species, especially Hyptis obtusiflora Presl ex Benth. and Hyptis. lantanaefolia Poit. have interesting inhibition potential efficiency (40%) on XO [26]. About antioxidant activity, extract of Orthosiphon pallidus showed a good reduction capacity on ferric ions (2.27 ± 0.06 EAA/g) which is better than those of the standard compound (ascorbic having 5.58± 0.32 mmol EAA/g as activity) [27]. The reduction activity (especially radical scavenging) of extract of the species is due to the presence of flavonoids and phenolic acids which are known for their strong antiradical activities [28] and could justify the use for anti-inflammatory purposes. From TLC analysis, caffeic acid (a phenolic acid) found in extract of Orthosiphon pallidus, is a well-known anti- inflammatory compound18 that could also justify the use of this species in traditional treatment of diseases with an inflammatory component. According to a recent research, high content in phenolic acids are positively correlated to human health benefit [29].
CONCLUSION
The study objectives were to collect ethnobotanical knowledge on Lamiaceae species in the central region of Burkina Faso, and to characterize pharmacognostically the three most cited species. The obtained results showed that at least thirteen species are usually implicated in traditional medicine treatment of different diseases. Screening of the main phytochemical groups present in the chloroform and methanol extracts showed high level of phenolic and terpenoids compounds. The antioxidant and xanthine oxidase inhibitory efficiencies are in relation with the plants chemical composition. The diversity of the secondary metabolites within the three species could explain their usages in the treatment of inflammatory diseases; diarrhea; and parasitic infections of the skin and could be a scientific support for their various usages made by traditional healers in central region of Burkina Faso. There is need to improve the chemical and pharmacological investigation on Orthosiphon pallidus Royle ex Benth.
ACKNOWLEDGEMENTS
The authors would like to thank the ‘Africa and beauty Forum’ 2nd edition and the West African Economic and Monetary Union (WAEMU) for their financial supports.
REFERENCES
- Nacoulma-Ouedraogo, O. G. Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso, cas du plateau central, Tome I et II Thèse de Doctorat d’Etat. thesis, Université de Ouagadougou., (1996).
- Institut National de la Statistique et de la Démographie Ministère de l’Économie et du Développement Ouagadougou, B. F. Enquête Démographique et de Santé 2003. (ORC Macro Calverton, Maryland, USA 2004).
- Martin, G. J. Ethnobotany: A Methods Manual. Vol. Volume 1 de People and plants conservation serie (1995).
- Maydell, H.-J. v. Arbres et arbustes du Sahel : leurs caractéristiques et leurs utilisations. 531 (1990).
- Ciulei, I. Methodology of analysis of vegetable drug. Practical manual on industrial utilization of medicinal and aromatic plants. the Ministry of chemical industry. BUCAREST, 16-27 (1982).
- Singleton, L. V., Orthofer, R., Lamuela- raventos, R.R. Analysis of total phenol and other oxidation substances and antioxidants by mean of Folin-Ciocalteu Reagent. Method in Enzymology., 547-551 (1999).
- Arvouet-Grand, A. B., Vennat, A., Legret, P. Standardization of propolis extract and identification of key components. Journal de Pharmacie de Belgique 49, 462-429 (1994).
- Abarca, N. A., Campos, M.G., Reyes, J- A.A., Jimenez, N.N., Corral, J.H., Valdez, S.G. Antioxidant activity of phenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis julifloral, Leguminoseae). . Journal of Food Composition and Analysis 20, 119-124 (2007).
- Velasquez E, T. H., Mordujovichole BP, Saadevra G, Scinella GR. Antioxidant activity of Paraguayan plants extracts. Fitoterapia 74, 91-97 (2003).
- Iris Hinneburg, H. J. D. D., Raimo Hiltunen. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chemistry 97, 122-129 (2006).
- Re R, P. N., Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26, 9-10 (1999).
- Ferraz-Filha Z.S., I. F. V., L.G. Fietto, J.A. Lombardi, D.A. Saúde-Guimarães. Xanthine oxidase inibitory activity of Lychnophora species from Brazil (“Arnica”). Journal of Ethnopharmacology 107, 79–82 (2006).
- Tonzibo Z.F., J. C. C. Y. T. N. G. Chemical Composition of Essential Oils of Ocimum canum Sims from Côte d’Ivoire. Journal of Essential Oil Bearing Plants 11, 530-535 (2013).
- Estifanos, E. Chemical Studies of Leucas martinicensis. M.Sc. Thesis submitted to the Addis Ababa University,, (2003).
- Hooker W.J.. Niger Flora. 489 (Wheldon & Wesley Ltd., 1996).
- Perera, D. F. T. N., Fernando, K.M.E.P., Wijendra, W.A.S. Efficacy of phytochemicals present in leaves of Punica granatum against Malassezia species. American Journal of pharmacology and pharmacotherapeutics 2 (2015).
- Kontogiorgis, C. A., Xu, Y., Hadjipavlou- Litina, D., Luo, Y. Coumarin derivatives protection against ROS production in cellular models of Abeta toxicities. Free radical research. 41, 1168-1180 (2007).
- Bruneton,J. Pharmacognosy: Phytochemistry, Medicinal Plant., (1999).
- Gundidza G. M., S. G. D., K. P. Svoboda and S. Mavi Antimicrobial Activity of Essential Oil from Hoslundia opposite. Central African Journal of Medicine 38, 290-300 (1992).
- Achenbach, W. R. N. M. H. H. W. H. Antimalarial compounds from Hoslundia opposita. Phytochemistry 31, 3781-3784 (1992).
- Ashokan, K. M., Meenakshi Sundaram. nticancer studies on Orthosiphon pallidus Royle. And Peristrophe bicalyculata Nees. Journal of Pharmacy Research 4, 2654 (2011).
- Ancilla Muhayimanaa, J.-C. C. R.-P. G. Chemical Composition of Essential Oils of Some Medicinal Plants from Rwanda. Journal of Essential Oil Research 10, 251- 259 (1998).
- Muhammad S., A. F., M. M. Yahaya. The Phytochemical Components of Leucas Martinicensis that Cause Repellence of Adult Mosquito. International Journal of Modern Botany 2, 1-5 (2012).
- Uche A. Eze, S. O. B., Emmanuel U. Etuk, George, Ameh, Oguejiofor M. Ugwah, Chinenye J. Ugwah-Oguejiofor. Phytochemical and preliminary toxicological studies of the aqueous leave extract of Leucas martinicensis in wistar rats. International Journal of Medicinal Plants Research 2, 166-169 (2013).
- Dawson, J., and Walters, M.. Uric acid and xanthine oxidase: future therapeutic targets in the prevention of cardiovascular disease? Br. J. Clin. Pharmacol 62, 633-644 (2006).
- Antonio, G., González, I.L., Bazzocchi, L.M., Angel, G.R., Mireya, D.C., Mahabir,P.G. Xanthine oxidase inhibitory activity of some Panamanian plants from Celastraceae and Lamiaceae. Journalof Ethnopharmacology. 46, 25-29 (1995).
- Dastmalchi, K., Dorman, H.J.D., Kosar, M., Hiltunen, R. Chemical composition and in vitro antioxidant evaluation of a water soluble Moldavian balm (Dracocephalum moldavica L.) extract.. Lebensmittel- Wissenschaft & Technology. 40, 239-248 (2007).
- Nijveldt, R. J., van Nood, E., van Hoorn, D.E., Boelens, P.G., van Norren, K., van Leeuwen, P.A. Flavonoids: a review of probable mechanisms of action and potential applications. . American Journal of Clinical Nutrition. 74, 418-425 (2001).
- Amitabh Singh, S. M., Mandavi Singh, Udai Pratap Singh Studies on the phenolic acid contents in different parts of raw and ripe jackfruit and their importance in human health. International Annals of Avanced Scientific Research 2 (2015).
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences