ISSN : 0976 - 8688
Der Pharmacia Sinica
Enhancement of Antibacterial Property of Ciprofloxacin and Isoniazid against Pseudomonas Aeruginosa and Mycobectirium Smegmatisin in Combination with Extracted Lycopene from Lycopersicon Esculentum
Jaya Maitra* and Sangeeta
Department of Applied Chemistry, Gautam Buddha University, Uttar Pradesh, India
- *Corresponding Author:
- Jaya Maitra, Department of Applied Chemistry, Gautam Buddha University, Uttar Pradesh, India, Tel: 212671247953; E-mail: maitra.jaya@gmail.com
Received date: February 07, 2022, Manuscript No. IPDPS-12638; Editor assigned date: February 10, PreQC No. IPDPS-12638 (PQ); Reviewed date: February 25, 2022, QC No. IPDPS-12638; Revised date: April 11, 2 022, Manuscript No. IPDPS-12638 (R); Published date: April 19, 2 022, DOI: 10.36648/0976-8688.22.13.001
Citation: Maitra J, Sangeeta (2022) Enhancement of Antibacterial Property of Ciprofloxacin and Isoniazid against Pseudomonas Aeruginosa and Mycobectirium Smegmatis in Combination with Extracted Lycopene from Lycopersicon Esculentum. Der Pharmacia Sinica Vol:13 No:4
Abstract
Tomato is one of the most important vegetables worldwide because of its high consumption, year round availability and large content of health related components. Tomato contains a variety of phytochemicals such as lycopene, carotene, vitamin-C, quercetin glycosides, and chlorogenic acid and has good health protective effects. The present work is to search antimicrobial activity of lycopene extracted from tomato in combination with ciprofloxacin and isoniazid. The antimicrobial property was evaluated using agar dilution and diffusion method using bacterial cultures Pseudomonas aeruginosa and M.smegmatis. It was found that extracted lycopene executed good antimicrobial activity against Pseudomonas aeruginosa and M.smegmatis in combination with ciprofloxacin and isoniazid. An increase in the zone of inhibition was observed by increasing the concentration of lycopene and decreasing the concentration of the drug in combination.
Keywords
Lycopene; Tomato; Extraction; Antibacterial properties
Introduction
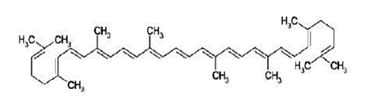
The main source of lycopene is tomatoes because tomato products have high concentrations of lycopene, but it is also present in gas, watermelon, apricots, red, grapes, pink grapefruit, pink guava, papaya, peaches, berry and cranberries. It is also one of the most abundant non-vitamin analogues present in human blood from food consumption [1]. Lycopene is one of about 600 naturally occurring carotenoids and is responsible for the red colour in fruits [2]. Carotenoids are natural antioxidants which protect the cells of the body from the harmful effects of oxidation due to free radicals [3]. Lycopene has 13 double bonds, of which 11 are conjugated, resulting in excellent antioxidant properties. The presence of unsaturated bonds in its molecular structure makes lycopene susceptible to oxidants, sensitive to light and heat. Because of its non-polarity, Lycopene is lipophilic, insoluble in water, and can be dissolved only in organic solvents and oils [4]. It is the most abundant carotenoid in tomatoes, followed by beta-carotene, gamma carotene and other minor carotenoids. The human body cannot produce lycopene so it must be obtain from food sources [5].
Materials and Methods
Extraction of lycopene from different method
Benzene extraction method: 50 gm of tamoto paste was taken in 250 mL beaker. It was warmed and added about 20 ml of warm benzene to it. This has been done about 5 times. Benzene was distilled off and lycopene residue was obtained. Residue was recrystallized by ether and weighed [6].
Methanol extraction method: 50 gm paste of tomato was taken and was dehydrated by adding 65 ml methanol. Mixture was immediately shaken vigrously to prevent the formation of hard lumps. After 2 hr, the thick suspension was filtered. The end cake was shaken for another 15 min with 75 ml mixture of equal volume of methanol and carbon tetrachloride and separated by filtration. The CCl4 phase was transferred to a separatory funnel added 1 vol of water and shake well. After phase sepration, the CCl4 phase was evaporated and the residue was diluted with about 2 ml of benzene.
Using a dropper, 1 ml of boiling methanol was added in portion, then crystal of crude lycopene were appeared immediately and the crystallization was completed by keeping the liquid at room temperature and ice bath, respectively. The crystals were washed 10 times using benzene and boiling methanol [7].
Coloum chromatography method: To 30 gm of tomato paste was added 50 ml acetone and dehydrated for 4-5 min for the removing all water and water soluble substance. The dehydrated tomato paste was taken in beaker and added 1:1 mixture of di chloro methane and ether and stirring was done for 4-5 min. After stirring added slowly small cubes of anhydrous MgSO4 for removing the water. The mixture was filtered and taken into a small beaker and solvent was evaporated at room temperature. Then Lycopene was separated from coloum firstly by removing the β-carotene and then Lycopene [8,9].
Evaluation and characterization of lycopene
Identification of lycopene
Thin Layer Chromatography (TLC) analysis
Sample details: lycopene
Adsorbent: Precoated silica gel
Solvent system: Pet ether;acetone (40:10)
Sample preparation: Lycopene (1 mg) was dissolved in n hexane (1 ml) and this solution was applied on the TLC plate with the aid of capillary tube.
Detection: Saturated Iodine Chamber.
Procedure: The lycopene was subjected on to the precoated and activated Silica gel TLC plates. (Plates were kept in oven for 1 hr at 70°C) The mobile phase is pet- ether:acetone ratios. After the TLC run and spraying the detecting agent reddish sports of lycopene were dentified visually. Rf value was calculated. Rf-value=0.35 (Figure 1)
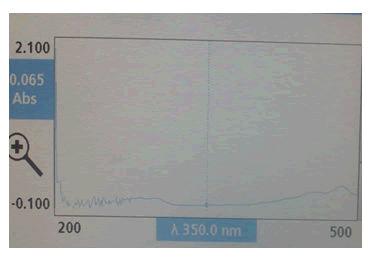
Ultra violet- spectrophotometer analysis: The 0.01% w/v solution of lycopene in methanol was prepared and λmax was determined (Figure 2).
λmax=475; Absorbance=2.100
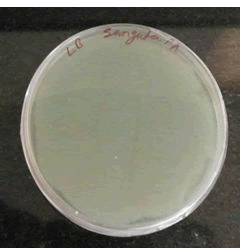
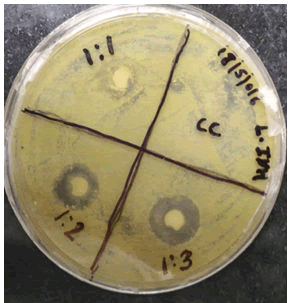
Antibacterial activity: Antibacterial activity was studied against two Bacteria Pseudomonasaeruginosa (MTCC-3541) and M.smegmatis. Antibacterial activity of lycopene against Pseudomonas aeruginosa (MTCC-3541) (Figures 3,4).
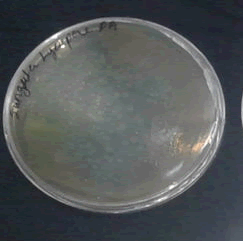
Antibacterial activity of lycopene against M.Smegmatis.
Synergistic effect of lycopene with ciprofloxacin and INH against P.aeurginosa & M. smegmatis.
By disc diffution method: Test organism: Gram-negative bacteria P.aeruginosa & M. smegmatis
Preparation of sub-culture: One day prior to these testing, inoculations of above bacterial cultures were made in the nutrient agar.
Preparation of base layer medium (nutrient ager): It was prepared by dissolving definite volumes of nutrient LB- agar 2 gm, in 25 ml of distilled water and sterilized by autoclaving at for 20 min.
Sterlization of equipments: Petri dishes, pipette, cork borer sterilized by dry heat sterilized at 160°C for 1 hr in hot air oven.
Preparation of test solution: Isolated lycopene (50 mg) were dissolved in n-Hexane to give a 50 μg/ml of these stock solution was pipette out with the help of micropipette and used for testing.
Method of testing
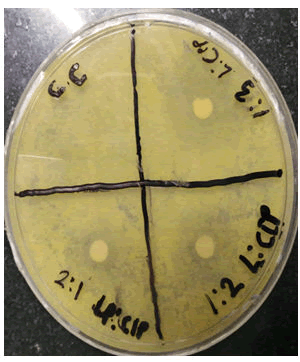
0.1 ml bacteria (P.aeruginosa) innoculatam was added into different petri dish having sterilized nutrient agar media. Immediately the cups were prepared with help of cork. In each cup then we added (0.1 ml) of test and std drug solution. Finally it was kept for diffution in a deep freezer for 2 hr and placed in incubator at 37°C for 48 hrs. After incubation measured the zone of inhibition (Table 1) (Figures 5,6 and Table 2).
Table 1: Zone of inhibition of lycopene against P.aeruginosa.
| Name of bacteria | Plant extract+drug | Conc of lycopene (μl) | Conc of drug (μl) | Zone of inhibition (mm) |
|---|---|---|---|---|
| P.aeruginosa | Lycopene + Ciprofloxacin | 50 | 50 | 9 |
| 75 | 25 | 13 | ||
| 66.5 | 33.5 | 16 |
Synergistic effect of lycopene with isoniazid against M. Smegmatis
Table 2: Zone of inhibition of lycopene with isoniazid against Mycobacterium smegmatis.
| Name of bacteria | Plant extract + drug | Conc of lycopene (μl) | Conc of drug (μl) | Zone of inhibition (mm) |
|---|---|---|---|---|
| M. smegmatis | Lycopene + INH | 50 | 50 | 8 |
| 75 | 25 | 18 | ||
| 66.5 | 33.5 | 20 |
Results
Synergistic effect of lycopene with ciprofloxacin against P.aeruginosa
In the present study the antibacterial effect of lycopene with ciprofloxacin on P.aeruginosa Table 1. It indicates that zone of inhibition increases as the concentration of lycopene increased. Lycopene has shown an antibacterial activity against gram negative bacteria with zone of inhibition ranged from (9 mm-16 mm).
Synergistic effect of lycopene with Antituberclosis drug (INH) against M. smegmetis
The antibacterial effect of lycopene in combination with Isoniazid against M.smegmatis is shown in (Table 2). It indicates that zone of inhibition increases as the concentration of lycopene increased. Lycopene has shown an antibacterial activity against M.smegmatis with zone of inhibition ranged from (8 mm-20 mm). The combination of Isonazid with lycopene was effective against M.smegmatis, which shows the synergism between Lycopene and drug.
Discussion
The extraction and isolation of lycopene has been carried out using different methods and the yields of lycopene showed to be high in natural sources of lycopene. A simple liquid- liquid extraction method was employed to extract lycopene in minimum organic solvent. Crystals were purified by recrystallization from ether. The study showed that extracted lycopene by different showed enhanced zone of inhibition with ciprofloxacin and isoniazid against P. aeruginosa and M. smegmatis.
Conclusion
The experimental report clearly showed that by increasing the concentration of lycopene which has, strong antioxidant and antibacterial property the dosage of synthetic drugs viz ciprofloxacin and isoniazid is reduced, if used in combination. This approach will reduce the cost and increase the effectiveness of the drug. A very detailed and comprehensive study is required to develop this type of combinatorial drug to benefit the society.
References
- Sunil S, Aditya D, Sakhare V (2012) Isolation of lycopene from tamoto and study of its antimicrobial activity. Biochem 3:671-673
- RM CB (2013) Functional properties and health benefits of Lycopene. Nutr Hosp 28:6-15
[Crossref] [Google Scholar] [Indexed]
- Pickett JP, Kleinedler SR, editors (2011) The American heritage dictionary of the English language (5th edition). Houghton Mifflin Harcourt
- Shi J, Le Maguer M (2000) Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit Rev Biotechnol 20:293-334
[Crossref] [Google Scholar] [Indexed]
- Agarwal S, Rao AV (2000) Tomato lycopene and its role in human health and chronic diseases. Can Med Assoc J 163:739-744
- Sathish T, Udayakiran D, Himabindu K, Lakshmi P, Sridevi D, et al. (2009) HPLC method for the determination of lycopene in crude oleoresin extracts. Asian J Chem 21:139-148
- Wang CY, Chen BH (2006) Tomato pulp as source for the production of lycopene powder containing high proportion of cis-isomers. Eur Food Res Technol 222:347-353
[Crossref] [Google Scholar] [Indexed]
- M Neelu (1014) Isolation and quantification of lycopene from watermelon, tamoto and papaya. Res J Recent Sci 3:68-70
- Lycopene (2012) Mayo foundation for medical education and research. Department of Medical Science.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences