ISSN : 2348-9502
American Journal of Ethnomedicine
Comprehensive Review on Potent Derivatives of Rutin towards Different Biological Activities
Shweta Saboo*, Suryawanshi Yashwanti, Virdhe Gauri, Solanke Sanskruti, Swami Sunita, Valekar Harshada and Vitekar Govind
Department of Pharmacy, Government College of Pharmacy, Mumbai, India
- *Corresponding Author:
- Shweta Saboo
Department of Pharmacy,
Government College of Pharmacy,Mumbai,
India,
E-mail: shweta.saboo1@gmail.com
Received date: August 19, 2024, Manuscript No. IPAJE-24-19549; Editor assigned date: August 21, 2024, PreQC No. IPAJE-24-19549 (PQ);Reviewed date: September 04, 2024, QC No. IPAJE-24-19549; Revised date: April 21, 2025, Manuscript No. IPAJE-24-19549 (R); Published date:April 28, 2025, DOI: 10.36648/2348-9502.12.2.107
Citation: Saboo S, Yashwanti S, Gauri V, Sanskruti S, Sunita S, et al. (2025) Comprehensive Review on Potent Derivatives of Rutin towards Different Biological Activities. Am J Ethnomed Vol:12 No:2
Abstract
The greatest class of plant-derived polyphenolic chemicals are called flavonoids; they have a wide range of biological effects in both in vitro and in vivo mammalian cell systems. rutin is one of more prominent flavonoid possessing wound healing, anti-inflammatory, antimicrobial, anti-mutagenic, anti-oxidant, and anti-HIV (Human Immunodeficiency Virus) property. The modification of rutin’s structure aims to enhance the bioavailability, potency and target specificity. The exploration of Structure Activity Relationship (SAR) has been instrumental in guiding the design of its derivatives. The continued development of rutin derivatives hold promise for novel therapeutic agents in the treatment of various diseases and health conditions. The chemical features, structure-activity connections, and therapeutic qualities of rutin and its strong derivatives that are helpful in drug development are briefly illustrated in this article.
Keywords
Polyphenols; Rutin derivatives; Chemical properties; Antioxidant; Antimicrobial; Anti-inflammatory
Introduction
With its antimicrobial, anti-inflammatory, and cytotoxic characteristics, rutin is a widely recognized bioactive plant flavonoid with substantial therapeutic importance for the treatment of numerous disorders [1-8]. Owing to its pharmacological characteristics, rutin has been extensively studied and has demonstrated notable efficacy in generating potential medicinal agents. Molecular docking techniques may be used to obtain novel compounds that have the potential to be druggable for the targeted enzyme, provided that the mechanism of action is accurately assessed. Often referred to as vitamin P, rutin (3',4',5,7-tetrahydroxyflavone-3-d-rutinoside) is a common flavonoid glycoside that is found in many different plants, including buckwheat plants. Contemporary pharmacological research has revealed that rutin controls the capillary wall's permeability and fragility, inhibits haemostasis and vascular rupture, and also has antibacterial, anti inflammatory, analgesic, anti-radiation, anti-oxidation, and antimyocardial hypoxic properties. It also lowers serum cholesterol, diuresis, spasmolytic, and platelet aggregation inhibitory effects. Thus, the development of straightforward, trustworthy, and sensitive rutin analysis methods is crucial [9-14].
With reducing lipid peroxidation as well as decreasing oxidative stress, rutin has been shown in multiple studies to have anti-inflammatory, anti-carcinogenic, neuroprotective effects, anti-proliferative, anti-metastatic, and anti-oxidative stress properties. Reactive Oxygen Species (ROS) have the ability to harm chromosomal mutations, DNA, uncontrolled gene expression, cell development, and division. Additionally, they might reduce the activity of several proteins that are part of the antioxidant systems. Numerous investigations have revealed a correlation between Reactive Oxygen Species (ROS) and various cancers, including breast, lung, colon, hepatic, leukaemia, and neuroblastoma. Lipid peroxidation is the reaction that occurs when oxygen reacts with lipids to create lipid hydroperoxides by first forming peroxyl radicals and then cleaving hydrogen. Lipid peroxidation can be quite harmful if it isn't inhibited by antioxidants like rutin. Prior research has demonstrated that Alteration in the content of lipids may cause cell death and other effects that rutin may be able to target. Additionally, rutin decreased ischemia-reperfusion damage by reducing oedema and sensitivity.
In an effort to improve rutin's pharmacokinetic profile, several research on the creation of innovative drug delivery systems have been carried out recently. The results indicate that these newly developed systems have not only enhanced rutin's pharmacokinetics but also increased its efficacy. To fully understand the therapeutic effects of rutin by increasing its lipid solubility, more research is required.
Materials and Methods
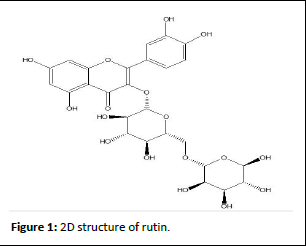
One of the primary secondary metabolites of plants, rutin is a naturally occurring flavonoid glycoside that is sometimes referred to as rutoside, quercetin-3-Orutinoside, sophorin, or vitamin P. It is known as 2-(3,4-dihydroxyphenyl) in the chemical sense. 5-[β-Dglucopyranosyloxy-(1-6)-α-L-rhamnopyranosyl].Formula for -5,7-dihydroxy-3-chromen-4H is C27H30O16. The powder is yellow-colored. Its molar mass is 610.521 g/mol. Structure of the lavonoid rutin. (e 15-carbon Flavonoids are mostly made up of a three-ring system with the con iguration C6-C3-C6. In many flavonoids, the third ring (ring C) is typically made up of two aromatic rings (rings A and B), which are frequently connected by a three-carbon chain when they are in polyphenolic forms. Flavonoids are categorized based on their oxidation state, the existence or lack of a double bond, and the manner in which ring C and ring B are joined. Diverse groups of flavonoids, such as flavonols, flavones, and flavanones, have been identified.
Chemical structure of rutin
Diagramatic representation of rutin shown in Figure 1.
Figure 1: 2D structure of rutin.
Chemical properties of rutin
Different type of chemical properties of rutin shown in Table 1.
| Property | Description |
| Chemical formula | C27H30O16 |
| Molecular weight | Approximately 610.51 g/mol |
| Melting point | 176-178°C |
| Solubility | Soluble in water, methanol, ethanol, acetone; slightly soluble in ethyl acetate; insoluble in chloroform and ether |
| UV-visible absorption | λmax: 254 nm (in acidic solution) and 355 nm (in basic solution) |
| Stability | Stable under normal conditions |
| pH | Stable over a wide pH range |
| Reactivity | Rutin can undergo hydrolysis in alkaline conditions to yield quercetin and rutinose. It reacts with various oxidizing agents. |
Table 1: Various chemical properties of rutin.
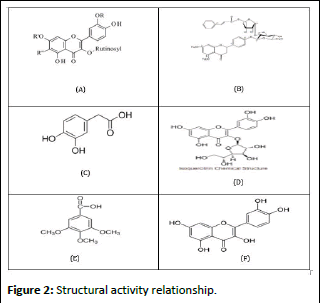
Stractural activity relationship
Quercetin's limited bioavailability may be caused by its low solubility in water and several pharmaceutically acceptable solvents. One of the main classes of flavonoids, quercetin (2- (3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one), has five hydroxyl groups at positions 3,5,7,3', and 4' of the flavanol basic skeleton. The majority of quercetin derivatives are made up of some of these hydroxyl groups, which are glycosylated to form different quercetin glycosides. Antioxidants are chemicals that can shield biological and chemical materials against Reactive Oxygen Species (ROS) that cause oxidative damage. ROS include singlet oxygen, Hydroxyl radicals (OH), superoxide radicals (O2-), and lipid peroxy-radicals. One type of flavonoid glycoside is rutin. The number of free hydroxyl groups, a C2–C3 double bond in the C ring, the presence of C-3, C-5, and C-7 hydroxyl groups, or the presence of a dihydroxyl group (catechol-type) or three adjacent hydroxyl groups (pyrogallol type) on the B ring of the flavonoid molecule are typically listed as requirements for antioxidant and antiradical activity. The primary cause of hyperglycaemia is the hydrolysis of the terminal 1,4- linked α-D-glucose residue by α-glucosidase, which liberates free α-D-glucose. Consequently, α-glucosidase inhibition is a crucial clinical treatment approach for diabetes mellitus. The substance outperforms quercetin in terms of DPPH radical scavenging ability, according to the experimental results. Based to the computed data, the substance has a lower IP and BDE than quercetin [15-20].
Apart from rutin, very little is known about the studies on flavanol glycosides' suppression of lipid autoxidation due to their restricted solubility in lipids. Based on earlier studies, rutin demonstrated potent antioxidant action due to its disaccharidebased glycosyl substitution. A quercetin diglycosidic was discovered to exhibit nearly the same antioxidative activity as rutin, as shown in Figure 2. This finding implies that other quercetin aminoglycosides may be involved in the antioxidative activity. The xanthine oxidase inhibitory activity suggests that the rutinoside moiety is crucial for the activity, as its deletion results in a loss of activity. It demonstrates that, out of all the compounds, RU3a3 (with the rutinoside group) showed the maximum activity with an IC50 value of 04.870 µM, while RU7c1 had the lowest activity and a fivefold decrease in activity with an IC50 value of 19.377 µM. It was discovered that hydrazine derivatives were more efficient than aniline derivatives, highlighting the significance of the NH–NH2 group. However, like in RU3a3 and RU3a2, substituting a sulfur group with hydrazine reduces activity, but substituting a phenyl group with sulfur increases activity (RU3a1). The inhibitory action decreases with substitution with an ester group.
Figure 2: Structural activity relationship.
Results and Discussion
Biological activity’s/significance
Antioxidant: The human body's internal metabolic activities and certain external substances can react with oxygen molecules to form highly Reactive Oxygen Species (ROS). Several chemically reactive compounds, such as hydroxyl radical (OH), superoxide (O2 -), and hydrogen peroxide (H2O2 ), are classified as ROS. Numerous pathophysiological events result when the body's antioxidant capacity is exceeded by the production of ROS brought on by different stressors. By evaluating the DPPH and ABTS radical scavenging activities, respectively, antioxidant activity was assessed. Increased DPPH radical scavenging activity was seen upon treatment with rutin and rutin glycoside in a concentration-dependent manner; rutin glycoside exhibited greater effects than rutin.
Anticancer: The most prevalent kind of kidney cancer in adults that develops in the renal proximal convoluted tubules is called Renal Cell Carcinoma (RCC). RCC has a high vascularization level and has the ability to spread to the liver, lungs, and bones, among other bodily parts. RCC is divided into several histopathological subtypes. Of these, 70-80% of RCC cases are of the clear cell RCC (ccRCC) type, which is the most prevalent. Age, gender, and ethnicity all have an impact on the occurrence of RCC, which differs among nations.
Anticancer: The most prevalent kind of kidney cancer in adults that develops in the renal proximal convoluted tubules is called Renal Cell Carcinoma (RCC). RCC has a high vascularization level and has the ability to spread to the liver, lungs, and bones, among other bodily parts. RCC is divided into several histopathological subtypes. Of these, 70-80% of RCC cases are of the clear cell RCC (ccRCC) type, which is the most prevalent. Age, gender, and ethnicity all have an impact on the occurrence of RCC, which differs among nations.
There are obvious drawbacks to the treatment, including refractoriness and low response rates to adjuvant therapies such radiation, targeted therapy, chemotherapy, hormone therapy, and immunotherapy. For circumscribed primary tumours, surgery is therefore the recommended course of treatment. However, the disease's grade, stage, and whether or not metastases are present all have a significant impact on how well the procedure works. Thus, the development of novel, secure, and potent anti-RCC therapies becomes imperative.
Xanthine oxidase inhibitors: The gout condition was elucidated by Hui Xue et al. One common type of arthritis is gout, which is characterized by the build-up of urate crystals in joints, causing pain and inflammation. An essential component of purine catabolism, xanthine oxidase, is involved in the etiology of gout. Furthermore, increased amounts of uric acid, a by-product of purine metabolism, aid in the crystallization of urate. Xanthine oxidase is indirectly linked to a number of metabolic diseases, such as diabetes and cancer, in addition to gout. We explore the potential of plant-found flavonoid molecules called rutin derivatives as anti-gout medicines in this review. An oxidoreductase enzyme with a molecular weight of 300 kDa, xanthine oxidase catalyzes reactions that result in the production of free radicals and uric acid. Excessive production of uric acid causes hyperuricemia, which in turn causes gout. The development of XO inhibitors from natural sources has been undertaken due to the numerous negative effects of purinederived xanthine oxidase inhibitors, such as Allopurinol. Former drug rutin. Rutin is a bioactive plant flavonoid that has antiinflammatory, antioxidant, and cytotoxic actions, among other therapeutic benefits, in various medical disorders. It has been discovered that rutin and its derivatives inhibit xanthine oxidase.
Anticonvulsant activity: A common and dangerous neurological disorder is epilepsy. Epidemiologic research has demonstrated the prevalence and significance of epilepsy in modern culture. Approximately 70% of epileptic patients have their seizures under control, primarily through pharmaceutical modulation of membrane ion channels or GABAergic or glutamatergic transmission. The molecular structure of flavonoids is phenylbenzopyran. Depending on the oxidative condition and the quantity and kind of substituents on the heterocyclic ring, they are separated into several types. The effects of flavonoids on the central nervous system have garnered a lot of interest lately. The most significant inhibitory neurotransmitter receptors in the Central Nervous System (CNS) are Γ-Aminobutyric Acid type A (GABAA) receptors, which are also essential targets for many relevant medications used to treat conditions like seizures, anxiety, sleeplessness, and alcoholism.
Antimicrobial activity: Numerous investigations have shown that two polyphenolic flavonoids that stand out among natural products are quercetin and rutin. Research on quercetin's antimicrobial action has revealed that it exhibits antibacterial activity against periodontal infections in vitro as well as activity against Clostridium botulinum and Staphylococcus aureus. The bacteria Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. One such source of naturally occurring compounds with biological activity could be RUTA species. RUTA extracts, essential oils, and isolated chemicals have demonstrated a wide range of potential applications in the management of various ailments and the eradication of pests, hence augmenting the value of these plants. Two methods exist for producing antibacterial polyamide fiber: Treating polyamide fiber with antibacterial agents during wet processing, or adding inorganic antibacterial agents (such as zinc oxide and silver nanoparticles) to polyamide during fiber spinning. The latter is most frequently used due to its ease of processing. In wet processing, cationic non-surfactant and surfactant agents (such as cetylpyridinium chloride and chlorhexidine), chitosan, silver nanoparticles, and metal salts are among the antibacterial agents utilized.
Quercetin's antibiofilm and antibacterial qualities against resistant S. aureus and S. saprophyticus were documented by Sergio Dias da Costa Junior et al. MIC values were calculated using the microdilution technique. Quercetin was found to exhibit antibacterial activity against the following strains of S. aureus: Methicillin-Susceptible (MSSA), Methicillin-Resistant (MRSA), Vancomycin-Intermediate (VISA) (MIC=125 and 150 µg/ ml), S. saprophyticus resistant to oxacillin, Vancomycin-Resistant Aureus (VRSA), and S. saprophyticus resistant to both oxacillin and vancomycin (MIC=500 to 1000 µg/ml). Quercetin reduced the S. aureus biofilm at MIC/2 and MIC/4, respectively, and the S. saprophyticus biofilm at MIC/2 and 46.9, at 5.5% and 46.5, at ± 2.7% and 39.4, respectively. Thus, it was established that quercetin functions admirably as a natural antibacterial agent.
S. Al-Majmaie et al. Rutin (1), rutin 3'-methyl ether (2), and 6- hydroxy-rutin 3',7-dimethyl ether (3), a novel flavonol glycoside, were identified from the methanol extract of Ruta chalepensis fruits that were harvested in Diyala, Iraq. They were able to clarify their structures by spectroscopic investigations, such as 1D, 2D, and HRESIMS NMR. Using a 96-well resazurin microtitre assay, the antimicrobial activity of compounds 1-3 was evaluated against four strains of both gram +ve and gram -ve bacteria as well as the single fungus strain, Candida albicans.
Han Y and others. From Artemisia PRINCEPS Pamp, rutin and other chemicals were extracted. A number of new rutin derivatives with a 1,4-pentadien-3-one moiety were then created and synthesized. Proton nuclear magnetic resonance spectroscopy (1H NMR), carbon nuclear magnetic resonance spectroscopy (13C NMR), and ESI-MS were used to analyze the target compounds. According to the results of the bioassay, at 500 μg/mL in vivo, some of the compounds had good to exceptional antiviral activity against the Tobacco Mosaic Virus (TMV) and the Cucumber Mosaic Virus (CMV).
The antibacterial activity of polyphenolic compounds derived from Ferocactus species was examined by O. Elansary H. and colleagues. Six polyphenols were found in the stem extracts of the Ferocactus sp. plants, out of the 21 compounds that were examined. Four phenolic acids protocatechuic acid, 3,4- dihydroxyphenylacetic acid, caffeic acid, and vanillic acid and two flavonoids rutoside and quercitrin were among these polyphenols. Two flavonoids, rutoside and quercitrin, and four phenolic acids, protocatechuic acid, 3,4-dihydroxyphenylacetic acid, caffeic acid, and vanillic acid, were among these polyphenols.
The antibacterial exertion of methanol extract and insulated factors of Mitracarpus scaber, a plant that native West Africans employ in folk medicine, was assessed by Bisignano G. et al. against strains of Candida albicans and Staphylococcus aureus. The methanol extract of Mitracarpus exhibits antibacterial and antimycotic parcels. The remaining linked chemicals, videlicet rutin, kaempferol-3-O-rutinoside, and psoralen, had negligible antibacterial and antimycotic parcels. Following this extract's separation and bioassay monitoring, seven mixes with antibacterial and antimycotic parcels were isolated. Gallic acid and trimethoxybenzoic acid were two of these substances that prevented Staph. aureus from growing.
Ivanov M, et al. excavated how flavanols affected Candida albicans. This study has examined the antifungal parcels of luteolin, apigenin, and flavonols; also, it has examined the goods of these mixes on genes garbling efflux pumps (CDR1) and the ergosterol biosynthesis enzyme (ERG11), as well as their glycosylated derivatives (quercitrin, isoquercitrin, rutin, and apigetrin). Also, the cytotoxicity of flavonoids to primary liver cells has been studied. Candida albicans growth was inhibited by luteolin, quercitrin, isoquercitrin, and rutin at the lowest inhibitory attention. The Minimum Inhibitory Attention (MIC) and minimum fungicidal attention were determined using the microdilution test, with some variations. Acinetobacter baumannii, Staphylococcus aureus, Enterococcus faecalis, Bacillus subtilis, and their drug-resistant isolates were tested against standard strains of Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, and fungi (Candida albicans, C. krusei) using the microdilution broth system by Orhan DD, et al. Strong antibacterial and antifungal exertion were demonstrated by all excavated mixes against isolated isolates of P. aeruginosa, A. baumanni, S. aureus, and C. krusei. Cinnamoyl-β-D-apiofuranosyl, 5,7- dimethoxyflavanone-4′-O-β-D-glucopyranoside, ′-trihydroxyflavanone-4 ′-O-β-D-glucopyranoside, and rutin were each effective against PI-3. HSV-1 was potently suppressed by β-Dglucopyranoside. The antiviral and antibacterial exertion of several flavonoids was estimated by Özçelik B, et al. Generally speaking, flavonoids have a spare sweet ring that comes from malonyl-CoA and p-coumaroyl-CoA connected to a chromane ring. More than 5000 flavonoids have been isolated to date from shops, including fruits, vegetables, and drinks. A significant amount of flavonoids are set up in nature as water-answerable glycosides. Due to their different natural conduct, including those of seditious, cardioprotective, antibacterial, anticancer, hepatoprotective, and antiviral parcels, flavonoids have gained attention in the nutraceutical and remedial domains. Four flavonoid derivatives were tested for their antibacterial, antifungal, and antiviral parcels scandenone, tiliroside, quercetin -3,7-O-α-l-dirhamnoside, and kaempferol -3,7-O-α-ldirhamnoside. The results showed that these four mixes were the most active against S. Aureus and E. Faecalis, with E. coli, K. pneumonia, A. baumanniiand, B. subtilis, P. mirabilis, and P. aeruginosa were themost resistant bacteria against mixes.
Quercetin inhibited the growth of six of the eleven tested microbes, including Streptococcus mutans, Streptococcus sobrinus, Lactobacillus acidophilus, Streptococcus sanguis, Actinobacillus actinomycetemocomitans, and Prevotella intermedia with MIC. Shu, et al. observed quercetin's antibacterial goods on the growth of eleven main oral pathogenic microbes. At varying minimum inhibitory pilules, quercetin and its derivatives displayed antibacterial action against distinct kinds of bacteria. also, it has been demonstrated that quercetin exhibits antibacterial parcels against a variety of Gram-positive bacteria, including Standard Enterococcus, Methicillin-sensitive S. aureus, and Methicillin-Resistant Staphylococcus aureus (MRSA). Also, it has been demonstrated that quercetin inhibits the growth of a variety of drug-resistant microbes, indicating the eventuality of quercetin as a important antimicrobial agent against drug-resistant strains.
Six flavonoids (5,7-dimethoxyflavanone-4'-O-β-Dglucopyranoside,5,7 dimethoxyflavanone-4'-O-(2''-O-(5'''-O trans-cinnamoyl)) were examined for their antibacterial exertion by Ozcelik B. et al. -β-D-apiofuranosyl) Extended-spectrum βlactamase producing multidrug-resistant bacteria Klebsiella pneumonia was screened against β-D glucopyranoside, naringenin-7-O-β-D-glucopyranoside,'- trihydroxy- flavanone- 4'- O- β- D-glucopyranoside, rutin, and nicotiflorin) isolated from Galium fissurense, Viscum anthology ssp. anthology, and Cirsium hypoleucum. At pilules of ampicillin, another control, all the flavonoids had no effect whereas all the isolated strains of K. pneumoniae displayed antimicrobial exertion in vitro analogous to the control antibiotic. Given the established resistance of ESβL-producing bacteria to all β-lactam antibiotics, it's noteworthy that our results lie within the antimicrobial exertion attention range.
Li B, et al. bandy how the attention of rutin affects the structure of fibers. Its limited water solubility and low bioavailability circumscribe its use, which still has to be addressed. In order to palliate these issues, Cellulose Acetate/poly (ethylene oxide) (CA/PEO) fiber was employed as a carrier. The process involved combining the bioactive fiber membrane with the CA/PEO result. The rutin-loaded fiber membranes' face shape, encapsulation effectiveness, antioxidant exertion, antibacterial exertion, and drug release were all examined. also, the mechanical, thermal stability and molecular relations of the membranes were assessed. This suggests that the CA/PEO fiber membrane loaded with rutin may be a bioactive substance.
The impact of rutin, a flavonoid molecule, on photodynamic inactivation by MB Pseudomonas aeruginosa and Staphylococcus aureus was examined by Motallebi M, et al. One of the many qualities of the flavonoid component rutin, which is derived from plants like tea, wheat, and apples, is its antibacterial action. Following the execution of the MTT and Minimum Inhibitory Concentration (MIC) assays, the impact of a PDT at 660 nm and rutin pre or post-treatment on bacteria in the forms of planktonic and biofilm were examined. The outcomes demonstrated that when rutin is combined with methylene blue as a photosensitizer and a PDT, bacterial biofilm formation and the quantity of bacteria in the planktonic state are reduced more than when MB is used alone. With the suggested approach, MB-a PDT had no adverse effect on human dermal fibroblast, which may indicate that it can be used in conjunction with rutin as a novel approach to treat wound infection bacteria.
Antioxidant property: Rutin's impact on neurodegenerative illnesses. Numerous Neurodegenerative Disorders (NDs) are related by common mechanisms, such as neuronal loss, apoptosis, mitochondrial dysfunction, oxidative stress, and inflammation. These diseases include Alzheimer's disease, Parkinson's disease, Huntington's disease, and prion diseases. Numerous studies have demonstrated the critical role that oxidative stress plays in the onset and progression of NDs. A review of quercitin's biological relevance was provided by Alexander Victor et al. Antioxidants are chemicals that have the potential to shield cells from harm from unstable molecules like free radicals, plant pigments, mostly found in onions, grapes, berries, cherries, broccoli, and citrus fruits, are strong antioxidant flavonoids, or more precisely, flavanols. It is a multipurpose antioxidant that has been shown to have protective properties against tissue damage brought on by a range of drug toxicity. Sung Sook Choi and colleagues' work on the in vitro antioxidant activity and anti-inflammatory effects, as well as the effects on platelet aggregation and blood coagulation, were examined between rutin and rutin glycoside with varying solubility. Rutin glycoside, which is made up of rutin di and mono-glucoside, was made usingrutin by enzymatic trans glycosylation. In antioxidant experiments, rutin glycoside exhibited a greater effect on radical scavenging activity than rutin. Rutin was more hazardous to murine macrophage RAW264.7 cells than rutin glycoside.
Tanza Pivec et al. clarified the relationship between waterbased rutin's antioxidant activity and its capacity to heal wounds. One well-known antioxidant that comes from plants is the flavonoid Rutin (RU). Because of its poor water solubility, its prospective use in the cosmetic and pharmaceutical industries is nevertheless restricted. Polymerization of the phenolic RU into Polyrutin (PR) helps get over this restriction. In this work, RU was polymerized enzymatically in water without the use of organic solvents. Moreover, 1H NMR and FTIR spectroscopy were used to examine the chemical structure of PR. PR's molecular weight was ascertained by Size-Exclusion Chromatography (SEC), and its acid/base characteristics were investigated using potentiometric charge titrations. Additionally, based on PR's capacity to (i) Scavenge non-biological stable free radicals (ABTS), (ii) Scavenge biologically significant oxidants like O2•, NO•, and OH•, and (iii) Chelate Fe2+, this work examined the antioxidant and free radical scavenging potential of PR with regard to its chemical structure. The anti-inflammatory, antitumour, and antioxidant characteristics of kaempferol were investigated by Jingqiu Wang et al. Few studies have contrasted kaempferol (D) and its glycosides, despite the fact that they are both widely distributed in nature and exhibit a variety of bioactivities. In this work, we provide the variations in the anticancer, antioxidant, and anti-inflammatory properties of kae, kae-3-O-rhamnoside (kae-3-O-rha), kae-7-O-glucoside (kae-7-O-glu), and kae-3-O-rutinoside (kae-3-O-rut).
Anticancer activity: According to research by Shirin Asgharian and colleagues, some flavonoids have been demonstrated to control a number of signal proto-oncogene pathways. It has been demonstrated that proto-oncogenes encode phosphokinases, growth factors, nuclear proteins, membrane proteins, and cell surface receptors. Three significant protooncogenes, c-Fos, c-Jun, and c-Myc, are linked to the processes of inflammation and carcinogenesis. Similarly, rutin caused cytotoxicity by genotoxicity, the mitochondrial apoptotic route, and a few relatively upstream apoptotic mechanisms, such as the increase of Antioxidant Enzyme (AOE) and the decrease of ROS production. In order to modify the solubility and lipophilicity of quercetin, Ayan Mukherjee et al. published a publication that outlines a synthetic process to selectively attach a distinct substitution pattern at C-3′ locations of quercetin. By using X-ray crystallography, the regioselectivity between the C-3′ and C-5 locations was demonstrated. Using both in vitro and in vivo colon cancer models, the likely mechanism of cytotoxicity was examined. The HCT-116 cell line was used for the in vitro experiment. Mice with CT-26 tumours, a common model utilized in drug development, were used for the in vivo assessment. When weak base and esters-containing alkyl chains are added to quercetin, it becomes more soluble and lipophilic, which effectively increases cell permeability. Significantly superior physicochemical features of the new semi synthetic compounds enhance the anticancer effectiveness against colon cancer.
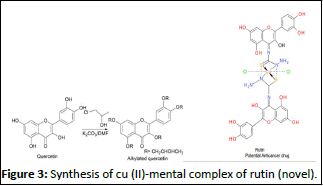
According to studies by Amir Imani et al., the polyphenolic bioflavonoid rutin's antioxidant qualities are well-established and have a variety of pharmacological uses in the study of cancer. Recent studies have been conducted to examine the processes underlying rutin's anticancer action because it is a safe anticancer agent with little side effects, in contrast to chemotherapy medicines, which have a wide spectrum of negative effects. Numerous studies have been conducted on the anticancer mechanisms of this natural substance, both in vitro and in vivo. The anticancer effects of this drug are mediated through the regulation of various cellular signalling pathways, including NF-θB, Wnt/β-catenin, p53-independent pathway, PI3K/Akt, JAK/STAT, MAPK, p53, and apoptosis. MMS Kinthada Prakash, et al. The major uses of quercetin derivatives of thiosemicarbazone, vincristine, and vinblastine are in the treatment of advanced testicular cancer, advanced breast cancer, Hodgkin's disease, and lymphocytic lymphoma. The chemicals thiosemicarbazide and thiosemicarbazone have drawn particular interest because of their ability to combat protozoa, influenza, and specific types of cancers. Because of their beneficial chemotherapeutic qualities, thiosemicarbazones have been investigated for their anti-malarial and anti-tumour activity (Figure 3).
Figure 3: Synthesis of cu (II)-mental complex of rutin (novel).
Agarose gel electrophoresis has been used to examine the nuclease activity of current ligands and their complexes on pBR 322 plasmid DNA in the presence or absence of H2O2. Both in the presence and absence of the oxidant, the ligands show no discernible activity at micro molar concentrations. According to Udaya Rajesh R. et al., quercetin inhibits pancreatic cell proliferation in both vitro and in vivo using the MIA PaCa-2 cell line. Researchers are looking for novel approaches to build a powerful medication against pancreatic cancer since pancreatic ductal adenocarcinoma is a very aggressive tumour type that has become resistant to gemcitabine and other cytotoxic medications. In this regard, quercetin has shown promise in treating pancreatic cancer. According to a study by Rajesh Prasad et al., rutin has been shown to have anticancer, chemo preventive, and chemo sensitizing properties against a range of cancers. The study also showed that rutin, when given ntraperitoneally (i.p.) once every four days at a dose of 120 mg/kg, was effective in inhibiting the growth of human leukaemia HL-60 tumours in a xenograft mouse model and had an anticancer effect on human neuroblastoma LAN-5 cells. It was discovered that rutin-mediated G2/M phase cell cycle arrest and apoptosis induction in LAN-5 cells were responsible for this anticancer effect. According to reports, rutin has no harmful effects on nude mice with SW480 tumours, but it exhibits strong cytotoxic effects against human colon adenocarcinoma SW480 cells in vitro as well as anticancer and anti-angiogenic actions in vivo.
Conclusion
In order to solve pharmacokinetic problems, phytochemicals are particularly important in the drug development process since they can be used to synthesize derivatives with changed structures. Significant therapeutic promise for rutin and its derivatives exists in pharmaceuticals. This review looks at several artificial derivatives of rutin, emphasizing how effective strong derivatives are at treating and preventing diseases like bacterial or microbial infections, inflammation, and oncogenesis.
Future research should focus on optimizing the synthesis processes, conducting comprehensive in vivo studies and evaluating the long-term safety and efficacy of these compound.
References
- Kicel A, Owczarek A, Michel P, Skalicka-Woźniak K, Kiss AK, et al. (2015) Application of HPCCC, UHPLC-PDA-ESI-MS3 and HPLC-PDA methods for rapid, one-step preparative separation and quantification of rutin in Forsythia flowers. Ind Crop Prod 76:86-94
- Pivec T, Kargl R, Maver U, BraÄÂÂiÄÂÂ M, Elschner T, et al. (2019) Chemical structure–Antioxidant activity relationship of water–based enzymatic polymerized rutin and its wound healing potential. Polymers 11:1566
[Crossref] [Google Scholar] [PubMed]
- Pyo SM, Meinke M, Keck CM, Müller RH (2016) Rutin-increased antioxidant activity and skin penetration by nanocrystal technology (smartCrystals). Cosmetics 3:9
- Lupascu D, Profire L, Apotrosoaei M, Tuchilus C, Vasincu Im, et al. (2020) Synthesis and Antimicrobial Activities of Novel Rutin Derivatives Carrying Quinoline Moiety.
- Enogieru AB, Haylett W, Hiss DC, Bardien S, Ekpo OE (2018) Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid Med Cell Longev 6241017
[Crossref] [Google Scholar] [PubMed]
- Ganeshpurkar A, Saluja AK (2017) The Pharmacological Potential of Rutin. Saudi Pharm J 25:149–164
[Crossref] [Google Scholar] [PubMed]
- Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G (2017) Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol 67:220–235
- Lue BM, Nielsen NS, Jacobsen C, Hellgren L, Guo Z, et al. (2010) Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem 123:221–230
- de Araújo MEMB, Moreira Franco YE, Alberto TG, Sobreiro MA, Conrado MA, et al. (2013) Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem 141:266–273
- Chen LY, Huang CN, Liao CK, Chang HM, Kuan YH, et al. (2020) Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 9:1122
- Gelen V, Å?engül E, Gedikli S, Atila G, Uslu H, et al. (2017) The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac J Trop Biomed 7:647–653
- Satari A, Ghasemi S, Habtemariam S, Asgharian S, Lorigooini Z (2021) Rutin: A Flavonoid as an Effective Sensitizer for Anticancer Therapy; Insights into Multifaceted Mechanisms and Applicability for Combination Therapy. 23:1–10 [Crossref]
- Malik N, Dhiman P, Khatkar A (2019) In silico design and synthesis of targeted rutin derivatives as xanthine oxidase inhibitors. BMC Chem 13:71
- Lin S, Bao M, Wang Z, Zou X, Ge S, et al. (2021) Morphological evaluation of the subaxial cervical spine in patients with basilar invagination: A CT-based study. Spine 46:1387-1393
[Crossref] [Google Scholar] [PubMed]
- He Q, Wu Y, Tian Y, Li G, Liu J, et al. (2019) Facile Electrochemical Sensor for Nanomolar Rutin Detection Based on Magnetite Nanoparticles and Reduced Graphene Oxide Decorated Electrode. Nanomaterials 9:115
- Prasad R, Prasad SB (2019) A review on the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J Pharm Pharmacol 5:1–20
- Narasagoudr SS, Hegde VG, Chougale RB, Masti SP, Vootla S, et al. (2020) Physico-chemical and functional properties of rutin induced chitosan/poly (vinyl alcohol) bioactive films for food packaging applications. Food Hydrocoll 109:106096
- Madkour D, Ahmed M, Elkirdasy A, Orabi S, Mousa A (2024) Rutin: Chemical properties, Pharmacokinetic properties and Biological activities. Matrouh J Vet Med 4:26–34
- Alizadeh SR, Ebrahimzadeh MA (2022) Quercetin derivatives: Drug design, development, and biological activities, a review. Eur J Med Chem 229:114068
- Magar RT, Sohng JK (2020) A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J Microbiol Biotechnol 30:11–20
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences