ISSN : 0976-8505
Der Chemica Sinica
Design, Synthesis, Biological and Molecular Docking Studies of Some O-Hydroxycyanopyridine Derivatives
Ahmed H Moustafa1*, Nermin A M El-Seadawy2, Allam A Hassan3, Shirin H Pasha2, Hassan A El-Sayed1*, Nadia A M Shimess2 and Nasser A Hassan4
1Departement of Chemistry, Faculty of Science, Zagazig University, Zagazig, Egypt
2Department of Chemistry, Faculty of Science, Al-azhar Univerisity, Cairo, Egypt
3Department of Chemistry, Faculty of Science, Suze Univerisity, Suze, Egypt
4Photochemistry Department, Synthetic unit, National Research Centre, Dokki, Cairo, Egypt
Abstract
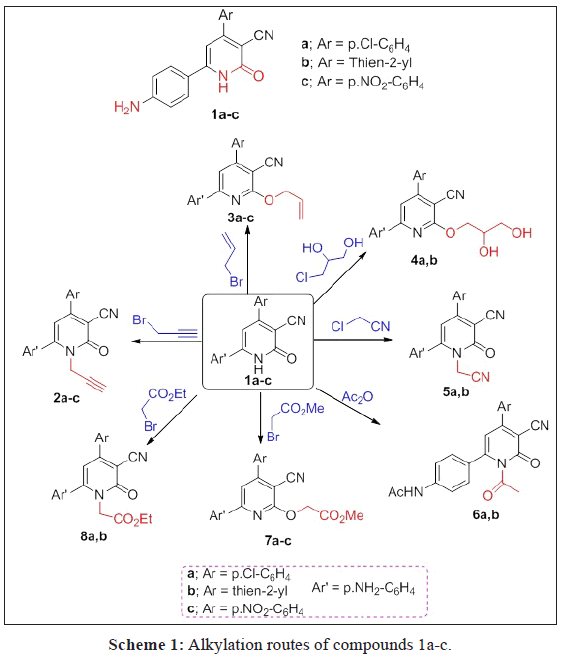
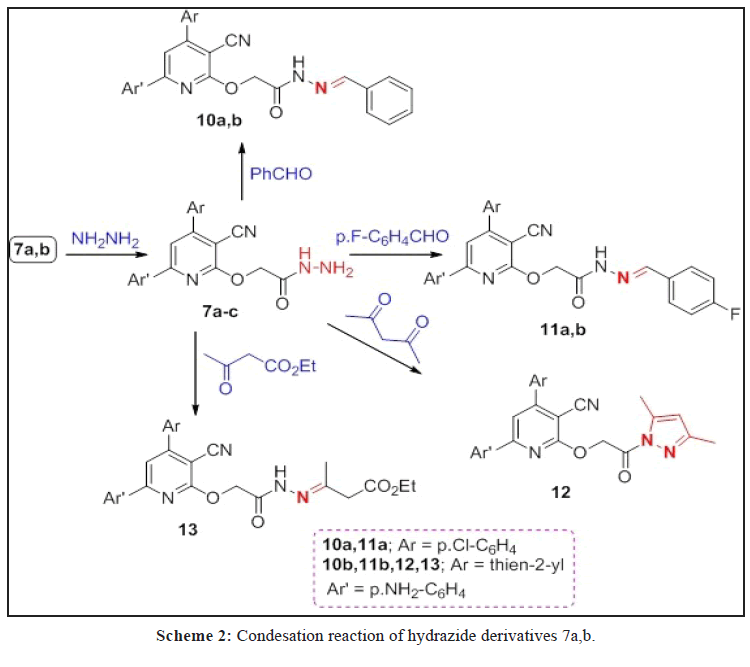
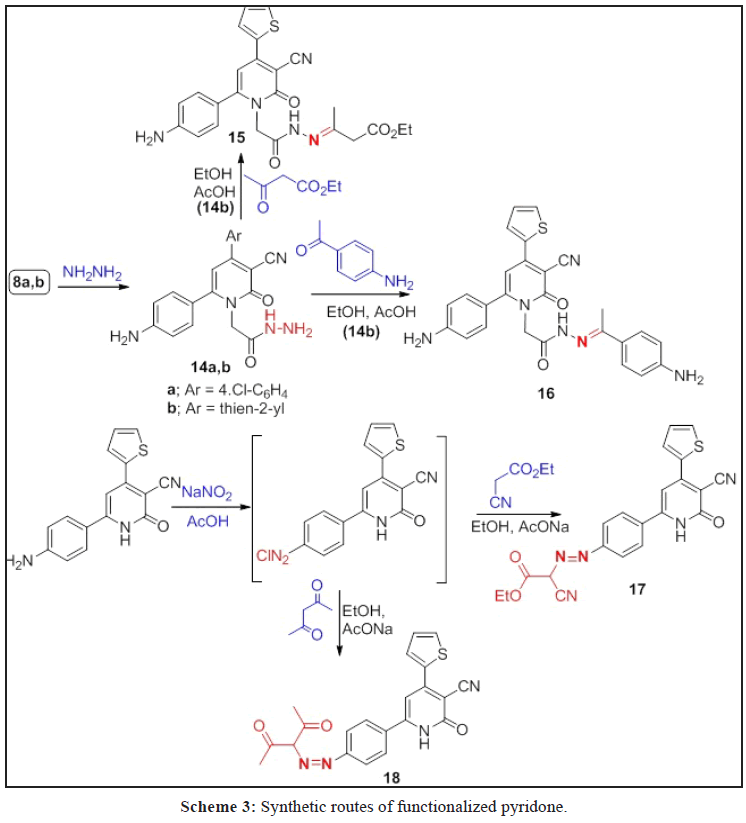
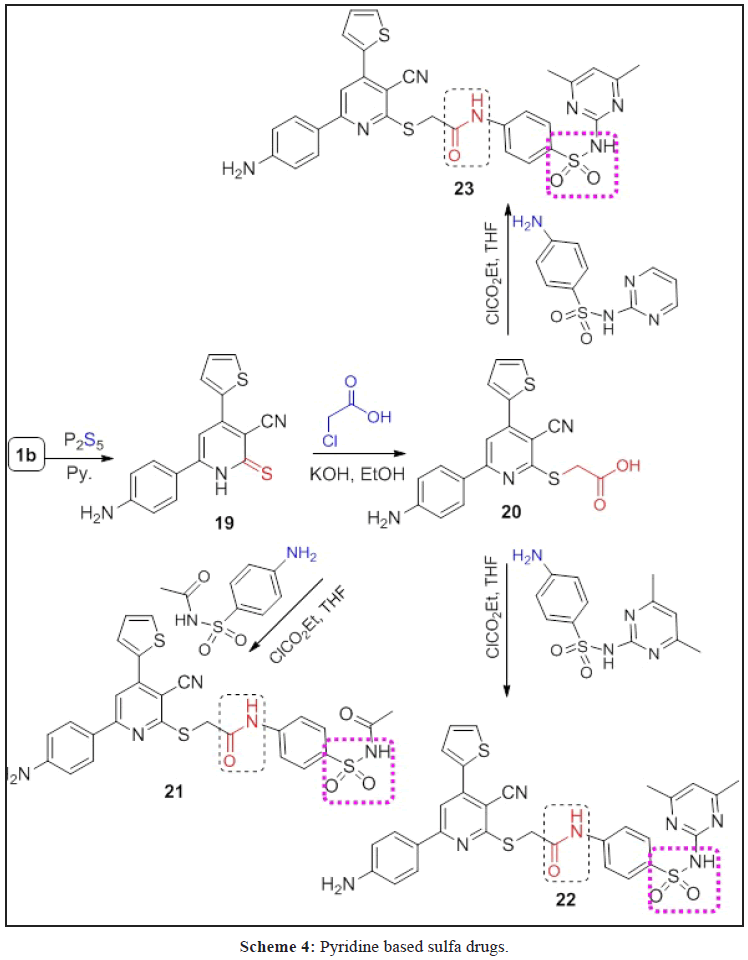
A series of O- and N-alkylated 2-pyridone derivatives 2a-c-8a,b were obtained from alkylation of 1a-c with different alkylating agents. Hydrazonolysis of 7a,b followed by reaction with aromatic aldehydes and active methylene reagents affroded the hydrazones 10a,b, 11a,b and 13, in addition to, pyrazole derivative 12. While, the hydrazide 14a,b obtained from hydrazonolysis of the ester 8a,b followed by reaction with ethyl acetoacetate and p-aminoacetophenone to give the corresponding hydrazones 15 and 16, respectively. Diazitization of 1b followed by reaction with active methylene reagents afforded hydrazonid derivatives 17 and 18. Sulpha-drugs 21-23 were obtained from alkylation of 2-thiooxopyridone 19 with chloroacetic acid followed by reaction with different sulphonamide derivatives. The triazole derivatives containing 2-pyridone moiety 24-26 were obtained from the reaction of 2a-c with ethyl 3-azidopropanoate via click reaction. All the newly synthesized compounds are elucidated by spectroscopic data (IR, 1H, 13C NMR, Mass spectrometry and elemental analyses. Some of these compounds evaluated against anti-cancer, antioxidant and antimicrobial and give a good and moderate effect.
Keywords
2-Pyridone derivatives, Alkylation, Hydrazone formation, Sulpha-drugs and Click reaction.
Introduction
The 2-pyridone derivatives are heterocyclic compounds with vital substructures of many naturally compounds and has a wide spread applications. Some synthetic 2-pyridone intermediates and its metabolites demonstrate a broad spectrum in biological applications [1-4]. Naturally 2-pyridone derivatives like Ricinine [5] remarkable as CNS stimulant activities and the analogs elfamycin [6,7], ilicolicin [8,9], and efratomycin [10] used as antibiotic naturally product discovery. The synthetic 2-pyridone analogs as amrinone and milrinone have excellent vasodilating agents [11], and also used in treatment of the acute congestive heart failure. The core of naturally alkaloids [12] is 2-pyridone used as intermediate in the bacterial metabolism [13]. The 2-pyridone derivatives containing amino fragment in position-4 are very interest potential biological as psychotropic, nootropic or antiepileptic activity [14-16]. Acyclic 2-pyridone nucleosides as 4-(hydroxyl/chloro or bromo)-1-(4-hydroxy-2-hydroxymethylbutyl)pyridon-2-one showed inhibitory properties against Klenow exo-polymerase, M. MuLV and HIV-1 reverse transcriptases and, while its nucleotides has efficiency incorporated into DNA by Klenow fragment [17]. Recently, N-methylcytisine and (-)-cytisine containing on the substituent’s secondary N atom and in the 2-pyridone nucleoside analogue core has antiviral activity against hepatitis C (HCV), hepatitis B (HBV) Herps simplex 1 (HSV-1), anti-SARS-CoV and Influenza type A (H5N1 and H1N1) activities [18-22]. Sulfonyl biscompounds carrying 2-pyridone moiety exhibited a good anticancer activity against human breast cell line (MCF7) [23]. Development of new 2-pyridone derivatives has a low toxic effect used as antihelminthic [24] anti-inflammatory agents [25]. In continuation of our efforts [26-29], we synthesized the new 2-pyridone derivatives containing a new substituent at position-4 and 6, which containing amino group moiety to evaluate the biological activity against antimicrobial, anti-cancer and antioxidant.
Materials and Methods
Experimental
All melting points were determined with Electro thermal IA 9100 series digital melting point apparatus with open capillary tube and are uncorrected. The IR spectra were recorded on a Parkin-Elmer model 1600 FTIR spectrometer as KBr (discs) USA. Elemental analysis was determined on a Perkin Elmer 240 at micro-analytical center Cairo University. Mass spectra were recorded at 75 eV on Kratos spectrometer. All diagrams and calculations were performed using maXus (Bruker Nonius, Delft & MacScience, Japan). 1H NMR spectra were recorded in the appropriate deuterated solvents using a BRUKER (400 MHz) and 13C NMR (100 MHz) spectrometer. The above data were recorded in Zagazig University, Faculty of Science (Nucleic Acid Center Research). The coupling constants (J) are given in Hertz. The chemical shifts are expressed on the δ (ppm) scale using TMS as the standard reference.
General procedure for preparation of pyridin-2-(1H)-one-3-carbonitriles (1a-c)
A mixture of 4-aminoacetophenone (10 mmol), aromatic aldehydes namely (4-chlorobenzaldehyde, 2-thiophenecarboxaldehyde and 4-nitrobenzaldehyde) (10 mmol), ethyl cyanoacetate (10 mmol), and ammonium acetate (80 mmol), in absolute ethanol (30 mL) was refluxed for 24 hours. The reaction mentioned by TLC using (methylene chloride/MeOH 10:1), leave to cooling at room temperature, the formed precipitate was filtered off, washed with ethanol, dried and crystallized from Ethanol.
6-(4-Aminophenyl)-4-(4-chlorophenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (1a)
Yellow powder; yield 58.5%; m. p. 305-307ºC. IR (KBr): 3445, 3318, 3195 cm-1 (NH2 and NH), 2207 cm-1 (C≡N) and 1673 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=5.98 (s, 2H, NH2, exchange with D2O), 6.61 (d, 2H, J=8.40 Hz, Ar- H), 7.61 (d, 2H, J=8.00 Hz, Ar-H), 7.66 (d, 2H, J=8.40 Hz, Ar-H), 7.71 (d, 2H, J=8.40 Hz, Ar-H), 7.95 (s, 1H, Ar-H, pyridone, H-5), 12.36 (s, 1H, NH, exchange with D2O). Anal. Calcd for C18H12ClN3O (321.76): C, 67.19; H, 3.76; N, 13.06. Found: C, 67.17; H, 3.78; N, 13.04.
6-(4-Aminophenyl)-2-oxo-4-(thien-2-yl)-1,2-dihydropyridine-3-carbonitrile (1b)
Brown powder; yield 61%; m. p. 300-302ºC. IR (KBr): 3464, 3357 cm-1 (NH2 and NH), 2212 cm-1 (C≡N) and 1650 cm-1 (C=O, amide). 1H NMR (DMSO-d6) δ=5.97 (s, 2H, NH2, exchange with D2O), 6.62 (d, 2H, J=8.80 Hz, Ar-H), 6.74 (s, 1H, Ar-H, pyridone, H-5), 7.29 (dd, 1H, J=3.60,4.00 Hz, thiophene), 7.64 (d, 2H, J=8.40 Hz, Ar-H), 7.94 (d, 1H, J=4.80 Hz, thiophene),7.97 (d,1H, J=3.61 Hz, thiophene), 12.25 (s, 1H, NH, exchange with D2O). 13C NMR (DMSO-d6) δ=113.4, 117.5 (C≡N), 128.5, 129.1, 130.7, 131.1, 137.3, 150.4, 152.4 (Ar-C) and 162.3 (C=O). Anal. Calcd for C16H11N3OS (293.34): C, 65.51; H, 3.78; N, 14.32. Found: C, 65.50; H, 3.79; N, 14.30.
6-(4-Aminophenyl)-4-(4-nitrophenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (1c)
Yellow powder; yield 59%; m. p. 243-245ºC. IR (KBr): 3450, 3322, 3191 cm-1 (NH2 and NH), 2210 cm-1 (C≡N) and 1670 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=6.02 (s, 2H, NH2, exchange with D2O), 6.61 (d, 2H, J=8.80 Hz, Ar-H), 6.71 (s, 1H, Ar-H, pyridone, H-5), 7.68 (d, 2H, J=8.80 Hz, Ar-H), 7.94 (d, 2H, J=8.80 Hz, Ar-H), 8.36 (d, 2H, J=8.80 Hz, Ar-H), 12.46 (s, 1H, NH, exchange with D2O). Anal. Calcd for C18H12N4O3 (332.31): C, 65.06; H, 3.64; N, 16.86. Found: C, 65.03; H, 3.65; N, 16.85.
General procedure for alkylation
A mixture of pyridin-2-(1H)-one-3-carbonitriles 1a-c (10 mmol) and (10 mmol) potassium carbonate or potassium hydroxide was stirred in dry DMF (15 mL) for 1H, followed by the addition of the appropriate alkyl halide (11 mmol) namely propagyl / allyl bromides, 3-chloro-1,2-propandiol, chloroacetonitrile, methylbromo acetate, ethylbromoacetate. The reaction mixture was refluxed from 24-30 h, cooling, then poured into ice-water to give the crude product as precipitate, which in turn was filtered off and dried. Except for propagyl and allyl derivatives, the reaction mixture was stirred at room temperature for 14 h and stirred for 30 h in the case of chloroacetonitrile. The product was crystallized from ethanol 95%.
6-(4-Aminophenyl)-4-(4-chlorophenyl)-2-oxo-1-(prop-2-yn-l-yl)-1,2-dihydro-pyridine-3-carbonitrile (2a)
Orange powder; yield 78.7%; m. p. 108-110ºC; IR (KBr): 3437, 3373 cm-1 (NH2) and 2217 cm-1 (C≡N), 1625 cm-1 (C=O, amide). 1H NMR (DMSO-d6) δ=3.59 (s, 1H, ≡CH), 5.23 (d, 2H, J=2.00 Hz, N-CH2), 5.85 (s, 2H, NH2), 6.64 (d, 1H, J=8.80 Hz, Ar-H), 7.62 (d, 2H, J=8.80 Hz, Ar-H), 7.72 (d, 2H, J=8.40 Hz, Ar-H),7.94 (s, 1H, Ar-H, pyridone), 7.99 (d, 1H, J=8.40 Hz, Ar-H). 13C NMR (DMSO-d6): δ=54.58 (N-CH2), 77.84, 79.03 (C≡C), 86.88, 111.2, 113.5, 115.67 (C≡N), 122.8, 127.4, 129.1, 129.5, 130.6, 136.9, 147.8, 151.9, 157.6 and 164.4 (Ar-C, and C=O). Anal. Calcd or C21H14ClN3O (359.81): C, 70.10; H, 3.92; N, 11.68. Found: C, 70.12; H, 3.89; N, 11.67.
6-(4-Aminophenyl)-2-oxo-1-(prop-2-yn-l-yl)-4-(thien-2-yl)-1,2-dihydropyridine-3-carbonitrile (2b)
Canary yellow crystals; yield 75.5%; m. p. 200-202ºC; IR (KBr): 3438, 3362 cm-1 (NH2) and 2215 cm-1 (C≡N), 1623 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=3.59 (s, 1H, ≡CH), 5.22 (d, 2H, J=2.40 Hz, N-CH2), 5.86 (s, 2H, NH2), 6.65 (d, 2H, J=8.80 Hz, Ar-H), 7.30 (t, 1H, J=4.40,4.00 Hz, thiophene), 7.66 (s, 1H, Ar-H, pyridoine), 7.91 (d, 1H, J=5.20 Hz, thiophene), 7.93(d,1H, J=4.00 Hz, thiophene), 7.99 (d, 2H, J=8.40 Hz, Ar-H). 13C NMR (DMSO-d6): δ=54.34 (N-CH2), 77.82, 79.00 (C≡C), 86.91, 110.2, 113.5, 115.9 (C≡N), 122.9, 128.4, 129.15, 129.8, 130.3, 136.9, 147.2, 151.9, 158.0 and 163.1 (Ar-C, thiophene and C=O). Anal. Calcd for C19H13N3OS (331.08): C, 68.86; H, 3.95; N, 12.68. Found: C, 68.88; H, 3.96; N, 12.65.
6-(4-Aminophenyl)-4-(4-nitrophenyl)-2-oxo-1-(prop-2-yn-l-yl)-1,2-dihydro-pyridine-3-carbonitrile (2c)
Red powder; yield 73%; m. p.107-109ºC; IR (KBr): 3442, 3373 cm-1 (NH2), 2218 cm-1 (C≡N) and 1664 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=3.60 (s, 1H, ≡CH), 5.25 (d, 2H, J=2.00 Hz, N-CH2), 5.89 (s, 2H, NH2), 6.64 (d, 2H, J=8.80 Hz, Ar-H), 7.67 (s, 1H, Ar-H, pyridone), 7.97 (d, 2H, J=8.40 Hz, Ar-H), 8.01(d, 2H, J=8.40 Hz, Ar-H), 8.38 (d, 2H, J=8.80 Hz, Ar-H). 13C NMR (DMSO-d6): δ=55.50 (N-CH2), 77.86, 79.13 (C≡C), 89.88, 112.2, 113.5, 115.54 (C≡N), 123.8, 127.2, 128.1, 129.5, 132.6, 136.9, 147.6, 152.9, 159.6 and 167.4 (Ar-C and C=O). Anal. Calcd for C21H14N4O3 (371.36): C, 68.10; H, 3.81; N, 15.13. Found: C, 68.12; H, 3.80; N, 15.13.
2-(Allyloxy)-6-(4-aminophenyl)-4-(4-chlorophenyl)nicotinonitrile (3a)
Yellow powder; yield 61.8%; m. p. 120-122ºC; IR (KBr): 3440, 3364 cm-1 (NH2) and 2215 cm-1 (C≡N). 1H NMR (DMSO-d6): δ=5.07 (d, 2H, J=5.20 Hz, OCH2), 5.29 (d, 1H, J=10.4 Hz,=CH H), 5.47 (d, 1H, J=17.2 Hz,=CH H), 5.83 (s, 2H, NH2), 6.11 (m, 1H, CH=), 6.64 (d, 2H, J=8.00 Hz, Ar-H), 7.55 (s, 1H, Ar-H, pyridone), 7.62 (d, 2H, J=8.40 Hz, Ar-H), 7.72 (d, 2H, J=8.40 Hz, Ar-H), 7.96 (dd, 2H, J=8.40 Hz, Ar-H). 13C NMR (DMSO-d6): δ=67.45 (OCH2), 86.98, 110.7, 113.5, 115.1 (C≡N), 118.8, 123.8, 127.4, 128.9, 129.5, 132.2, 133.1, 137.1, 148.1, 153.9, 159.1 and 165.9 (Ar- C carbon). Anal. Calcd for C21H16ClN3O (361.82): C, 69.71; H, 4.46; N, 11.61. Found: C, 69.70; H, 4.45; N, 11.63.
2-(Allyloxy)-6-(4-aminophenyl)-4-(thien-2-yl)nicotinonitrile (3b)
Yellow crystals; yield 80.8%; m. p. 180-182ºC. IR (KBr): 3478, 3373 cm-1 (NH2) and 2207 cm-1 (C≡N). 1H NMR (DMSO-d6): δ=5.06 (d, 2H, J=5.20 Hz, OCH2), 5.29 (d, 1H, J=10.4 Hz,=CH H), 5.47 (d, 1H, J=17.2 Hz,=CH H), 5.84 (s, 2H, NH2), 6.11 (m, 1H, CH=), 6.65 (d, 2H, J=8.80 Hz, Ar-H), 7.29 (t, 1H, J=4.41 Hz, thiophene), 7.62 (s, 1H, pyridione), 7.90 (d, 1H, J=5.20 Hz, thiophene), 7.92 (d, 1H, J=3.62 Hz, thiophene), 7.95 (d, 2H, J=8.40 Hz, Ar-H). 13C NMR (DMSO-d6): δ=67.03 (OCH2), 86.94, 109.7, 113.5, 116.1 (C≡N), 117.8, 123.1, 128.4, 128.9, 129.7, 130.2, 133.1, 137.1, 147.1, 151.9, 158.1 and 163.9 (Ar-C and thiophene carbon). Anal. Calcd for C19H15N3OS (333.41): C, 68.45; H, 4.53; N, 12.60. Found: C, 68.46; H, 4.52; N, 12.62.
2-(Allyloxy)-6-(4-aminophenyl)-4-(4-nitrophenyl)nicotinonitrile (3c)
Yellow crystals; yield 54%; m. p. 140-142ºC; IR (KBr): 3479, 3374 cm-1 (NH2) and 2216 cm-1 (C≡N). 1H NMR (DMSO-d6): δ=5.09 (d, 2H, J=5.60 Hz, OCH2), 5.30 (d, 1H, J=10.4 Hz,=CH H), ), 5.48 (d, 1H, J=17.2 Hz,=CH H), 5.85 (s, 2H, NH2), 6.12 (m, 1H, CH=), 6.66 (d, 2H, J=8.80, Ar-H), 7.65 (s, 1H, Ar-H, pyridone), 7.97 (d, 2H, J=8.80 Hz, Ar-H), 8.03 (d, 2H, J=8.80 Hz, Ar-H), 8.38 (d, 2H, J=8.80 Hz, Ar-H). 13C NMR (DMSO-d6): δ=66.98 (OCH2), 87.98, 111.7, 113.3, 115.5 (C≡N), 119.8, 124.8, 127.2, 128.1, 128.5, 132.6, 133.9, 139.1, 148.4, 154.9, 159.6 and 168.9 (Ar-C, carbon). Anal. Calcd for C21H16N4O3 (372.38): C, 67.73; H, 4.33; N, 15.05. Found: C, 67.74; H, 4.32; N, 15.06.
6-(4-Aminophenyl)-4-(4-chlorophenyl)-2-(2,3-dihydroxypropoxy) nicotinonitrile (4a)
Yellow powder; yield 82%; m. p. 140-1420C. IR (KBr): 3397 cm-1 (broad, OH and NH2), 2215 cm-1 (C≡N). 1H NMR (DMSO-d6/D2O): δ=2.96-3.05 (m, 2H, CH2(c)), 3.63-3.71 (m, 1H, CH(b)), 4.41 (dd, 1H, J=5.61 Hz, CH H(a), diasterotropic proton), 4.52 (dd, 1H, J=3.60, 4.10 Hz, CH H (a), diasterotropic proton), 7.53 (s, 1H, pyridone), 7.55-8.04 (m, 8H, Ar-H). 13C NMR (DMSO-d6): δ=62.69 (CH(c)), 68.30 (CH(b)), 69.50 (CH(a)), 87.11, 113.50, 116.1 (C≡N), 123.1, 128.4, 129.0, 129.6, 130.1, 137.2, 147.1, 151.8, 158.0 and 164.5 (Ar-C). Anal. Calcd. for C21H18ClN3O3 (395.84): C, 63.72; H, 4.58; N, 10.62. Found: C, 63.70; H, 4.57; N, 10.60.
6-(4-Aminophenyl)-2-(2,3-dihydroxypropoxy)-4-(thiophen-2-yl)-nicotinonitrile (4b)
Brown powder; yield 81%; m. p. 138-1400C. IR (KBr): 3431 cm-1 (broad, OH and NH2), 2211 cm-1 (C≡N). 1H NMR (DMSO-d6/D2O): δ=3.49 (m, 2H, CH2(c)), 4.38 (m, 1H, CH(b)), 4.52 (dd, 1H, J=6.0 Hz, CH H(a), diasterotropic proton), 4.56 (dd, 1H, J=4.00, 4.40 Hz, CH H (a), diasterotropic proton), 6.65 (d, 2H, J=8.4 Hz, Ar-H), 7.27 (t, 1H, J=4.0 Hz, thiophene), 7.50 (s, 1H, Ar-H, pyridone), 7.82 (d, 1H, J=4.81 Hz, thiophene), 7.85 (d, 1H, J=3.62 Hz, thiophene), 7.91 (d, 2H, J=8.40 Hz, Ar-H). 13C NMR (DMSO-d6): δ=62.69 (CH(c)), 68.30 (CH(b)), 69.50 (CH(a)), 87.11, 113.50, 116.1 (C≡N), 123.1, 128.4, 129.0, 129.6, 130.1, 137.2, 147.1, 151.8, 158.0 and 164.5 (Ar-C). Anal. Calcd. for C19H17N3O3S (367.42): C, 62.11; H, 4.66; N, 11.44. Found: C, 62.13; H, 4.66; N, 11.45.
6-(4-Aminophenyl)-4-(4-chlorophenyl)-2-oxo-1-(cyanomethyl)-1,2-dihydro-pyridine-3-carbonitrile (5a)
Yellow powder; yield 79%; m. p.180-182ºC; IR (KBr): 3448, 3363 cm-1 (NH2) and 2210 cm-1 (2 C≡N), 1678 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=5.47 (s, 2H, N-CH2), 5.98 (s, 2H, NH2), 6.63 (d, 2H, J=8.40 Hz, Ar-H), 7.33 (s, 1H, Ar-H, pyridone), 7.46 (d, 2H, J=8.00 Hz, Ar-H), 7.63-7.70 (m, 4H, Ar-H). 13C NMR (DMSO-d6): δ=58.82 (N-CH2), 114.4, 115.6, 115.6 (2C≡N), 127.2, 128.4, 131.2, 132.0, 138.3, 151.2, 153.2 (Ar-C) and 167.3 (C=O). Anal. Calcd for C20H13ClN4O (360.80): C, 66.58; H, 3.63; N, 15.53. Found: C, 66.54; H, 3.60; N, 15.55.
6-(4-Aminophenyl)-1-(cyanomethyl)-2-oxo-4-(thien-2-yl)-1,2-dihydropyridine-3-carbonitrile (5b)
Black powder; yield 72%; m. p.200-202ºC; IR (KBr): 3491, 3394 cm-1 (NH2), 2218 cm-1 (2C≡N) and 1631cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=5.45 (s, 2H, NCH2), 5.95 (s, 2H, NH2), 6.67 (d, 2H, J=8.40 Hz, Ar-H), 7.31 (t, 1H, J=4.40, 4.00 Hz, thiophene), 7.74 (s, 1H, Ar-H, pyridone), 7.94 (d, 1H, J=6.80 Hz, thiophene), 7.96 (d,1H, J=2.41 Hz, thiophene), 8.03 (d, 2H, J=8.00 Hz, Ar-H). 13C NMR (DMSO-d6): δ=57.87 (N-CH2), 113.4, 115.3, 115.8 (2C≡N), 127.5, 128.1, 130.2, 132.1, 137.3, 151.4, 152.2 (Ar-C) and 165.3 (C=O). Anal. Calcd for C18H12N4OS (332.38): C, 65.04; H, 3.64; N, 16.86. Found: C, 65.01; H, 3.66; N, 16.86.
N-(4-(4-(4-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyridin-2-yl)phenyl)-acetamide (6a)
Yellow powder; yield 87%; m. p. 250-252 0C. IR (KBr): 3450, 3388 cm-1 (2NH), 2214 cm-1 (C≡N) and 1779 (COCH3), 1671 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=2.08 (s, 3H, CH3), 6.80 (s, 1H, Ar-H, pyridone), 7.47 (d, 2H, J=8.40 Hz, Ar-H), 7.63 (d, 2H, J=8.80 Hz, Ar-H), 7.70 (d, 2H, J=8.00 Hz, Ar-H), 7.86 (d, 2H, J=8.40 Hz, Ar-H), 10.24 (s, 1H, NHCOCH3), 12.75 (s, 1H, NH). 13C NMR (DMSO-d6): δ=32.83 (COCH3), 112.4, 114.3, 115.2 (C≡N), 127.3, 129.1, 131.2, 132.0, 138.3, 151.2 (Ar-C), 156.2 and 167.3 (2C=O). Anal. Calcd for C20H14ClN3O2 (363.80): C, 66.03; H, 3.88; N, 11.55. Found: C, 66.05; H, 3.85; N, 11.55.
1-Acetyl-6-(4-aminophenyl)-2-oxo-4-(thien-2-yl)-1,2-dihydropyridine-3-carbonitrile (6b)
Yellow powder; yield 85%; m. p. 260-2610C. IR (KBr): 3449, 3339 cm-1 (2NH), 2205 cm-1 (C≡N) and 1751, 1653 cm-1 (2C=O). 1H NMR (DMSO-d6): δ=2.08 (s, 3H, CH3), 6.88 (s, 1H, Ar-H, pyridone), 7.31 (t, 1H, J=3.61 Hz, thiophene), 7.71 (d, 2H, J=8.40 Hz, Ar-H), 7.84 (d, 2H, J=8.00 Hz, Ar-H),7.97 (d, 1H, J=4.40 Hz, thiophene), 8.03 (d,1H, J=2.00 Hz, thiophene), 10.24 (s, 1H, NHCOCH3), 12.59 (s, 1H, NH). Anal. Calcd for C18H13N3O2S (335.38): C, 64.46; H, 3.91; N, 12.53. Found: C, 64.44; H, 3.91; N, 12.50.
Methyl 2-((6-(4-aminophenyl)-4-(4-chlorophenyl)-3-cyanopyridine-2-yl)oxy)acetate (7a)
Brown powder; yield 99%; m. p.120-1220C. IR (KBr): 3471, 3374 cm-1 (NH2), 2217 cm-1 (C≡N) and 1751 (C=O acetoxy). 1H NMR (DMSO-d6) δ=3.71 (s, 3H, OCH3), 5.12 (s, 2H, OCH2), 5.87 (br, 2H, NH2), 6.62 (d, 2H, J=8.80 Hz, Ar-H), 7.61 (s, 1H, Ar-H, pyridone), 7.64 (d, 2H, J=8.40 Hz, Ar-H), 7.74 (d, 2H, J=8.80 Hz, Ar-H), 7.87 (d, 2H, J=8.80 Hz, Ar-H). 13C NMR (DMSO-d6): δ=51.83 (OCH3), 63.39 (OCH2), 88.77, 111.92, 112.06, 113.51, 115.41 (C≡N), 122.85, 123.78, 128.85, 129.02, 130.45, 134.88, 150.89, 152.04, 154.48), 157.85, 162.76, 168.92 and 171.29 (Ar-C and C=O). Anal. Calcd for C21H16ClN3O3 (393.82): C, 64.05; H, 4.09; N, 10.67. Found: C, 64.08; H, 4.08; N, 10.66.
Methyl 2-((6-(4-aminophenyl)-3-cyano-4-(thien-2-yl)pyridin-2-yl)oxy)acetate (7b)
Yellow powder; yield 60%; m. p. 95-970C. IR (KBr): 3454, 3374 cm-1 (NH2) and 2214 cm-1 (C≡N), 1755 cm-1 (C=O acetoxy). 1H NMR (DMSO-d6): δ=3.70 (s, 3H, OCH3), 5.11 (s, 2H, OCH2), 5.88 (s, 2H, NH2), 6.63 (d, 2H, J=8.42 Hz, Ar-H), 7.30 (t, 1H, J=3.61 Hz, thiophene), 7.66 (s, 1H, pyridone), 7.87 (d, 2H, J=8.40 Hz, Ar-H), 7.92 (d, 1H, J=3.2 Hz, thiophene), 7.95 (d, 1H, J=4.00 Hz, thiophene). Anal. Calcd for C19H15N3O3S (365.41): C, 62.45; H, 4.14; N, 11.50. Found: C, 62.48; H, 4.15; N, 11.55.
Methyl 2-((6-(4-aminophenyl)-4-(4-nitrophenyl)-3-cyanopyridine-2-yl)oxy)acetate (7c)
Brown powder; yield 90%; m. p. 110-1120C. IR (KBr): 3461, 3373 cm-1 (NH2), 2219 cm-1 (C≡N) and 1754 (C=O acetoxy). 1H NMR (DMSO-d6): δ=3.80 (s, 3H, OCH3), 5.13 (s, 2H, OCH2), 5.89 (br, 2H, NH2), 6.65 (d, 2H, J=8.80 Hz, Ar-H), 7.65 (s, 1H, Ar-H, pyridone), 7.68 (d, 2H, J=8.40 Hz, Ar-H), 7.71 (d, 2H, J=8.80 Hz, Ar-H), 7.91 (d, 2H, J=8.80 Hz, Ar-H). 13C NMR (DMSO-d6): δ=51.39 (OCH3), 63.39 (OCH2), 88.77, 111.92, 112.06, 113.51, 115.41 (C≡N), 122.85, 123.78, 128.3, 129.1, 131.4, 135.8, 150.2, 152.2, 154.2, 158.8, 162.6, 169.0 and 170.9 (Ar-C and C=O). Anal. Calcd for C21H16N4O5 (404.38): C, 62.37; H, 3.99; N, 13.86. Found:C, 62.33; H, 3.97; N, 13.86.
Ethyl 2-(6-(4-aminophenyl)-4-(4-chlorophenyl)-3-cyano-2-oxopyridin-1(2H)-yl)acetate (8a)
Yellow powder; yield 88%; m. p. 190-1920C. IR (KBr): 3454, 3363 cm-1 (NH2), 2214 cm-1 (C≡N), 1735 cm-1 (C=O acetoxy) and 1658 cm-1 (C=O amide). 1H NMR (DMSO-d6): δ=1.18 (t, 3H, J=6.82 Hz, CH3), 4.00 (s, 2H, NCH2), 4.11 (q, 2H, J=6.82 Hz, OCH2CH3), 5.91 (s, 2H, NH2), 6.62 (d, 2H, J=8.40 Hz, Ar-H), 6.85 (s, 1H, pyridone), 7.89-8.02 (m, 6H, Ar-H). Anal. Calcd for C22H18ClN3O3 (407.85): C, 64.79; H, 4.45; N, 10.30. Found: C, 64.81; H, 4.45; N, 10.34.
Ethyl 2-(6-(4-aminophenyl)-3-cyano-2-oxo-4-(thien-2-yl)pyridin-1(2H)-yl)acetate (8b)
Yellow powder; yield 77%; m. p. 180-1820C. IR (KBr): 3464, 3383 cm-1 (NH2), 2214 cm-1 (C≡N), 1728 cm-1 (C=O acetoxy) and 1627 cm-1 (C=O amide).1H NMR (DMSO-d6): δ=1.20 (t, 3H, J=7.23 Hz, CH3), 4.02 (s, 2H, NCH2), 4.12 (q, 2H, J=7.22 Hz, OCH2CH3), 5.82 (s, 2H, NH2), 6.65 (d, 2H, J=8.00 Hz, Ar-H), 6.78 (s, 1H, pyridone), 7.30 (t, 3H, J=3.60 Hz, thiophene), 7.70 (d, 2H, J=8.00 Hz, Ar-H), 7.95 (d, 1H, J=4.81 Hz, thiophene), 7.99 (d, 1H, J=3.61 Hz, thiophene). Anal. Calcd for C20H17N3O3S (379.43): C, 63.31; H, 4.52; N, 11.07. Found: C, 63.35; H, 4.52; N, 11.11.
General method for preparation of hydrazide (9a and 9b)
Hydrazin hydrate (10 mmol) was added to a solution of compound ester of 7a,b and (10 mmol) in ethanol (10 mL), the reaction mixture was refluxed for 24 h, and followed by TLC, cool then flittered off the formed precipitate and re-crystallized it from ethanol.
2-((6-(4-Aminophenyl)-4-(4-chlorophenyl)-3-cyanopyridine-2-yl)oxy)aceto-hydrazide (9a)
Yellow crystals; yield 85%, m. p. 160-1620C. IR (KBr): 3455, 3369 cm-1 (NH and 2NH2), 2211 cm-1 (C≡N) and 1627 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=2.78 (s, 2H, NH2), 4.84 (s, 2H, OCH2), 5.97 (s, 2H, NH2), 6.61 (d, 2H, J=8.80 Hz, Ar-H), 6.65 (s, 1H, Ar-H, pyridone), 7.61 (d, 2H, J=8.40 Hz, Ar-H), 7.66 (d, 2H, J=8.40 Hz, Ar-H),7.70 (d, 2H, J=8.40 Hz, Ar-H), 9.09 (br, 1H, NH). Anal. Calcd for C20H16ClN5O2 (393.83): C, 60.99; H, 4.09; N, 17.78. Found: C, 60.97; H, 4.09; N, 17.75.
2-((6-(4-Aminophenyl)-3-cyano-4-(thiophen-2-yl)pyridin-2-yl)oxy)acetohydrazide (9b)
Yellow powder; yield 82%, m. p. 165-1670C. IR (KBr): 3286 cm-1 (br, NH and 2NH2), 2213 cm-1 (C≡N) and 1661 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=2.75 (s, 2H, NH2), 4.84 (s, 2H, OCH2), 5.96 (s, 2H, NH2), 6.64 (d, 2H, J=8.80 Hz, Ar-H), 7.31 (t, 1H, J=4.00 Hz, thiophene), 7.63 (s, 1H, Ar-H, pyridone), 7.90 (d, 1H, J=6.20 Hz, thiophene), 7.91 (d, 1H, J=4.40 Hz, thiophene), 7.99 (d, 2H, J=8.80 Hz, Ar-H), 9.04 (br, 1H, NH). Anal. Calcd for C18H15N5O2S (365.41): C, 59.16; H, 4.14; N, 19.17. Found: C, 59.19; H, 4.14; N, 19.15.
2-((6-(4-Aminophenyl)-4-(4-chlorophenyl)-3-cyanopyridin-2-yl)oxy)-N'-benzylideneacetohydrazide (10a)
Brown powder; yield 79%; m. p. 290-292ºC; IR (KBr): 3429 cm-1 (NH) and 2216 cm-1 (C≡N), 1638 cm-1 (C=O, amide). 1H NMR (DMSO-d6) δ=4.83 (s, 2H, OCH2), 6.01 (s, 2H, NH2), 6.61 (d, 2H, J=8.80 Hz, Ar-H), 6.64 (s, 1H, Ar-H, pyridone), 7.25-7.50 (m, 5H, Ar-H), 7.59 (d, 2H, J=8.40 Hz, Ar-H), 7.65 (d, 2H, J=8.80 Hz, Ar-H), 7.69 (d, 2H, J=8.40 Hz,Ar-H), 8.70 (s, 1H, CH=N), 10.11 (s, 1H, NH). 13C NMR (DMSO-d6): δ=66.65 (OCH2), 101.3, 112.9, 115.5 (C≡N), 116.5, 118.0, 128.7, 129.0, 129.2, 129.6, 130.0, 130.4, 130.6, 130.7, 131.9, 134.9, 136.4, 151.5, 161.4, 162.1 and 165.3 (Ar-C and C=O). Anal. Calcd for C27H20ClN5O2 (481.9): C, 67.29; H, 4.18; N, 14.53. Found: C, 67.25; H, 4.14; N, 14.53.
2-((6-(4-Aminophenyl)-3-cyano-4-(thien-2-yl)pyridin-2-yl)oxy)-N'-benzylidene-acetohydrazide (10b)
Yellow powder; yield 81%; m. p. 245-247ºC; IR (KBr): 3429 cm-1 (br, NH, NH2) and 2215 cm-1 (C≡N), 1632 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=5.02 (s, 2H, OCH2), 5.84 (s, 2H, NH2), 6.65 (d, 2H, J=8.40 Hz, Ar-H), 7.72 (s, 1H, Ar-H, pyridone), 7.35-8.26 (m, 10H, Ar-H), 8.71 (s, 1H, CH=N), 10.23 (s, 1H, NH). 13C NMR (DMSO-d6): δ=65.89 (OCH2), 102.3, 113.2, 115.7 (C≡N), 116.2, 119.0, 128.3, 128.8, 129.1, 129.7, 130.1, 130.4, 130.8, 130.9, 132.9, 134.7, 136.6, 152.5, 162.4, 163.1 and 166.7 (Ar-C and C=O). Anal. Calcd for C25H19N5O2S (453.52): C, 66.21; H, 4.22; N, 15.44. Found: C, 66.20; H, 4.22; N, 15.48.
2-((6-(4-Aminophenyl)-4-(4-chlorophenyl)-3-cyanopyridin-2-yl)oxy)-N'-(4-fluorobenzylidene)acetohydrazide (11a)
Brown powder; yield 78.7%; m. p. 140-142ºC; IR (KBr): 3427 cm-1 (broad, NH and NH2) and 2216 cm-1 (C≡N), 1635 cm-1 (C=O, amide). 1H NMR (DMSO-d6) δ=4.34 (s, 2H, OCH2),6.02 (s, 2H, NH2), 6.61 (d, 2H, J=8.40 Hz, Ar-H), 7.13 (s, 1H, Ar-H, pyridone), 7.24 (d, 2H, J=8.80 Hz, Ar-H), 7.34 (d, 2H, J=8.80 Hz, Ar-H), 7.60 (d, 2H, J=8.40 Hz, Ar- H), 7.65 (d, 2H, J=8.80 Hz, Ar-H), 7.70 (d, 2H, J=8.40 Hz, Ar-H), 8.70 (s, 1H, CH=N), 12.37 (br, 1H, NH).13C NMR (DMSO-d6): δ=67.10 (OCH2), 113.4, 100.3, 115.9 (C≡N), 116.21, 117.0, 128.7, 129.1, 129.2, 129.3, 130.0, 130.4, 130.6, 130.7, 131.9, 134.9, 135.4 152.5, 160.4, 162.1 and 162.3 (Ar-C and C=O). Anal. Calcd for C27H19ClFN5O2 (499.92): C, 64.87; H, 3.83; N, 14.01. Found: C, 64.85; H, 3.80; N, 14.01.
2-((6-(4-Aminophenyl)-3-cyano-4-(thien-2-yl)pyridin-2-yl)oxy)-N'-(4-fluorobenzylidene)acetohydrazide (11b)
Yellow powder; yield 81%; m. p.145-146ºC; IR (KBr): 3428 cm-1 (br, NH and NH2) and 2217 cm-1 (C≡N), 1632 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=4.98 (s, 2H, OCH2), 5.85 (s, 2H, NH2), 6.64 (d, 2H, J=8.80 Hz, Ar-H), 7.28 (s, 1H, Ar-H, pyridone), 7.22-7.37 (m, 5H, Ar-H), 7.39-8.21 (m, 5H, Ar-H), 8.83 (s, 1H, CH=N), 10.21 (s, 1H, NH). 13C NMR (DMSO-d6): δ=66.78 (OCH2), 113.2, 101.3, 115.5 (C≡N), 116.6, 117.1, 128.5, 129.1, 129.2, 129.3, 130.1, 130.5, 130.6, 130.6, 132.9, 134.5, 135.4, 152.4, 161.4, 162.2 and 165.3 (Ar-C and C=O). Anal. Calcd for C25H18FN5O2S (471.51): C, 63.68; H, 3.85; N, 14.85. Found: C, 63.68; H, 3.85; N, 14.85.
6-(4-Aminophenyl)-2-(2-(3,5-dimethyl-1H-pyrazol-1-yl)-2-oxoethoxy)-4-(thien-2-yl)nicotinonitrile (12)
Brown powder; yield 75.5%; m. p.137-139ºC; IR (KBr): 3430 cm-1 (NH2) and 2214 cm-1 (C≡N), 1649 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=2.10 (s, 3H, CH3), 2.45 (s, 3H, CH3), 4.79 (s, 2H, OCH2), 5.94 (s, 2H, NH2), 6.04 (s, 1H, CH, pyrazole proton), 6.63 (t, 1H, J=4.40,4.00 Hz, thiophene), 7.29 (d, 1H,thiophene), 7.64 (s, 1H, Ar-H, pyridone), 7.87 (m, 5H,Ar-H). Anal. Calcd for C23H19N5O2S (429.49): C, 64.32; H, 4.46; N, 16.31. Found: C, 64.35; H, 4.46; N, 16.30.
Ethyl 3-(2-(2-((6-(4-aminophenyl)-3-cyano-4-(thien-2-yl)pyridin-2-yl)oxy)acetyl)-hydrazono)butanoate (13)
Brown powder; yield 75%; m. p. 153-154ºC; IR (KBr): 3431 cm-1 (br, NH, NH2) and 2214 cm-1 (C≡N), 1737 (C=O, ester), 1630 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=1.14 (t, 3H, J=6.00 Hz, CH2CH3), 1.76 (s, 3H, CH3), 2.45 (s, 2H, CH2COO), 4.13 (q, 2H, J=6.00 Hz, OCH2CH3), 5.04 (s, 2H, OCH2CO), 5.94 (s, 2H, NH2), 6.63 (t, 1H, J=7.60 Hz, thiophene), 7.29 (s, 1H, Ar-H, pyridone), 7.63 (d, 1H, thiophene), 7.85 (d, 2H, J=8.40 Hz, Ar-H). 7.92 (m, 3H, Ar-H and thiophene), 10.11(s, 1H, NH). Anal. Calcd for C24H23N5O4S (477.54): C, 60.36; H, 4.85; N, 14.67. Found: C, 60.39; H, 4.83; N, 14.66.
2-(6-(4-Aminophenyl)-4-(4-chlorophenyl)-3-cyano-2-oxopyridin-1(2H)-yl)aceto-hydrazide (14a)
Brown powder; yield 79%; m. p. 200-202ºC; IR (KBr): 3436, 3372 cm-1 (NH and NH2) and 2212 cm-1 (C≡N), 1650 cm-1 (C=O, amide). 1H NMR (DMSO-d6) δ=2.91 (s, 2H, NH2), 5.98 (s, 2H, NH2), 6.61 (d, 2H, J=8.40 Hz, Ar-H), 6.65 (s, 1H, Ar-H, pyridone), 7.61 (d, 2H, J=8.00 Hz, Ar-H), 7.65 (d, 2H, J=8.40 Hz, Ar-H), 7.71 (d, 2H, J=8.00 Hz, Ar-H), 10.75 (br, 1H, NH). Anal. Calcd for C20H16ClN5O2 (393.83): C, 60.99; H, 4.09; N, 17.78. Found: C, 60.95; H, 4.09; N, 17.77.
2-(6-(4-Aminophenyl)-3-cyano-2-oxo-4-(thien-2-yl)pyridin-1(2H)-yl)aceto-hydrazide (14b)
Yellow powder; yield 73%; m. p. 220-222ºC; IR (KBr): 3384 cm-1 (broad, NH, NH2) and 2209 cm-1 (C≡N), 1641 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=2.90 (s, 2H, NH2), 5.89 (s, 2H, NH2), 6.66 (d, 2H, J=8.40 Hz, Ar-H), 6.72 (s, 1H, Ar-H, pyridone), 7.31 (t, 1H, J=4.00 Hz, thiophene), 7.65 (d, 2H, J=8.40 Hz, Ar-H), 7.82 (d, 1H, J=6.00 Hz, thiophene), 7.93 (d, 1H, J=4.00 Hz, thiophene), 10.51 (br, 1H, NH). Anal. Calcd for C18H15N5O2S (365.41): C, 59.16; H, 4.14; N, 19.17. Found: C, 59.16; H, 4.12; N, 19.16.
Ethyl 3-(2-(2-(6-(4-aminophenyl)-4-(4-chlorophenyl)-3-cyano-2-oxopyridin-1(2H)-yl)acetyl)hydrazono)butanoate (15)
Yellow powder; yield 77%; m. p. 260-262ºC; IR (KBr): 3442 cm-1 (NH and NH2) and 2217 cm-1 (C≡N), 1735 (C=O, ester), 1644 cm-1 (C=O, amide). 1H NMR (DMSO-d6): δ=1.17 (t, 3H, J=5.81 Hz, CH3), 1.77 (s, 3H, CH3), 2.41 (s, 2H, CH2CO), 4.12 (q, 2H, J=5.80 Hz, OCH2CH3), 4.81 (s, 2H, N-CH2), 5.94 (s, 2H, NH2), 6.60 (d, 2H, J=8.40 Hz, Ar-H), 7.28 (s, 1H, Ar-H, pyridone), 7.81-7.96 (m, 6 H, Ar-H), 9.94 (s, 1H, NH). Anal. Calcd for C26H24ClN5O4 (505.95): C, 61.72; H, 4.78; N, 13.84. Found: C, 61.75; H, 4.74; N, 13.84.
2-(6-(4-Aminophenyl)-3-cyano-2-oxo-4-(thien-2-yl)pyridin-1(2H)-yl)-N'-(1-(4-aminophenyl)ethylidene) acetohydrazide (16)
Yellow powder; yield 78%; m. p.240-242ºC; IR (KBr): 3428 cm-1 (br, 2NH2), 2207 cm-1 (C≡N), 1638 cm-1 (2C=O, amide). 1H NMR (DMSO-d6): δ=2.37 (s, 3H, CH3), 4.32 (s, 2H, N-CH2), 5.44 (s, 2H, NH2), 6.01 (s, 2H, NH2), 6.54 (d, 2H, J=8.40 Hz, Ar-H), 6.71(d, 2H, J=8.80 Hz, Ar-H), 7.30 (t, 1H, J=4.00 Hz, thiophene), 7.53 (d, 2H, J=8.40 Hz, Ar-H), 7.64 (d, 2H, J=8.40 Hz, Ar-H). 7.72 (s, 1H, Ar-H, pyridone), 7.94 (d, 1H, J=4.80 Hz, thiophene), 7.98 (d,1H, J=3.20 Hz, thiophene), 12.33 (s, 1H, NH). Anal. Calcd for C26H22N6O2S (482.56): C, 64.71; H, 4.60; N, 17.42. Found: C, 64.75; H, 4.60; N, 17.40.
General procedure for preparation of hydrazonide derivatives (17 and 18)
Asolution of NaNO2 (4.2 g, 0.06 mol) in H2O (10 mL) was added to a soln of 1b (0.02 mol) in (20 mL) glacial AcOH, in ice bath in portion-wise, leave the mixture stirring in ice-bath for 10 min., then added active methylene compounds namely (ethyl cyanoacetate, acetylacetone, respectivily) (0.02 mol) in (30 mL) ethanol, and leave to stirring at room temperture for 2 h, the precipitate was formed collected by filtration, dry, crystallized from ethanol.
Ethyl 2-cyano-2-(2-(4-(5-cyano-6-oxo-4-(thien-2-yl)-1,6-dihydropyridin-2-yl)phenyl)hydrazono)acetate (17)
Yellow powder; yield 79%; m. p. 140-142ºC; IR (KBr): 3437 cm-1 (NH) 2267, 2217 cm-1 (2C≡N), and 1728 cm-1 (C=O). 1H NMR (DMSO-d6): δ=1.28 (t, 3H, J=7.21 Hz, CH3), 4.28 (q, 2H, J=7.22 Hz, CH2,), 6.80 (s, 1H, Ar- H, pyridone), 6.89 (d, 2H, J=8.80 Hz, Ar-H), 7.31 (t, 1H, J=3.61 Hz, thiophene), 7.57 (d, 2H, J=8.80 Hz, Ar-H), 7.75 (d, 1H, J=6.80 Hz, thiophene), 7.86 (d, 1H, J=4.80 Hz, thiophene), 12.31 (s, 1H, NH), 12.41 (s, 1H, NH). 13C NMR (DMSO-d6): δ=14.10 (CH2CH3), 61.75 (CH2CH3), 113.3, 115.7, 116.1, (2 C≡N), 105.5, 117.2, 118.5, 120.0, 127.7, 128.5, 130.1, 131.5, 144.2, 145.2 and 150.6 (Ar-C, thiophene carbon), 160.6, 162.2 (2C=O). Anal. Calcd for C21H15N5O3S (417.44): C, 60.42; H, 3.62; N, 16.78. Found: C, 60.45; H, 3.62; N, 16.79.
6-(4-(2-(2,4-Dioxopentan-3-ylidene)hydrazinyl)phenyl)-2-oxo-4-(thien-2-yl)-1,2-dihydropyridine-3-carbonitrile (18)
Yellow powder; yield 79%; m. p. 190-192ºC; IR (KBr): 3448 cm-1 (NH) and 2211 cm-1 (C≡N), 1632 cm-1 (C=O). 1H NMR (DMSO-d6): δ=2.44 (s, 3H, CH3), 2.54 (s, 3H, CH3),6.93 (s, 1H, Ar-H, pyridone), 7.30 (t, 1H, J=4.40 Hz, thiophene), 7.67 (d, 2H, J=8.80 Hz, Ar-H), 7.95 (d, 3H, J=6.80 Hz, Ar-H + thiophene), 8.02 (d, 1H, J=3.20 Hz, thiophene), 13.76 (s, 1H, NH), 13.79 (s, 1H, NH). 13C NMR (DMSO-d6): δ=24.10 (2COCH3), 105.5, 113.3, 115.7 (C≡N), 116.2, 117.7, 118.2, 121.0, 127.7, 128.2, 130.0, 131.5, 144.5, 145.2 and 152.6 (Ar-C, thiophene carbon), 165.6, 169.2 (C=O, amide and 2C=O, sym. ketone). Anal. Calcd for C21H16N4O3S (404.44): C, 62.36; H, 3.99; N, 13.85. Found: C, 62.33; H, 3.99; N, 13.82.
6-(4-Aminophenyl)-4-(thien-2-yl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (19)
A mixture of 6-(4-aminophenyl)-2-oxo-4-(thien-2-yl)-1,2-dihydropyridine-3-carbonitrile (1b) (0.01 mol) and P2S5 (0.01 mol) was refluxed in dry pyridine (20 mL) for 10 hrs. The solvent was evaporated and the residue was treated with dil. acetic acid. The solid product was filtered and crystallized from absolute ethanol to give 19, as black powder, yield 88%; m. p. 120-122°C. IR (KBr): 3423 cm-1 (broad, NH and NH2) and 2214 cm-1 (C≡N) and 1258 cm-1 (C=S). Anal. Calcd for C16H11N3S2 (309.41): C, 62.11; H, 3.58; N, 13.58. Found: C, 62.13; H, 3.55; N, 13.58.
2-((6-(4-Aminophenyl)-3-cyano-4-(thien-2-yl)pyridine-2-yl)thio)acetic acid (20)
This intermediate was prepared through the S-alkylation reaction using chloroacetic acid to alkylate the sulfhydroxyl group of 19. A solution of 19 (10 mmol) in abs. ethanol (30 mL), KOH (10 mmol) and chloroacetic acid (10 mmol) were added and the mixture was refluxed for 15 hrs. The hot mixture was filtered and the ethanolic solution was evaporated under reduced pressure. The residue was dissolved in distilled water, acidified with diluted hydrochloric acid to (pH=3). The precipitate was collected by filtration, washed with cold distilled water and dried in an oven at 40 - 45oC to provide 20, which crystallized from ethanol, as dark brown powder, yield 71%; m. p. 127-129°C. IR (KBr): 3439 cm-1 (broad, OH and NH2) and 2209 cm-1 (C≡N) and 1725 cm-1 (C=O, acid). 1H NMR (DMSO-d6): δ=4.22 (s, 2H, SCH2), 5.80 (s, 2H, NH2), 6.97 (dd, J=7.62 Hz, 1H, thiophene), 7.32 (s, 1H, pyridone), 7.82 (d, 1H, J=4.40 Hz, thiophene), 7.95 (d, 1H, J=3.60 Hz, thiophene), 8.08 (d, 2H, J=8.00 Hz, Ar-H), 8.17 (d, 2H, J=8.00 Hz, Ar-H), 11.0 (br, 1H, OH). Anal. Calcd for C18H13N3S2 (367.44): C, 58.84; H, 3.57; N, 11.44. Found: C, 58.80; H, 3.57; N, 11.42.
General procedure for preparation of sulphadrug derivatives (21-23)
An anhydrous solution of 20 (10 mmol.) and TEA (10 mmol) in THF (30 mL) was cooled to -100C. To this solution an aliquot of ethyl chloroformate (10 mmol) was added dropwise with continuous stirring. The resulting mixture was left for 30 min., with continuous stirring at 0°C. A cold aqueous solution (10 mmol) of sulphacetamide, 4-amino-N- (4,6-dimethylpyrimidin-2-yl)benzensulfonamide and sulphadiazine, respectively, and TEA (10 mmol) was added to the above mixture. The final mixture was vigorously stirred for 2 hrs at room temperature, diluted with water (30 mL) and then was extracted with diethyl ether (2 × 20 mL). The aqueous phase was acidified with diluted HCl to pH=3, and extracted with ethyl acetate (3 × 20 mL). The extracted were pooled together, dried over anhydrous sodium sulfate and then evaporated under reduced pressure. The residue was treated with petroleum ether (60/80), the precipitate was collected, dried and crystallized from ethanol to give the corresponding (21-23) in good yields, respectively.
N-(4-(N-acetylsulfamoyl)phenyl)-2-((6-(4-aminophenyl)-3-cyano-4-(thien-2-yl)pyridine-2-yl)thio)acetamide (21)
Yellow powder, yield 62%, m. p. 150-152°C. IR (KBr): 3436 cm-1 (broad, NH and NH2), 2211 cm-1 (CN), 1713 cm-1 (COCH3) and 1633 cm-1 (CH2CONH).1H NMR (DMSO-d6): δ=2.71 (s, 3H, CH3CO), 4.20 (s, 2H, SCH2),5.90 (s, 2H, NH2), 6.70 (s, 1H, Ar-H, pyridone), 7.11 (s,1H, NHCOCH3), 7.30 (t, 1H, J=4.00 Hz, thiophene), 7.72 (d, 1H, J=8.00 Hz, Ar-H),7.92 (d, 1H, J=4.01 Hz, thiophene), 7.98-8.27 (m, 7H, Ar-H), 10.31 (s, 1H, CONHAr). Mass spectrometry: M+2 (m/e)=563.13 (7.72%) as a parent ion, m/e=71.10 (82.65%), 77.07 (69.76%). 128.37 (87.73%), 145.10 (84.25%), 276.18 (100%) as a base peak and m/e=338.04 (46.96%). Anal. Calcd for C26H21N5O4S3 (563.67): C, 55.40; H, 3.76; N, 12.42. Found: C, 55.43; H, 3.74; N, 12.40.
2-((6-(4-Aminophenyl)-3-cyano-4-(thien-2-yl)pyridin-2-yl)thio)-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl) phenyl)acetamide (22)
Yellow powder, yield 66%, m. p. 165-167°C. IR (KBr): 3470, 3370 cm-1 (NH and NH2), 2211 cm-1 (CN), 1730 cm-1 (SCONH). 1H NMR (DMSO-d6): δ=2.45 (s, 6H, 2 CH3), 4.27 (s, 2H, SCH2),5.88 (s, 2H, NH2), 6.65 (s, 1H, Ar-H, pyridone), 7.11 (s,1H, NHSO2), 7.29 (t, 1H, J=4.00 Hz, thiophene), 7.65 (d, 1H, J=8.00 Hz, Ar-H),7.92 (d, 1H, J=4.01 Hz, thiophene), 7.42-8.29 (m, 8H, Ar-H), 10.31 (s, 1H, CONHAr). Mass spectrometry: M+2 (m/e)=629.59 (0.74%) as a parent ion, m/e=43.10 (36.54%), 95.11 (28.75%). 243.16 (36.59%), 321.08 (22.46%), 322.07 (100%) as a base peak and m/e=324.08 (31.85%). Anal. Calcd for C30H25N7O3S3 (627.76): C, 57.40; H, 4.01; N, 15.62. Found: C, 57.37; H, 4.01; N, 15.66.
2-((6-(4-Aminophenyl)-3-cyano-4-(thien-2-yl)pyridin-2-yl)thio)-N-(4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl) acetamide (23)
Yellow powder, yield 67%, m. p. 155-157°C. IR (KBr): 3430 cm-1 (broad, NH and NH2), 2207 cm-1 (CN), 1729 cm-1 (CONH). 1H NMR (DMSO-d6): δ=4.53 (s, 2H, SCH2),5.98 (s, 2H, NH2), 6.87 (s, 1H, Ar-H, pyridone), 7.43 (s,1H, NH), 7.34 (t, 1H, J=4.00 Hz, thiophene), 7.52 (d, 1H, J=8.00 Hz, Ar-H),7.82 (d, 1H, J=4.01 Hz, thiophene), 7.82-8.48 (m, 11H, Ar-H), 10.57 (s, 1H, CONHAr). Mass spectrometry: M+2 (m/e)=599.62 (1.11%) as a parent ion, 44.05 (100%) as a base peak, m/e=45.04 (77.10%), 97.07 (43.19%). 178.02 (58.57%), 202.07 (40.63%) and m/e=329.13 (48.85%). Anal. Calcd for C28H21N7O3S3 (599.71): C, 56.08; H, 3.53; N, 16.35. Found: C, 56.08; H, 3.55; N, 16.33.
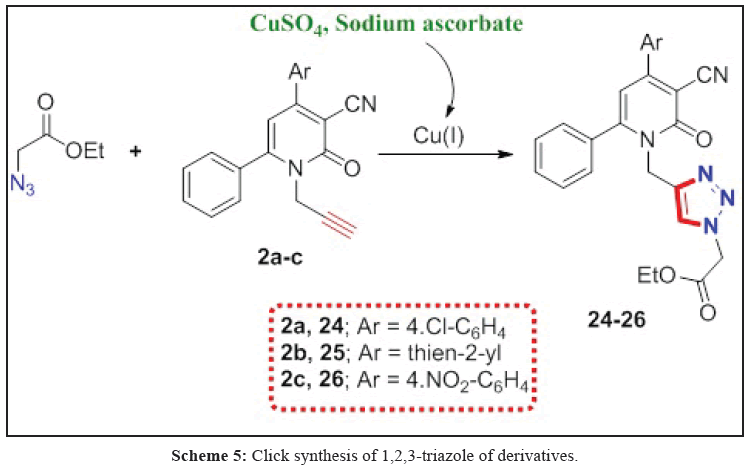
Preparation of ethyl 2-azidoacetate
To a stirred solution of the corresponding ethyl bromoacetate and (1.0 eq) in a (50 mL) water/acetone mixture (1:4) was added NaN3 (1.5 eq). The resulting suspension was stirred at room temperature for 24 h. Dichloromethane (DCM) was added to the mixture and the organic layer was separated. The aqueous layer was extracted with 3 × 10 mL aliquots of DCM and the combined organic layers were dried over dry MgSO4. Solvent was removed under reduced pressure, and the azide was sufficiently pure to use without further work up [30,31]. Colorless oil; yield 99%. 1H NMR (CDCl3): δ=1.21 (t, 2H, J=7.20 Hz, CH3), 3.76 (d, 2H, J=1.21 Hz, CH2N3), 4.16 (q, 2H, J=6.91 Hz, CH2CH3).
General procedure for preparation of 1,4-disustituted triazoles (24-26)
Ethyl 3-azidopropanoate (0.011 mol) and alkylated 2-pyridone derivatives 2a-c (0.01 mol) were dissolved in water/ tetrahydrofuran (30:70 (10 mL)). The reaction mixture was stirred at room temperature for 10 minutes, while an aqueous solution of CuSO45H2O (2.0 mL, 5%) and an aqueous solution of (+)-sodium L-ascorbate (2.0 mL, 10%) were added. The reaction mixture was stirred until complete consumption of the starting material indicated by thin layer chromatography (TLC; 3–5 hours). The reaction mixture was evaporated under reduced pressure, extracted with dichloromethane and the organic phase was dried over anhydrous Na2SO4. After filtration, the solvent was evaporated to dryness under reduced pressure [32-40] and the residue was crystallized from ethanol.
Ethyl 2-(4-((6-(4-aminophenyl)-4-(4-chlorophenyl)-3-cyano-2-oxopyridin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl) acetate (24)
Yellow powder; yield 88%; m. p.95-97ºC; IR (KBr): 3450, 3370 cm-1 (NH2), 2218 cm-1 (C≡N) and 1749 cm-1 (C=O, ester). 1H NMR (DMSO-d6): δ=1.18 (t, 3H, J=7.21 Hz, CH3CH2), 4.14 (q, 2H, J=6.80 Hz, CH2CH3), 5.41 (s, 2H, N-CH2), 5.72 (s, 2H, NCH2CO), 5.85 (s, 2H, NH2), 6.66 (d, 2H, J=8.40 Hz, Ar-H), 7.59 (s, 1H, Ar-H, pyridone), 7.62 (d, 2H, J=8.40 Hz, Ar-H), 7.71 (d, 2H, J=8.40 Hz, Ar-H), 8.03 (d, 2H, J=8.40 Hz, Ar-H), 8.23 (s, 1H, Ar-H, triazole). 13C NMR (DMSO-d6): δ=13.68 (CH2CH3), 50.32 (N-CH2), 61.43 (CH2CH3), 65.44 (N-CH2CO), 111.9, 113.2, 114.2, 115.2 (C≡N), 123.6, 124.5, 127.1, 129.0, 129.1, 130.1, 143.3, 148.1, 151.0, 154.6, 158.3 (Ar-C) and 165.1, 167.8 (2C=O). Anal. Calcd for C25H21ClN6O3 (488.93): C, 61.41; H, 4.33; N, 17.19. Found: C, 61.40; H, 4.33; N, 17.17.
Ethyl 2-(4-((6-(4-aminophenyl)-3-cyano-2-oxo-4-(thien-2-yl)pyridin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl) acetate (25)
Yellow powder; yield 80%; m. p.80-82ºC; IR (KBr): 3449, 3369 cm-1 (NH2), 2213 cm-1 (C≡N) and 1745 cm-1 (C=O, ester). 1H NMR (DMSO-d6): δ=1.18 (t, 3H, J=6.82 Hz, CH3CH2), 4.13 (q, 2H, J=6.80 Hz, CH2CH3), 5.35 (s, 2H, N-CH2), 5.41 (s, 2H, NCH2CO), 5.71 (s, 2H, NH2), 6.77 (d, 2H, J=8.80 Hz, Ar-H), 7.29 (t, 1H, J=4.00 Hz, thiophene), 7.31 (s, 1H, Ar-H, pyridone), 7.65 (d, 1H, J=3.60 Hz, thiophene), 7.90 (d, 1H, J=4.00 Hz, thiophene), 7.96 (d, 2H, J=8.00 Hz, Ar-H), 8.07 (d, 2H, J=8.80 Hz, Ar-H), 8.22 (s, 1H, Ar-H, triazole). Anal. Calcd for C23H20N6O3S (460.51): C, 59.99; H, 4.38; N, 18.25. Found: C, 59.95; H, 4.38; N, 18.22.
Ethyl 2-(4-((6-(4-aminophenyl)-3-cyano-4-(4-nitrophenyl)-2-oxopyridin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl) acetate (26)
Red powder; yield 76%; m. p.120-122ºC; IR (KBr): 3436, 3374 cm-1 (NH2), 2217 cm-1 (C≡N) and 1749 cm-1 (C=O, ester). 1H NMR (DMSO-d6): δ=1.18 (t, 3H, J=6.81 Hz, CH3CH2), 4.14 (q, 2H, J=6.80 Hz, CH2CH3), 5.40 (s, 2H, N-CH2), 5.42 (s, 2H, NCH2CO), 5.75 (s, 2H, NH2), 6.83 (d, 2H, J=8.40 Hz, Ar-H), 7.70 (s, 1H, Ar-H, pyridone), 7.96 (d, 2H, J=8.00 Hz, Ar-H), 8.12 (d, 2H, J=8.40 Hz, Ar-H), 8.25 (s, 1H, Ar-H, triazole), 8.38 (d, 2H, J=8.00 Hz, Ar-H). 13C NMR (DMSO-d6): δ=13.90 (CH2CH3), 50.41 (N-CH2), 61.42 (CH2CH3), 65.50 (N-CH2CO), 111.9, 113.2, 113.5, 115.2 (C≡N), 123.7, 124.5, 126.1, 129.0, 129.3, 130.1, 142.3, 148.1, 150.0, 153.6, 158.1 (Ar-C) and 163.1, 167.1 (2C=O). Anal. Calcd for C25H21N7O5 (499.48): C, 60.12; H, 4.24; N, 19.63. Found: C, 60.14; H, 4.23; N, 19.63.
Biology
In-vitro anticancer activity
Cell culture of HepG-2 (human liver carcinoma), PC-3 (human prostate adenocarcinoma) and HCT116 (human colorectal carcinoma) cell lines were purchased from the American Type Culture Collection (Rockville, MD) and maintained in RPMI-1640 medium which was supplemented with 10% heat-inactivated FBS (fetal bovine serum), 100U/mL penicillin and 100 U/mL streptomycin. The cells were grown at 370C in a humidified atmosphere of 5% CO2.
MTT cytotoxicity assay
The antitumor activity against HepG-2, PC-3 and HCT-116 human cancer cell lines was estimated using the 3-[4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, which is based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells [32-34]. Cells were dispensed in a 96 well sterile microplate (5 × 104 cells/well), and incubated at 37oC with series of different concentrations, in DMSO, of each tested compound or Doxorubicin® (positive control) for 48 h in a serum free medium prior to the MTT assay. After incubation, media were carefully removed, 40 μL of MTT (2.5 mg/mL) were added to each well and then incubated for an additional 4 h. The purple formazan dye crystals were solubilized by the addition of 200 μL of DMSO. The absorbance was measured at 590 nm using a SpectraMax® Paradigm® Multi-Mode microplate reader. The relative cell viability was expressed as the mean percentage of viable cells compared to the untreated control cells.
Statistical analysis
All experiments were conducted in triplicate and repeated in three different days. All the values were represented as mean ± SD. IC<sub>50</sub>s were determined by probit analysis using SPSS software program (SPSS Inc., Chicago, IL).
In-vitro antioxidant activity
Antioxidant activity of each compound and standard (ascorbic acid) was assessed based on the radical scavenging effect of stable DPPH free radical [32,35]. 10 μL of each tested compound or standard (series of different concentrations) was added to 90 μL of a 100 μM methanolic solution of DPPH in a 96-well microtitre plate. After incubation in dark at 37°C for 30 min, the decrease in absorbance of each solution was measured at 520 nm using an ELISA micro plate reader. Absorbance of blank sample containing the same amount of DMSO and DPPH solution was also prepared and measured. All experiments were carried out in triplicate. The scavenging potential was compared with a solvent control (0% radical scavenging) and the standard compound. Radical scavenging activity was calculated by the following formula:
% Reduction of absorbance=[(AB-AA)/AB] 100, where: AB-absorbance of blank sample and AA-absorbance of tested compound (t=30 min). The concentration of each compound required to scavenge 50% of DPPH (IC<sub>50</sub>) was determined as well [33,34].
Antimicrobial activity
Media and chemicals
Müller-Hinton broth was obtained in dehydrated form from Oxoid, Hampshire, England. Agar agar was supplied by Biolife, Milano, Italy. Dimethyl formamide (DMF) used as a negative control.
Microorganisms
A total of three standard microbial strains were used in this study. They were obtained from the Egyptian Pharmaceutical Industries Company (EPICO), Egypt which were Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 10536), and Candida albicans (ATCC 10231).
Antimicrobial activity
The antibacterial activities of the samples were determined by the agar well diffusion method as modified from NCCLS [34]. Mueller-Hinton agar plates were surface-inoculated with the tested strains suspensions adjusted to match 0.5 McFarland standard and the inoculate were spread over the surfaces of plates using sterile cotton swabs. After drying of the plates, cups (10 mm diameter) were punched in the agar and 1000 μg of the samples in DMF or the antimicrobial agents were added into the wells. The plates were incubated at 37ºC for 24 hours. The antibacterial activity was determined by measuring the diameter of the zone of inhibition. The test was repeated three times and the mean inhibition zones were calculated.
Results and Discussion
Chemistry
In the present work, we synthesized a new 2-pyridone derivatives 1a-c by four component system from the reaction of 4-aminoacetophenone, aromatic aldehydes namely (4-chlorobenzaldehyde, 2-thiphencarboxaldehyde and 4-nitobenzaldehyde) and ethyl cyanoacetate in the presence of ammonium acetate as procedure reported in literture [18,26,27].
The structures of 1a-c were confirmed by spectroscopic analysis (IR, 1H, 13C NMR and elemental analysis). IR spectra of 1a-c showed the characteristic bands at between 3464-3465, 3195-3191, 2212-2207 and 1673-1650 cm-1 for NH2, NH, C≡N and C=O amides, respectively. While, the 1H, 13C NMR spectra and their elemental analysis are agreement with the structures and reported in the experimental part.
Alkylation of 2-pyridone derivatives 1a-c with alkylating agents namely (propargyl/allyl bromides, 3-chloro-1,2- propandiol, chloroacetonitrile, acetic anhydride, methyl bromoacetate and ethy bromoacetate) in anhydrous potassium carbonate and dry DMF afforded the O-alkylated products 3a-c, 4a,b, 7a-c and N-alkylated 2a-c, 5a,b, 6a,b and 8a,b, respectively (Scheme 1). The alkylation of 2-pyridone derivatives at O- and N- owing to the ambient anion of two sites of the salts 2-pyridone 1a-c, are the oxygen atom at position-2 and nitrogen atom at position-1 [28].
The structures of the alkylated products are confirmed by the IR, 1H, 13C NMR and elemental analysis. The IR spectra of 3a-c, 4a,b and 7a-c confirmed the absence of amidic carbonyl C=O groups, while for 2a-c, 5a,b, 6a,b and 8a,b showed the presence of the amidic carbonyl C=O groups, this indicate the alkylation at O- and N-products. 1H NMR spectrum of 2b revealed the signals at δ=3.59 and 5.22 ppm for acetylenic proton (≡C-H) and N-CH2, respectively, while its 13C NMR spectrum showed the signals at δ=54.34 and 77.82, 79.00 ppm characteristic for N-CH2 and C≡C carbon, respectively. 1H NMR spectrum of 3b revealed the characteristic signals for OCH2 and terminal=CH2 protons at δ=5.06 and 5.29, 5.47 ppm as singlet and doublet. Its 13C NMR spectrum showed signals at δ=67.03 and 116.1 ppm for OCH2 and C≡N groups. The characteristic signals of 4a appeared as doublet of doublet in 1H NMR for at δ=4.41 and 4.52 ppm for OCH2(a) as diasterotropic protons. While 13C NMR spectrum of 4b showed signals at δ=62.69, 68.30 and 69.50 ppm for CH(c), CH(b) and CH(a), respectively. 1H NMR spectra of 5a,b revealed signals at δ=5,47, 5.45 ppm for N-CH2, respectively. In compounds 6a,b the IR indicate the presence of 2NH with absence of NH2 bands, in addition to the amidic C=O groups, while in 1H NMR spectra the presence of acetoxy methyl groups at δ=2.08 ppm with absence of the signals for NH2 protons. The IR, 1H, 13C NMR and elemental analysis of the rest of compounds are in agreement with the elucidated structures and see the experimental section.
Hydrazonolysis of alkylated 2-pyridones 7a,b using hydrazine hydrates in absolute ethanol afforded 9a,b in high yields, followed by condensation with benzaldehyde, 4-flourobenzaldehyde, acetyl acetone and ethylacetoacetate gave the hydrazones 10a,b, 11a,b and 13, in addition to, pyrazole derivative 12 (Scheme 2).
All the newly synthesized compounds are in agreement with the spectroscopic analysis. IR spectra of 9a,b revealed the presence of bands characterized for 2NH2, NH and amidic C=O groups with absence of the ester C=O groups, while, their 1H NMR spectra showed the absence of ethyl CH3CH2 groups. 1H NMR spectrum of 10a showed signals at δ=4.83 and 8.70 ppm for OCH2 and CH=N groups, while in 11a give signals at δ=4.34 and 8.70 ppm for OCH2 and CH=N, its 13C NMR spectrum showed signals at δ=67.10, 115.6, 160.4, 162.1 and 162.3 ppm characterized for OCH2, C≡N, N=C-O, C-F and C=O, groups, respectively. IR spectrum of 12 gave bands at 3430, 2214 and 1649 cm-1 for NH2, CN and C=O amide. Its 1H NMR spectrum revealed signals at δ=2.10, 2.45, 4.79 and 6.04 ppm characteristic for 2 CH3, OCH2 and CH of pyrazole ring. The analysis of compound 13 and other products are in agreement with the structures and were written in the experimental part.
The ester of 2-pyridone derivatives 8a,b reacted with hydrazine hydrate to give hydrazides 14a,b which condensed with ethyl acetoacetate and 4-aminoacetophenone afforded hydrazones 15 and 16, respectively (Scheme 3), while the diazotization of 1a,b followed by coupling with active methylene reagents namely (ethyl cyanoacetate and acetyl acetone) gave the hydrazonoid 17 and 18 (Scheme 3).
The IR spectra of 14a,b revealed the absence of ester bands and presence of 2C=O amidic carbonyl as a broad band at 1650 and 1641 cm-1 with other bands for 2NH2 and NH bands. Also, the absence of ethyl group for ester and presence of 2 NH2 and NH protons in 1H NMR. 1H NMR spectrum of 15 showed signals at δ=1.17, 1.77, 2.41, 4.12 and 4.81 ppm characteristic for CH3CH2, CH3, CH2CO, CH2CH3, and N-CH2 ppm, respectively.
The IR spectrum of compound 17 gave bands at 3437, 2267, 2217 and 1728 cm-1 for 2NH, 2CN and C=O, ester, respectively. Its 1H NMR spectrum revealed the presence of ethyl group signals at δ=1.28 and 4.28 ppm as triplet and quartet. Also, its 13C NMR spectrum showed signals at δ=14.10, 61.75, 115.7, 116.1, 160.6 and 162.2 ppm for CH3CH2, 2C≡N and 2C=O, respectively. Full analysis for compounds 16 and 18 and all the elemental analysis is confirmed the deduced structures and see the experimental section.
Sulpha-drugs products 21-23 were prepared from alkylation of 6-(4-Aminophenyl)-4-(thien-2-yl)-2-thioxo-1,2- dihydropyridine-3-carbonitrile (19) with chloroacetic acid to give compound 20, followed by with sulphamides namely (sulphacetamide, sulphamedene methazine and sulphadiazine) in the presence of THF/TEA and ethylchloroformate in yields between 62-67%, respectively (Scheme 4).
Compound 19 elucidated using IR and elemental analysis, which show the presence of C=S in IR at 1258 cm-1, while compound 20 proved by IR, 1H NMR and elemental analysis (see the experimental part). IR spectrum of sulphadrug 21 revealed the presence of bands at 3436, 2211, 1713 and 1633 cm-1 characteristic for NH, NH2, C≡N, COCH3 and C=O amide, respectively. Its 1H NMR spectrum showed signals at δ=2.71, 4.20, 7.11 and 10.31 ppm assigned the presence of CH3CO, SCH2, NHCOCH3 and CONHAr, respectively. Also, its Mass spectrometry: M+2 (m/e)=563.13 (7.72%) as a parent ion, m/e=71.10 (82.65%), 77.07 (69.76%). 128.37 (87.73%), 145.10 (84.25%), 276.18 (100%) as a base peak and m/e=338.04 (46.96%).
The IR, 1H NMR and elemental analysis of compounds 22 and 23 are in agreement with the structures and can see the experimental section.
Click reaction for the synthesis of 1,2,3-triazoles derivatives 24-26 via the reaction of 2-pyridone derivatives 2a-c with ethyl 2-azidoacetate (prepared according to lit [29,30] in the presence of CuSO4, sodium ascorbate/THF as show in Scheme 5 [31]. The IR spectra of triazoles compounds 24-25 revealed the presence of bands at 1749, 1745 and 1749 cm-1 assigned the C=O of ester moiety, respectively. 1H NMR spectrum showed the presence of signals at δ=1.18, 4.14 ppm as triplet and quartet characteristic for CH3CH2 moiety, in addition to, signals at δ=5.41, 5.72 ppm for N-CH2 and N-CH2CO protons. While, the 1H NMR spectrum of 26 revealed the presence of characteristic signals for CH2CH3, N-CH2 and N-CH2CO at δ=1.18, 4.14, 5.40 and 5.42 ppm, respectively. Its 13C NMR spectrum gave signals at δ=13.90, 50.41, 61.42 and 65.50 ppm characteristic for CH3, CH2, N-CH2 and N-CH2CO carbons, respectively, in addition to, signals at 163.1 and 167.1 ppm for 2 C=O groups.
Biological evaluation
Anticancer activity
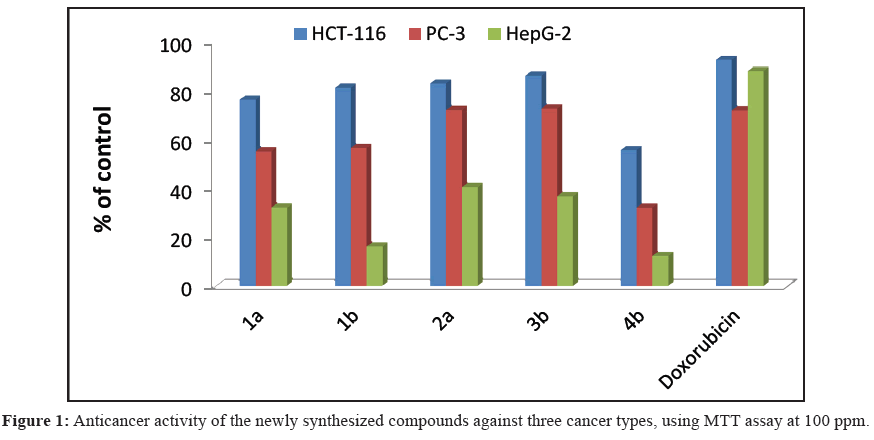
Five compounds were examined in-vitro for their anti-tumor activities against HepG-2, PC-3 and HCT-116 human carcinoma cell lines using MTT assay. The percentage of the intact cells was measured and compared to the control (Figure 1). The activities of these compounds against the three carcinoma cells were compared with that of Doxorubicin®. The obtained results showed that all compounds showed dose-dependent anticancer activities against the three cancer cells. The IC<sub>50</sub> values of these compounds are shown in Table 1. From Figure 1 and Table 1 we can deduce that, four compounds (1a,b, 2a, 3b and 4b) showed good anticancer activities against HCT-116 carcinoma cells and one compound 4b showed moderate activity against HCT-116 cells. In addition, two compounds 2a and 3b showed good anticancer activities, two compounds 1a and 1b showed moderate antitumor activity and one compound 4b showed weak antitumor activities against PC-3 cancer cells. Furthermore, all the compounds showed weak or no anticancer activities against HepG-2 liver cancer. Compounds (1a, 1b, 2a, 3b and 4b) which showed good anticancer activities (Table 1). The good anticancer activities of these compounds may be attributed by the presence of aryl hydrophobic group (thienyl or phenyl) in position-4 of pyridine ring, which enhances the binding energy as aryl hydrophobic group in position-4 occupies the unoccupied hydrophobic region binding pocket, and also the activity due to the presence of NH and CN groups at phenyl pyridine ring system in H-bond with the target inhibtor compounds and (N-atom).
| Compound | HCT-116 | PC-3 | HepG-2 |
|---|---|---|---|
| IC50 (µg/mL) ± SD | |||
| 1a | 65.2 ± 2.7 | 90.1 ± 7.1 | 153.9 ±9.2 |
| 1b | 61.4 ± 3.1 | 88.0 ± 2.6 | 302.1 ± 8.9 |
| 2a | 60.2 ± 3.9 | 69.1 ± 4.9 | 122.4 ± 5.9 |
| 3b | 57.9 ± 2.7 | 68.5 ± 3.8 | 134.9 ± 6.1 |
| 4b | 89.5 ± 3.7 | 154.3 ± 4.3 | 393.9 ± 8.9 |
| Doxorubicin | 73.50 ± 2.9 | 75.24 ± 4.1 | 67.9 ± 3.2 |
Table 1: The anticancer IC50 values of the five compounds using MTT assay against the three cancer types.
Molecular modeling
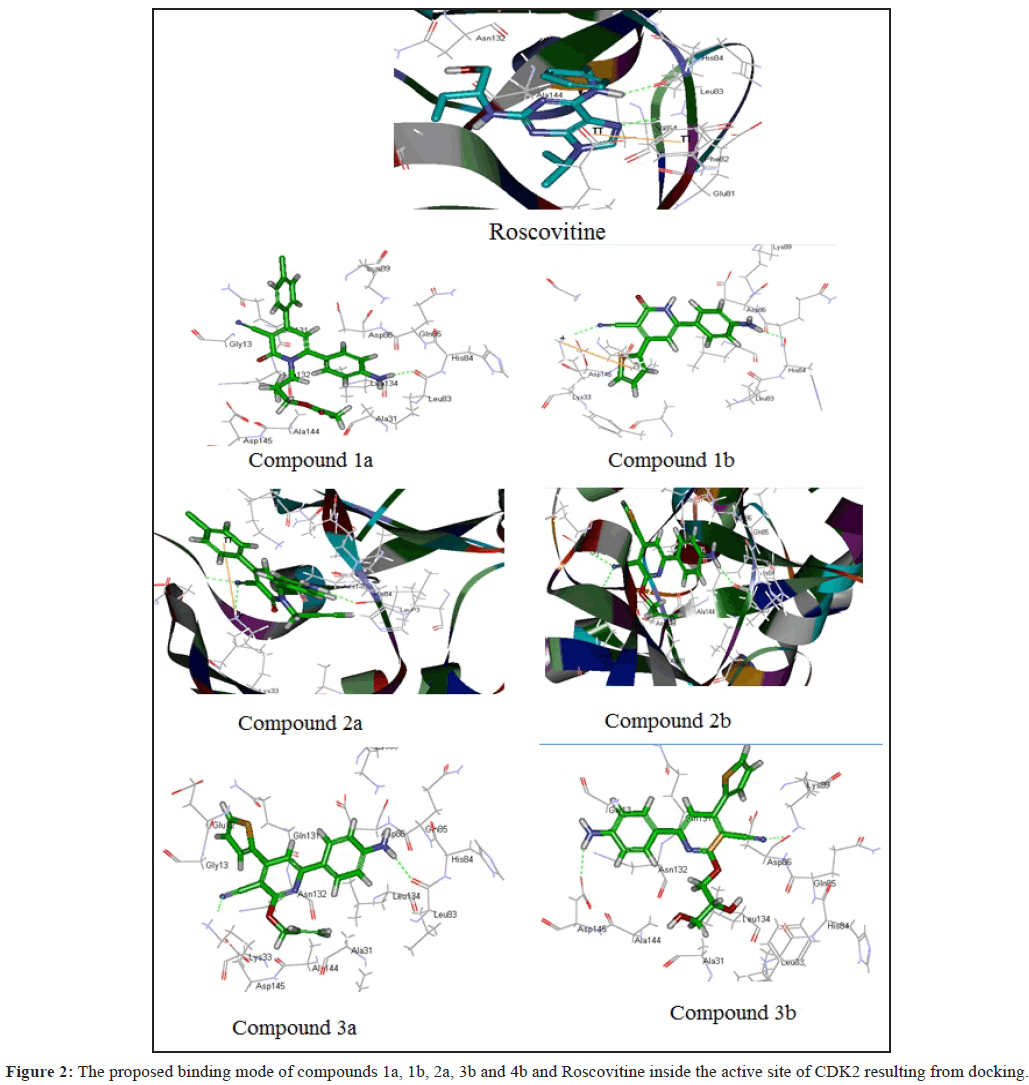
Molecular modelling study was initiated in order to support the assumed mode of action for tested compounds and optimize a reliable model for predicating novel effective anti-tumor hits. Docking study was carried out for the target compounds into CDK2 using Discovery Studio 2.5 software (Accelrys Inc., San Diego, CA, USA). The coordinate for the protein structure was obtained from the RCSB Protein Data Bank (PDB; 2a4l) [35]. Protein Structure was prepared and the invalid or missing residues were added [36]. The proposed compounds were optimized by semiempirical method (AM1) using Chem3D to eliminate bond length and bond angle biases and saved to be used in docking and binding energy calculations. Root mean square deviation (RMSD) between the positions of heavy atoms of the ligand 1 in the calculated and experimental structures of CDK2 active sites was used as a tool to evaluate the docking process reliability.
Docking studies of the proposed compounds in CDK2 active site revealed that most of the proposed compounds conserved the coordination of NH and CN groups at phenyl pyridine ring system in H-bond with LEU83 and (N-atom) and His84 as in compounds (1a, 1b, 2a, 3b and 4b) which showed good anticancer activities (Table 1). The good anticancer activities of these compounds may be attributed to the same binding mode as the lead compound Roscovitine (Figure 1). Introduction of aryl hydrophobic group (thienyl or phenyl) in position-4 of pyridine (Figure 1) enhances the binding energy as aryl hydrophobic group in position-4 occupies the unoccupied hydrophobic region binding pocket.
Conclusion of molecular modelling
The above molecular docking study provides useful information for understanding the structural features of CDK2 inhibitor and the effect of binding mode on the biological activity (Figure 2). Compounds 1a, 1b, 2a and 3b are veiled the highest biological inhibitory activity against CDK2 showed the highest docking scores and binding energy values (Table 2). This was extended to the successful designing of highly active analogs of pyridine derivatives with antitumor activities.
| Comp. | -C-DOCKER Interaction energy (kcal/mol) |
-Binding energy (kcal/mol) | Hydrogen bonds between compounds and amino acid | RMAD (A°) | ||
|---|---|---|---|---|---|---|
| Atom of comp. | amino acid | distance | ||||
| 1a | 38.3 | 21 | Ar-NH2 | Lue83 | 1.92 | 1.65 |
| 1b | 41.1 | 20 | Ar-NH2 Pyrine-CN |
Lue83 Lys33 |
2.12 2.32 |
1.02 |
| 2a | 44.5 | 19 | Ar-NH2 Pyrine-CN |
Lue83 Lys33 |
2.16 1.99 |
0.88 |
| 3b | 46.1 | 25 | Ar-NH2 Pyrine-CN |
Lue83 Lys33 |
1.86 2.47 |
0.57 |
| 4b | 39.1 | 17 | Pyrine-CN Ar-NH2 |
Lys89 Asp145 |
2.25 1.84 |
1.02 |
| Roscovitine | 46.67 | 17 | Imidazole-N -NH |
Lue83 His84 |
2.13 1.97 |
0.21 |
Table 2: The best docking score and binging energy of compounds docked into CDK2, and the distances and angles of hydrogen bonds between compounds and amino acids involved in CDK2.
Anti-oxidant activity
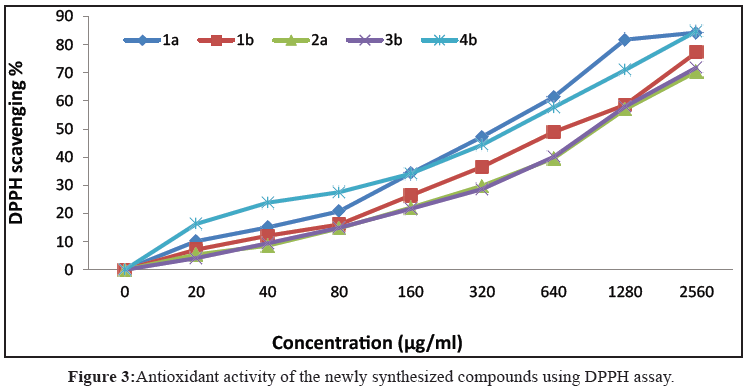
In this study, five newly synthesized compounds have been investigated for their antioxidant activity using DPPH assay. The results reveal that all the tested compounds show a dose dependent activity (Figure 3). Their corresponding IC<sub>50</sub>s are shown in Table 3. From these results we obtain that, all the investigated compounds showed week antioxidant activity compared to ascorbic acid (Table 1).
| Compound | IC50(µg/ml) ± SD |
|---|---|
| 1a | 382.5 ±5.3 |
| 1b | 711.5 ± 6.9 |
| 2a | 1023 ± 9.4 |
| 3b | 994.2 ± 8.2 |
| 4b | 445.2 ± 7.9 |
| Ascorbic Acid | 14.2 ± 1.5 |
Table 3: The Antioxidant activities of the newly synthesized compounds.
Antimicrobial activity
The newly synthesized compounds were tested for their antimicrobial activity using cup plate diffusion method [36-38] against Staphylococcus aureus as Gram positive bacteria and Escherichia coli as Gram negative bacteria. In addition Candida albicans was used as an example for fungi. The results were reported as zone of inhibition compared to standard Gemifloxacin as antibacterial drug and Fluconazole as antifungal drug. The results illustrated in (Table 4) revealed that:
| Tested samples | Diameter (mm) of inhibition zones against the corresponding standard strains of different microorganisms | ||
|---|---|---|---|
| Gm (+ve) bacteria | Gm (-ve) bacteria | Fungi | |
| Staphylococcus aureus (ATCC 6538) | Escherichia coli (ATCC 10536) | Candida albicans (ATCC 10231) | |
| 1a | 1.1 | - | 1.3 |
| 1b | - | 1.2 | 1.5 |
| 1c | 1.1 | 1.1 | 1.5 |
| 2a | - | 1.4 | 1.5 |
| 2c | - | - | 1.1 |
| 4a | 1.1 | - | 1.1 |
| 5a | - | 1.4 | 1.5 |
| 5b | - | 1.4 | 1.5 |
| 11a | 1.1 | 1.3 | 1.5 |
| 13 | - | 1.1 | 1.5 |
| 17 | 1.2 | 1.1 | 1.8 |
| 21 | 1.3 | - | 1.5 |
| 22 | 3 | 2.5 | 1.3 |
| 23 | 1.7 | 2 | 1.8 |
| Gemifloxacin | 4 | 4 | - |
| Fluconazol | - | - | 3.2 |
| DMF | - | - | - |
Table 4: Antimicrobial activity evaluation of the newly synthesized compounds.
• Compound 17, 23 showed the highest antifungal activity against Candida albicans. Moreover, compounds 1b, 1c, 2a, 5a, 5b, 11a, 13 and 21 have moderate inhibitory activity against Candida albicans. In addition to, compounds 1a, 2c, 4a and 22 exhibited weak activity against Candida albicans.
• Some of the tested compounds are inactive against Staphylococcus aureus except compound 22 which havethe highest activity and compounds 21, 23 have moderate activity. In addition compounds 1a, 1c, 4a, 11a and 17 exhibited weak activity against Staphylococcus aureus.
• Compounds 22, 23 have moderate activity against Gram negative bacteria (Escherichia coli). The remaining tested compounds showed weak activity, while compounds 1a, 2c, 4a and 21 are inactive against Escherichia coli.
Conclusion
In this article, we syntheses 2-pyridone derivatives and their alkylation, sulph-drugs formation which have a different substituent at position 4 and 6 which contain the active amino moiety, and studied the anticancer, antioxidant and antimicrobial activities which gave a good and moderate activity. In the other hand, we synthesized 1,2,3-triazole derivatives via click reaction between the azide of ethyl bromoacetae and N-alkylated 2-pyridone derivatives which obtained in a high yields.
References
- Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, et al. (2009) Novel Tacrine−Melatonin Hybrids as Dual-Acting drugs for alzheimer disease, with improved Acetylcholinesterase inhibitory and antioxidant properties.J Med Chem52: 3597-3617.
- Sasaki T, Guerrero JM, Leonard AD, Tour JM (2008) Nanotrains and self-assembled two-dimensional arrays built from carboranes linked by hydrogen bonding of dipyridones. Nano Res1: 412-419.
- Stoncius S, Orentas E, Butkus E, Öhrström L, Wendt OF, et al. (2006) An approach to Helical Tubular self-aggregation using c2-symmetric self-complementary Hydrogen-bonding cavity molecules.J Am ChemSoc128: 8272-8285.
- Cocco MT, Congiu C, Onnis V (2000) Synthesis and antitumour activity of 4-hydroxy-2-pyridone derivatives. Eur J Med Chem35: 545-552.
- Richard TV (1964) Note on an alkaloïd contained in the seeds of the Ricinuscommunis, or castor-oil plant. J ChemSoc17: 195-197.
- Nagarajan M, Xiao XS, Antony S, Kohlhagen G, Pommier Y, et al. (2003) Design, synthesis, and biological Evaluation of indenoisoquinoline topoisomerase I inhibitors featuring polyamine side chains on the Lactam Nitrogen. J Med Chem 46: 5712-5724.
- Li Q, Mitscher LA (2000) The 2-pyridone antibacterial agents: bacterial topoisomerase inhibitors. Med Res Rev20:231-293.
- Cox RJ, O’Hagan D (1991) Synthesis of isotopicallylabelled 3-amino-2-phenylpropionic acid and its role as a precursor in the biosynthesis of tenellin and tropic acid. J ChemSoc Perkin Trans1 1: 2537-2540.
- Dolle RE, Nicolaou KC (1985) Total synthesis of elfamycins: aurodox and efrotomycin 1 Strategy and construction of key intermediates. J Am ChemSoc107:1691-1694.
- Gary D, Byron AK (1991) The biosynthetic origin of the pyridone ring of efrotomycin. J IndMicrobiol 8: 265-271.
- Presti EL, Boggia R, Feltrin A, Menozzi G, Dorigo P, et al. (1999) 3-Acetyl-5-acylpyridin-2(1H)-ones and 3-acetyl-7,8-dihydro-2,5(1H,6H)-quinolinediones: synthesis, cardiotonic activity and computational studies. IL Farmaco54:465-474.
- Jessen HJ, Gademann (2010) 4-Hydroxy-2-pyridone alkaloids: Structures and synthetic approaches. Nat Prod Rep 27:1168-1185.
- Kaiser JP, Feng Y, Bollag JM (1996) Microbial metabolism of pyridine, quinoline, acridine, and their derivatives under aerobic and anaerobic conditions.MicrobiolRev60:483-498.
- Rodriguez-Franco MI, Fernandez-Bachiller MI, Perez C, Hernandez-Ledezma B, Bartolome B (2006) Novel Tacrine−Melatonin Hybrids as Dual-Acting Drugs for Alzheimer Disease, with Improved Acetylcholinesterase Inhibitory and Antioxidant PropertiesJ MedChem49:459-462.
- Munoz-Ruiz P, Rubio L, Garico-Plamero E, Dorronsoro I, Del Monte-Millan M, et al. (2005) Design, synthesis, and biological evaluation of dual binding site Acetylcholinesterase Inhibitors: New disease-modifying agents for Alzheimer's Disease.J Med Chem48:7223-7233.
- [No authors listed] (2000) New anticonvulsant action of 4-amino-1-aryl-pyridin-2-one and processes for their preparation. Germ Pat, DE 19835918A1.
- Tavraite D, Razanas R, Mikalenas A, Serva S, Meskys R (2016)Synthesis of Pyridone-based Nucleoside analogues as substrates or inhibitors of DNA Polymerases.Nucleosides Nucleotides and Nucleic Acids53:163-177.
- Abou-Elkhair RAI, Moustafa AH, Haikal AZ, Ibraheem AM (2014) Synthesis and biological evaluation of 2-oxonicotinonitriles and 2-oxonicotinonitrile based nucleoside analogues.Eur J MedChem74:388-397.
- Tsypysheva IP, Kovalskaya AV, Labou AN, Zarubaev VV, Karpinskaya LA (2013) Search for compounds with antiviral activity among synthetic (-)-cytisine derivatives.Chem Nat Comp Jan48:1042-1046.
- Rehman S, Ashfaq UA, Raiz S, Javed T, Riazuddin S (2011) Antiviral activity of Acacia nilotica against Hepatitis C Virus in liver infected cells. Virol J8: 220.
- Ding PL, Liao ZX, Huang H, Zhou P, Chen DF (2006) (+)-12α-Hydroxysophocarpine, a new quinolizidine alkaloid and related anti-HBV alkaloids from Sophoraflavescens. Bioorg Med ChemLett16: 1231-1235.
- Attu-ur-Rahman, Choudhary MI, Parvez K, Ahmed A, Akhtar F (2000) Quinolizidine Alkaloids from Sophoraalopecuroides.J Nat Prod63:190-192.
- Al-Said MS, Ghorab MM, Nissan YN (2012) Dapson in heterocyclic chemistry, part VIII: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active 1,3-dihydropyridine, chromene and chromenopyridine moieties. Chem Cent J6:64-76.
- Kosulina TP, Kaigorodova EA, Kul’nevich VG, Sapunov AY, et al. (1997) GovorovaSynthesis and study of antihelminthic activity of new 2-pyridone derivatives. Pharm Chem J 31:191-193.
- Rai SK, Singh P, Khanam S, Tewari AK (2016) Polymorphic study and anti-inflammatory activity of a 3-cyano-2-pyridone based flexible model. New JChem40: 5577-5587.
- El-Sayed HA, Ouf NH, Moustafa AH (2014) An efficient and facile multicomponent synthesis of 4,6-diarylpyridine derivatives under solvent-free conditionsRes ChemIntermed40:407-412.
- Haggam RA, El-Sayed HA, Said SA, Ahmed HM, Moustafa AH, et al. (2017) O-Glycosylation/Alkylation and Antimicrobial Activity of 4,6-Diaryl-2-Oxonicotinonitrile Derivatives.J Heterocyclic Chem54: 375-383.
- Moustafa AH, El-Sayed HA, Rasha AAE, Haikal AZ, El- Hashash MA (2016)Synthesis, antiviral, and antimicrobial activity of N- and S-Alkylated Phthalazine derivatives. J Heterocyclic Chem53:789-799.
- Shi F, Waldo JP, Chen Y, Larock RC (2008)Benzyne Click Chemistry: Synthesis of Benzotriazoles from Benzynes and Azides. Orglett10:2409-2412.
- Alvarez SG, Alvarez MT (1997) A Practical procedure for the synthesis of Alkyl Azides at Ambient Temperature in Dimethyl Sulfoxide in high purity and yield. Synthesis 1997: 413-414.
- Moustafa AH, El-Sayed HA, Haikal AEZ, El Ashry EH (2011)Synthesis and evaluation of antimicrobial activity of some pyrimidine glycosides. Nucleosides Nucleotides and Nucleic Acids30:340-352.
- Nehal AH, Manal MA, Khadiga MA, Hanem MA (2013) Synthesis, tumor inhibitory and antioxidant activity of new polyfunctionally 2-substituted 5,6,7,8-tetrahydronaphthalene derivatives containing pyridine, thioxopyridine and pyrazolopyridine moieties. Acta Pol Pharm 70:987-1001.
- Soliman HA, Yousif MNM, Said MM, Hassan NA, Ali MM, et al. (2014) Synthesis of novel 1, 6-naphthyridines, pyrano [3, 2-c] pyridines and pyrido [4, 3-d] pyrimidines derived from 2, 2, 6, 6-tetramethylpiperidin-4-one for in vitro anticancer and antioxidant evaluation. Der PharmaChemica 6: 394-410.
- Awad HM, Abd-Alla HI, Mahmoud KH, El-Toumy SA (2014)In vitro anti-nitrosative, antioxidant, and cytotoxicity activities of p lant flavonoids: a comparative study. MedChem Res 23:3298-3307.
- Michael HN, Awad HM,Nabil HE, Paul WP (2010)Chemical and antioxidant investigations: Norfolk pine needles (Araucaria excelsa).Pharm Biol 48:534-538.
- Barry AL (1977) The antimicrobial assay susceptibility test; principle and practices. BiolAbstr64: 251-283.
- William H (1977) Microbiological assay, an introduction to quantitative principles and evaluation. Academic press, New York, pp:1-68.
- Bauer RW, Kirby MDK, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method.Am JClinPathol 45: 49-54.
- National committee for clinical laboratory standards (NCCLS) (2002) "Methods for dilution antimicrobial susceptibility tests of bacteria that grow aerobically"; Approved standard M100-512, PA Wayne.
- Ding H,Yang R, Song Y, Xiao Q, Wu J (2008) A Highly efficient and selective synthesis of 1,2,3-Triazole Linked Saccharide Nucleosides via “Click Chemistry”. Nucleosides Nucleotides and Nucleic Acid27:368-375.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences