Assessment of Nephrotoxicity, Anti-Inflammatory and Antioxidant Properties of Epigallocatechin Epicatechin and Stigmasterol Phytosterol (Synergy) Derived from Ethyl Acetate Stem Bark Extract of Spondias Mombin on Wistar Rats Using Molecular Method of Analysis
Oludare Temitope Osuntokun1*, AO Oluduro2, TO Idowu3 and AO Omotuyi4
1Department of Microbiology, Adekunle Ajasin University, Nigeria
2Department of Microbiology, Obafemi Awolowo University, Nigeria
3Department of Pharmaceutical Chemistry, Obafemi Awolowo University, Nigeria
4Centre for Biocomputing and Drug Development (CBDD), Adekunle Ajasin University, Nigeria
- *Corresponding Author:
- Oludare Temitope Osuntokun
Department of Microbiology
Faculty of Science, Adekunle Ajasin University
Akungba Akoko, P.M.B 001, Ondo State, Nigeria
Tel: +234 806 381 3635
E-mail: osuntokun4m@yahoo.com
Received Date: 13 September 2017; Accepted Date: 23 October 2017; Published Date: 30 October 2017
Citation: Osuntokun OT, Oluduro AO, Idowu TO, Omotuyi AO (2017) Assessment of Nephrotoxicity, Anti-Inflammatory and Antioxidant Properties of Epigallocatechin, Epicatechin and Stigmasterol Phytosterol (Synergy) Derived from Ethyl Acetate Stem Bark Extract of Spondias Mombin on Wistar Rats Using Molecular Method of Analysis. J Mol Microbiol. Vol. 1 No. 1: 5.
Copyright: © 2017 Osuntokun OT, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The purpose of this research work is to assess the effect of nephrotoxicity, anti-inflammatory and antioxidant properties of epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) isolated from ethyl acetate stem bark extract of Spondias mombin on wistar rats using molecular method of analysis. The selected biomarkers used for this research work are GapdH, KIM-1, IL-1B, GSR, CAT, GPX-1 and IL-1Alpha at 30, 100 and 1000 mg/ml concentration. Seven week-old, male wistar rats from the Centre for Biocomputing and Drug Development (CBDD) Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria, weighing 50 g were housed and grouped into four groups (2 animals per group), 30 mg/g, 100 mg/g and 1000 mg/g group. they were acclimatized for one week and 14-day treatment regimen were given, wistar rats received daily oral administration of extract. At the end of the 14th day period of stable administration, wistar rats were subjected to fasting over-night and were sacrificed. Tissues (kidney and liver) were excised into an eppendorf tubes containing 50 μl molecular biology reagent (Trizol). Tissues is then stored in temperature of -7°C. RNA was extracted from the cells of the kidney and liver of wistar rats and homogenized in the eppendorf tubes containing Trizol reagent, 2 μl reverse transcriptase enzymes were added, to performed Reverse transcription with Polymerase Chain Reaction assay (PCR). Template (cDNA) of 5 μl nucleas-free water and 3.5 μl, forward primers of 2 μl and reverse primers of 2 μl, master mix 2 μl were added. 0.5% of agarose gel using 0.5x TBE buffer (2.6 g of Tris base, 5 g of Tris boric acid and 2 ml of 0.5 M EDTA) were used to perform gel electrophoresis were electrophoresed in gel electrophoresis tank. It was observed that epigallocatechin, epicatechin and stigmasterol phytosterol reduce the nephrotoxic effect induced by the selected biomarkers and it can be deduced that the isolated compound has the ability to reduce the activity of free radical (ROS) generated by the selective biomarker on the kidney and liver cell during metabolic activity. Epigallocatechin, epicatechin and stigmasterol phytosterol isolated from stem bark of Spondias mombim extract have proven itself to be nature’s extraordinary nephrotoxicity, anti-Inflammatory, antioxidant agent.
Keywords
Epigallocatechin; Epicatechin; Stigmasterol; Phytosterol; Nephtoxicity; Anti-inflammatory; Antioxidant properties
Introduction
Spondias Mombin is a fructiferous tree having habitat in Nigeria, Brazil and several other tropical forests in the world. This plant is readily common around in South West of Nigeria (Yoruba) and is commonly used in folk medicine. Various cultures frequently maintain within their collection of traditional medicine substances valued as drugs for treating diseases. Spondias Mombin Linn belongs to the family Anacardiacae. It grows in the rain forest and in the coastal area. It can reach a height of 15-22 m. The trunk has deep incisions in the bark, which often produces a brown resinous substance. The leaves and the flowers are at the end of the branches. Before the tree starts to flower, it strips itself from most of the leaves. The fruit, and 1½ inch long oval yellow plum, has a leathery skin and a thin layer of fruit pulp with a very exotic taste. It hangs in numerous clusters of more than a dozen on the tree. Very rich in vitamins B1 and C, the fruit mostly exists as an oval seed. The mode of propagation of the plant is by seeds and cuttings.
Catechins inhibited replication of influenza virus on MDCK cells. The inhibitory effect is in a concentration-dependent manner and throughout the virus infection cycle after initial infection. Antiviral effects exerted not only on initially infecting viruses but on newly propagated viruses as well [1]. Epicatechin is a type of flavonoid which is mainly found in green tea (Camellia sinensis) and dark chocolate; it has now been discovered in Spondias Mombin which is good alternative to green tea in places like West Africa where green tea is not found in large quantity. Polyphenols comprise of 30-40% of extractable solids from dried Spondias Mombin stem bark [2]. Epicatechins have been proven to have diverse benefits to human health; it reduces the risks of diabetes mellitus and cardiovascular diseases. Pharmalogical effects such as antihyperlipidemic, anti-inflammatory, antioxidative effects, anticarcinogenic, and cytoprotective should also be mentioned. These flavonoids can be used as therapeutic agents individually or in combination with other synthetic drugs and antibiotics to produce a new generation of phytopharmaceuticals [3]. Natural product like epicatechin is cost effective, highly biocompatible and has low toxicity [4]. Epicatechin (EC) helps in the inhibition of HIV-1 reverse transcriptase in vitro and binds directly to CD4 molecule with consequent inhibition of gp120 binding [5]. Epigallocatechin (EGC) inhibited plaque forming activity of influenza A and B virus whereas EC exhibited little inhibition. A polyphenol mixture is more efficient than a single compound in plaque inhibition and epicatechin had no antiviral effect and only a little inhibition on plaque formation, epicatechin had no antiviral effect and only a little inhibition on plaque formation [4]. (+)-Epicatechin and (-)-epicatechin was found to inhibit Hepatitis C virus (HCV) replication by inactivating Cyclooxgenase-2-depandant signalling pathway. Cyclooxygenase-2 (COX-2) produces various prostaglandins (PG) which result in chronic inflammation and fibrosis. PGE2, thromboxane B2 and prostacylin gives rise to cellular proliferation, cancer invasiveness, angiogenesis and is anti-apoptotic. Therefore, leading to hepatocarcinogenesis, (+)-Epicatechin and (-)-epicatechin may suppress the gene expressions of pro-inflammatory enzymes and cytokines that are induced by HCV [6].
Epigallocatechin (EGC) increases lysosomal acidification, regulates autophagy and lipid clearance in liver due to its antisteatotic property [7]. EGC, in dose dependent manner can reduce the release of cytokines/chemokines responsible for inflammation showing anti-inflammatory property [8]. Its antiallergic property strongly inhibits activation of mast cells and expression of high-affinity IgE receptor, which produces an allergic reaction on exposure to certain foreign antigens [9].
The most important antioxidant property is very crucial in treating chronic diseases which are related to oxidative stress, cardiovascular, neurodegenerative diseases and cancer. Research on this property revealed information about its anticardiovascular and anti-hypertensive activity which enables epigallocatechin, epicatechin (EGC, EC) and polysteroids, to prevent platelet aggregation, lower cholesterol level and inhibit lipid peroxidation [10]. Epigallocatechin, Epicatechin (EGC, EC) and polysteroids is also beneficial for preventing aging of brain and other neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases as depicted from mouse model studies [11]. EGC and EC also show antihypercholesterolemic (anti-obesity) activity and promote weight loss through fat oxidation [12]. Animal studies have demonstrated that EGC, EC and polysteroids is an efficient agent in preventing the development of diabetes, type 1 or type 2 [13].
EGC affects each step of the HIV life cycle, from cell attachment, entry of virus, replication cycle to the expression of mRNA. It also interferes with the infectivity of the virus by binding to the surface of viral envelope and deforming the phospholipids followed by lysis of the virus particle [5]. The attachment of the gp120 envelope protein to the CD4 receptor on T-helper cells initiates the entry of HIV-1 into the host. EGC blocks the interaction of gp120 and CD4 and prevents the attachment of HIV-1 virions, EGC also inhibits the viral production from infected cells and the level of expression of viral mRNA [14].
Materials and Methods
Nephrotoxicity, anti-inflammatory and antioxidant of selective biomarker on pure compound from Spondias Mombin on wistar rats using molecular method of analysis
Seven week-old, male wistar rats from Centre for Biocomputing and Drug Development (CBDD), Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria. The wistar rats weighing 50 g were housed and grouped into four groups (2 wistar rats per group) including the control group, 30 mg/g, 100 mg/g and 1000 mg/g group in a temperature and humidity controlled environment under a 12 hours light/dark cycle, with food and portable water available adlibitum. All procedures were performed in strict accordance with protocols approved by University of Newcastle Animal Care and Ethics Committee, the New South Wales Animal Research Act and Regulations, and the Australian code of practice for care and use of animals for scientific purposes.
Methods and treatment
After one week of acclimatization, 14-day treatment regimen were given, wistar rats received daily oral admiration of extract. Extract dose concentration for each group were calculated based on their body weight. 0.02 g of extract was weighed and dissolved in 14 ml of distilled water for each group 0.5 ml of the solution was given to each animal consecutively orally across the groups for 14 days except the control group that receives no treatment except their normal ration and portable water.
Animal sacrifice and tissue harvesting
At the end of the 14th day period of stable treatment regimen, animals were subjected to fasting over-night and were sacrificed in the next morning. Tissues (kidney and liver) were excised into eppendorf tubes containing 50 μl molecular biology reagents (Trizol) across the groups. Tissues is then stored in temperature of -70°C.
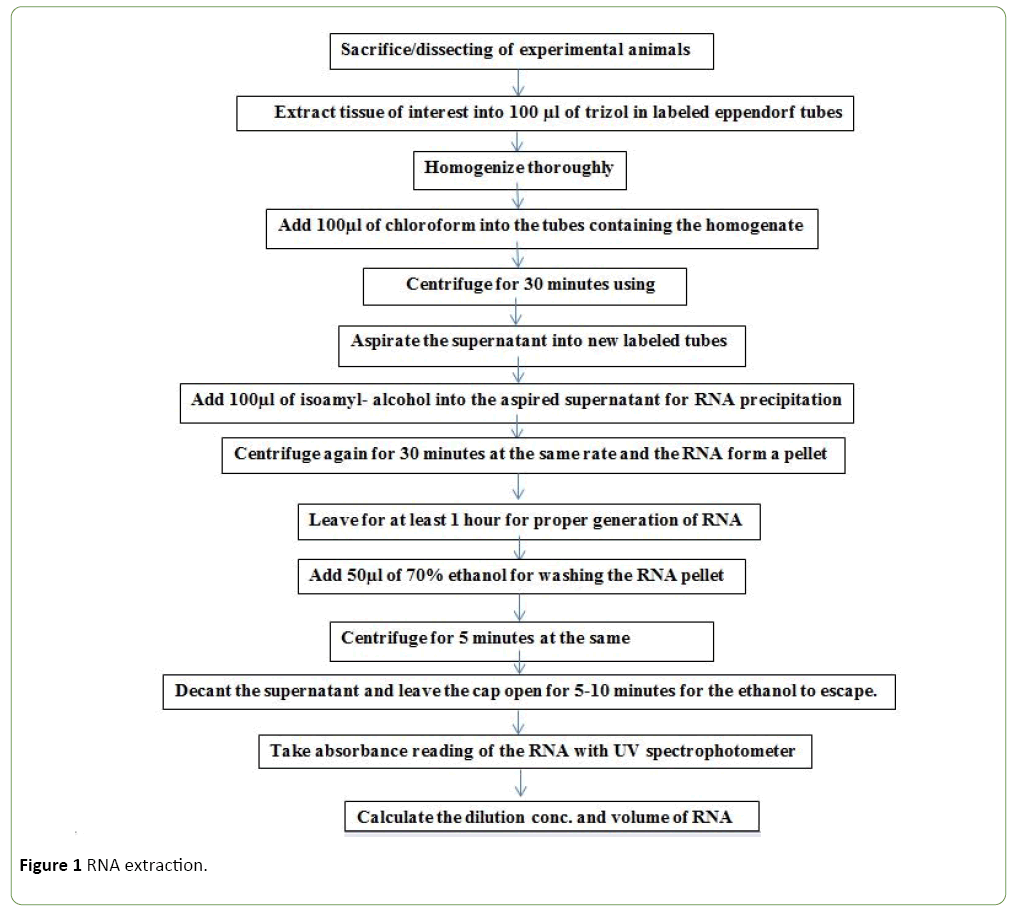
RNA extraction
High quality RNA was isolated using a modified hot SDS/hot phenol method previously described previously [15,16]. The cells were pelleted by centrifugation at 5000g for 5 min. To the pellets were rinsed twice in PBS buffer. 1/10 volume of 95% ethanol plus 5% saturated phenol were added to the pellets to stabilize cellular RNA. The cells were then re-harvested by centrifugation (8200 g, 4°C, 2 minutes). The supernatant was aspirated and pellets resuspended in 800 μl of lysis buffer (10 mM Tris, adjusted to pH 8.0 with HCl, 1 mM EDTA) and 8.3 U/ml Ready-LyseTM Lysozyme Solution.
After the pellets were resuspended, 80 μl of a 10% SDS solution was added, mixed and incubated for 2 minutes at 64°C. 88 μl of 1 M NaOAc (pH 5.2) was mixed with the lysate followed by an equal volume of water saturated phenol was added and incubated at 64°C for 6 min whilst inverting the tubes every 40 sec. The aqueous phase was separated following centrifugation at 21,000 g for 10 min at 4°C. RNA was precipitated from the aqueous layer using 1/10 volume of 3 M NaOAc (pH 5.2), 1 mM EDTA and 2 volumes cold EtOH and centrifugation at 21,000 g for 25 min at 4°C. Pellets were washed with ice cold 80% EtOH and centrifuged at 21,000 g for 5 minutes at 4°C. The ethanol was carefully removed and the pellets were air dried for 20 min. The pellets from each split sample were resuspended in a total of 100 μl of RNase-free water and combined into one microfuge tube (Figure 1).
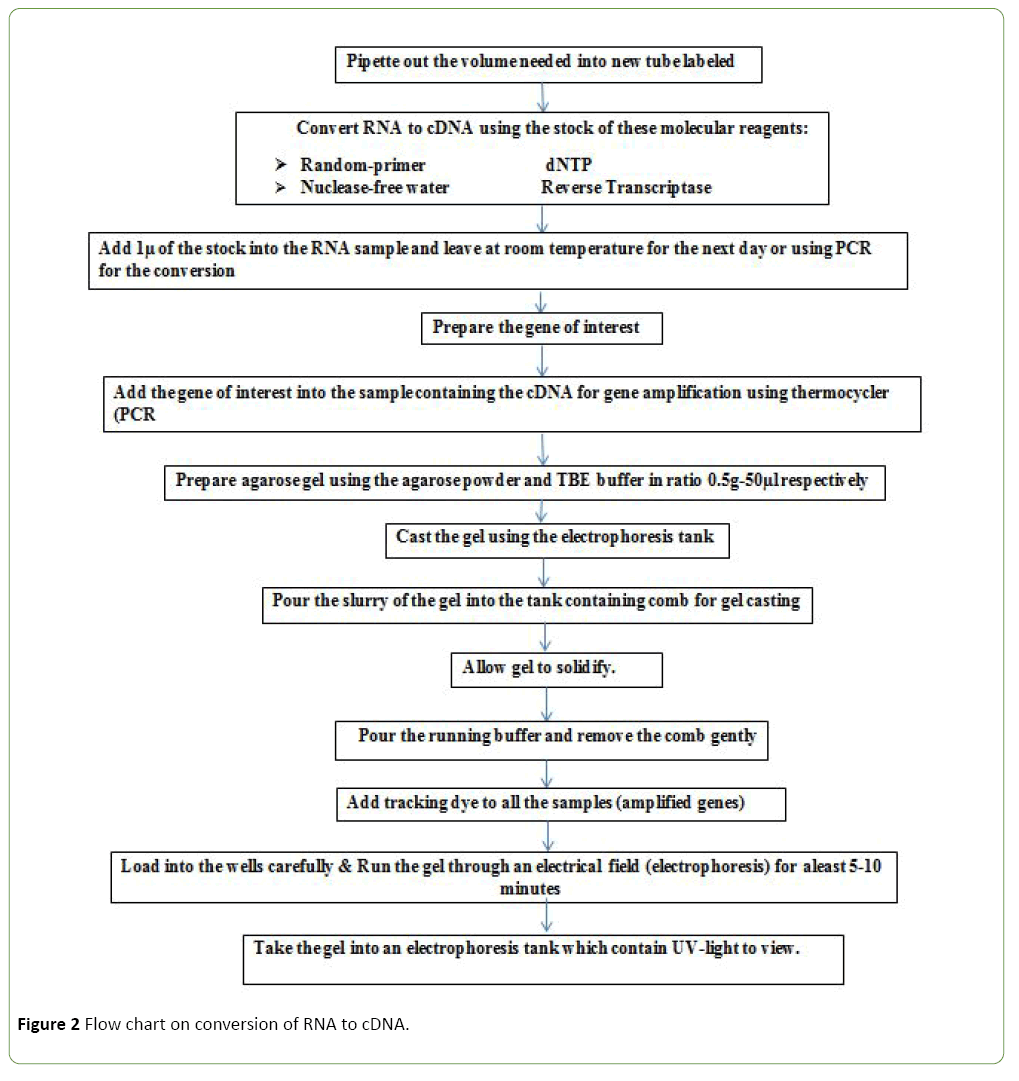
Reverse transcription
Reverse transcription was performed by adding 2 μl reverse transcriptase from the cocktail which contain (1) the random oligo primer (2) the dNTP's (3) the reverse transcriptase and (4) the reverse transcriptase buffer (5) nuclease-free water. Total RNA was quantified using spectrophotometric absorbance at 260 nm DNA was removed with Turbo DNA-free (Ambion, Inc.) using the rigorous protocol. Removal of DNA from the RNA samples was performed using DNA-free™ DNA Removal Kit (ThermoFisher) following manufacturer’s protocol. Purified DNA-free RNA was converted to cDNA immediately using ProtoScript® First Strand cDNA Synthesis Kit (NEB). The cDNA was diluted to a final volume of 286 μl and stored at 4°C (Figure 2).
Polymerase Chain Reaction (PCR)
The assay performs using an optimization. Template (cDNA) 5 μl, nucleas-free water 3.5 μl, forward primers 2 μl and reverse primers 2 μl and master mix 2 μl (Table 1), Reverse Transcription–PCR reaction was performed in a 15.0 μl final volume. Briefly, 1 μl template cDNA (~40 ng) was combined with 1.0 μl of forward primer (5 nM), 1.0 μl of reverse primer (5 nM), 4.5 μl nuclease-free water and 7.5 μl of Taq 2X Master Mix. Primers used for the RT–PCR. Thermal cycling was performed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 15 sec. Analysis of the PCR products was performed using 1.5% agarose gel solution in TBE buffer and visualization was enabled by soaking gel in ethidium bromide solution for 10 min and UV-transilluminator, Amplification conditions were: 94°C pre-denaturation for 5 min, 94°C for 30 sec, annealing 55°C for 30 sec and Extension 72°C for 30 sec and then 5 min at 72°C by 30 cycles.
Table 1 List of Primers used for PCR.
| Target genes Or Biomarkers |
GapdH (Glyceraldehyde-3-phosphate dehydrogenase) |
KIM-1 (Kidney injury molecule 1) |
IL-1B (Interleukin 1B) |
GSR (Glutathione-disulfide reductase) |
CAT (Catalase) |
GPX-1 (Glutathione Peroxidase-1) |
IL-1A (Interleukin 1, alpha) |
|---|---|---|---|---|---|---|---|
| Forward 5'-3' | GTCTCACCCCATTCTACCGC | AAGCCGCGCAAACATTAGTGC | TTGAGTCTGCACAGTTCCCC | GGAAGTCAACGGGAAGAAGTTCACTG | CCGACCAGGGCATCAAAA | AGTTCGGACATCAGGAGAATGGCA | TGGCAGGCCATAGGTTAAGG |
| Reverse 5'-3' | AGTTTGGCTACTCCAACCGC | TGAGCTAGAATTCAGCCACACA | TCCTGGGGAAGGCATTAGGA | CAATGTAACCGGCACCCACAATAAC | GAGGCCATAATCCGGATCTTC | TCACCATTCACCTCGACTTCTCA | GCGAGTGACTTAGGACGAGG |
| References | Butterfield DA et al. [17] | Anders MW [18] | Heuk S et al. [19] | Walley JW et al. [20] | Pinho C et al. [21] | Judy B et al. [22] | SomiglianaE et al. [23] |
Gel electrophoresis
Assessment of Polymerase Chain Reaction products (amplicons) were electrophoresed in 0.5% of agarose gel using 0.5 × TBE buffer ( 2.6 g of Tris base, 5 g of Tris boric acid and 2 ml of 0.5M EDTA and adjusted to pH 8.3 with the sodium hydroxide pellet) with 0.5 μl ethidum bromide. The expression product was visualized as bands by UV-transilluminator.
Results
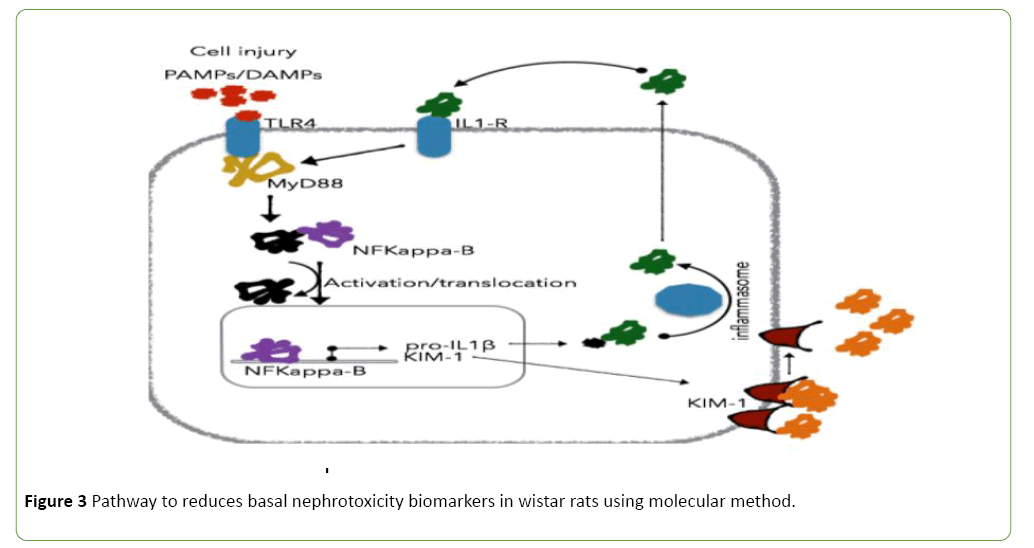
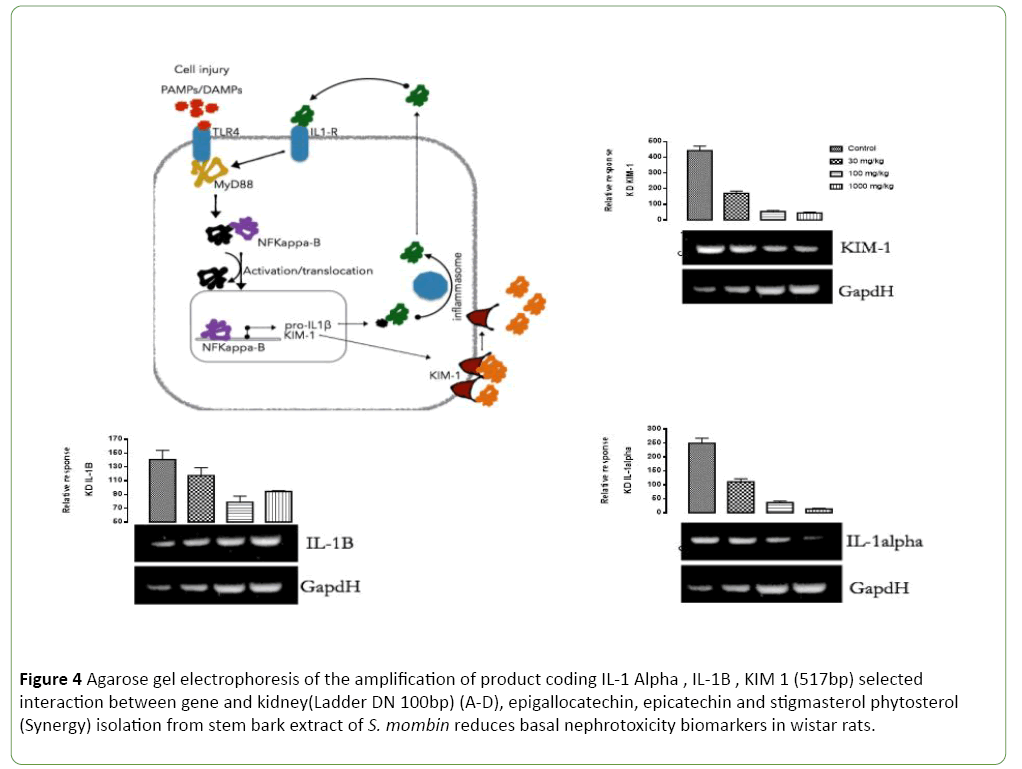
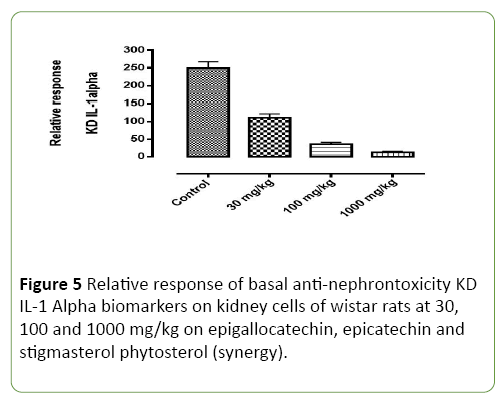
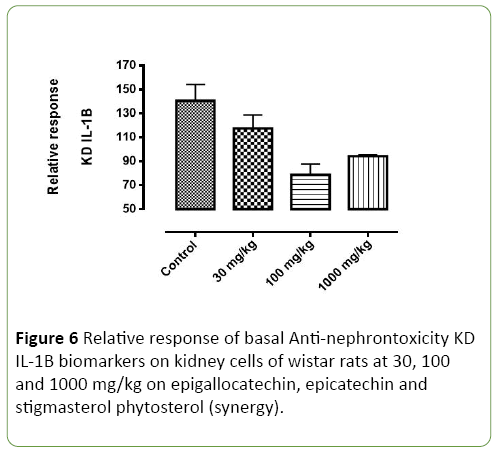
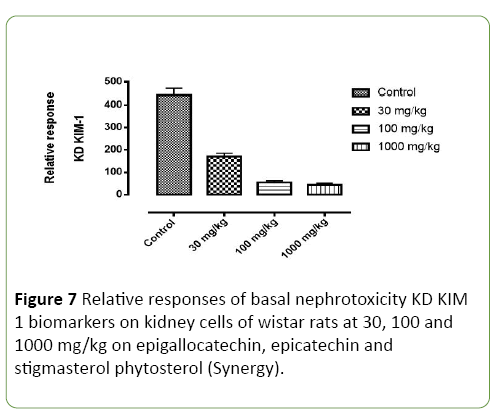
Basal nephrotoxicity of epigallocatechin (Figure 3), epicatechin and stigmasterol phytosterol on selective bio markers on kidney of Wister rats at 30, 100 and 1000 mg/ml using molecular method. Figures 4-7 show the relative response of epigallocatechin, epicatechin and stigmasterol phytosterol isolated from the Stem bark of S. mombin on the kidney of Wister rat at 30, 100 and 1000 mg/ml concentration. The biomarkers used are IL-1 ALPHA, IL-1B and KIM 1. In Figure 3, it can be deduced that, there is a relative response of epigallocatechin, epicatechin and phytosterol on the biomarker at 100 and 1000 mg/ml concentration. Epigallocatechin, epicatechin and stigmasterol phytosterol reduce the toxic effect induced by the selected biomarkers.
Figure 3: Pathway to reduces basal nephrotoxicity biomarkers in wistar rats using molecular method.
Figure 4: Agarose gel electrophoresis of the amplification of product coding IL-1 Alpha , IL-1B , KIM 1 (517bp) selected interaction between gene and kidney(Ladder DN 100bp) (A-D), epigallocatechin, epicatechin and stigmasterol phytosterol (Synergy) isolation from stem bark extract of S. mombin reduces basal nephrotoxicity biomarkers in wistar rats.
Figure 5: Relative response of basal anti-nephrontoxicity KD IL-1 Alpha biomarkers on kidney cells of wistar rats at 30, 100 and 1000 mg/kg on epigallocatechin, epicatechin and stigmasterol phytosterol (synergy).
Figure 6: Relative response of basal Anti-nephrontoxicity KD IL-1B biomarkers on kidney cells of wistar rats at 30, 100 and 1000 mg/kg on epigallocatechin, epicatechin and stigmasterol phytosterol (synergy).
Figure 7: Relative responses of basal nephrotoxicity KD KIM 1 biomarkers on kidney cells of wistar rats at 30, 100 and 1000 mg/kg on epigallocatechin, epicatechin and stigmasterol phytosterol (Synergy).
In vivo antioxidant potency of epigallocatechin, epicatechin and stigmasterol phytosterol on the liver of wistar rats at 30, 100 and 1000 mg/ml using molecular method
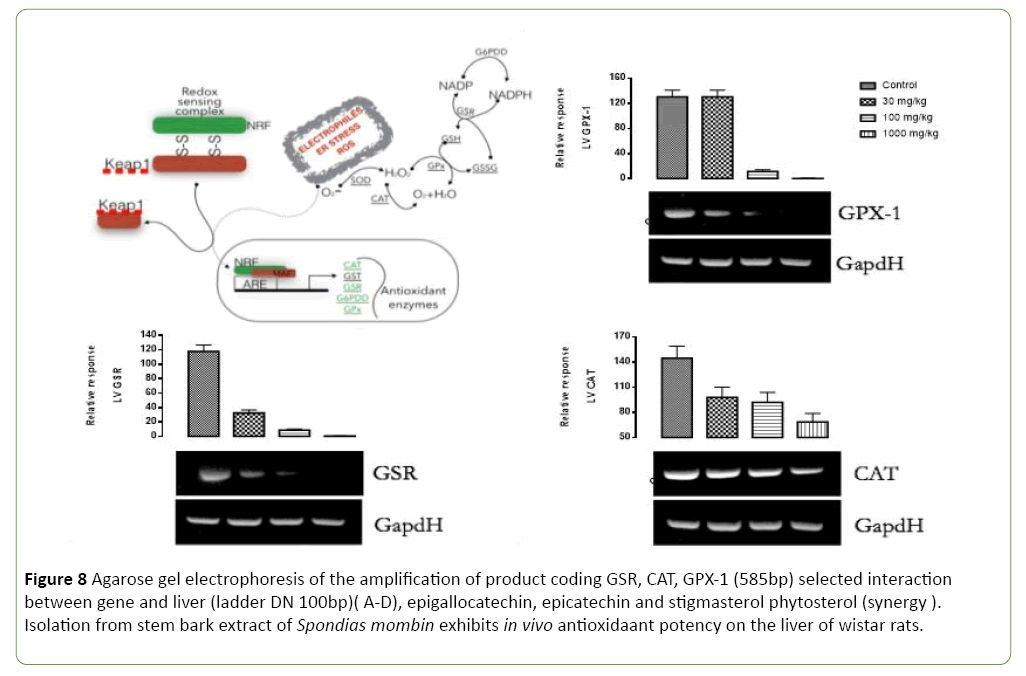
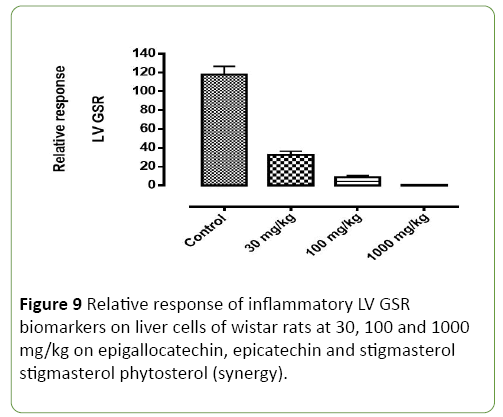
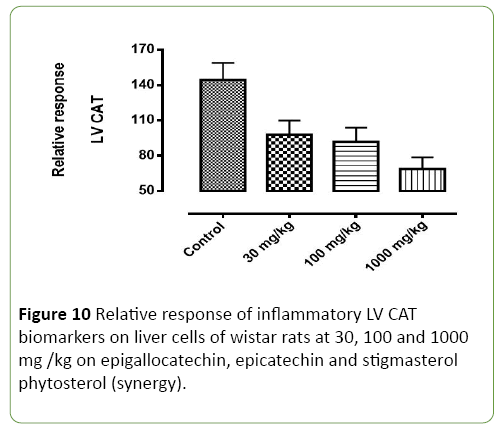
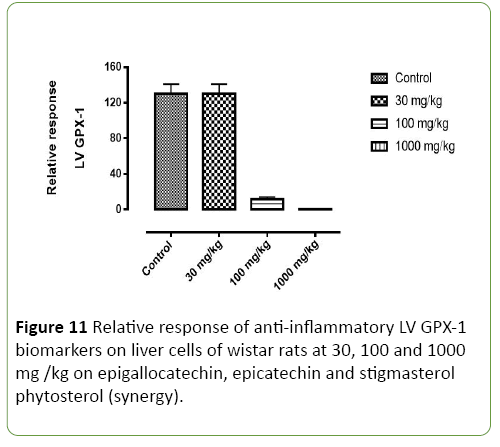
Figures 8-11 shows the relative response of epigallocatechin, epicatechin and stigmasterol phytosterol isolated from the Stem bark of S. mombin on the liver of Wister rat at 30, 100 and 1000mg/ml concentration. The Selected biomarkers used were GSR, GPX-1 and CAT. In Figures 9-11 it was observed that isolated epigallocatechin, epicatechin and stigmasterol phytosterol has antioxidant properties to reduce inflammation in the liver. The reduction effect was represented in the (Figure 9) (GPX and GSR) it can be seen at 1000 mg/ml. it can be deduced that the isolated compound has the ability to reduce the activity of free radical(ROS) generated by the kidney cell during metabolic activity.
Figure 8: Agarose gel electrophoresis of the amplification of product coding GSR, CAT, GPX-1 (585bp) selected interaction between gene and liver (ladder DN 100bp)( A-D), epigallocatechin, epicatechin and stigmasterol phytosterol (synergy ). Isolation from stem bark extract of Spondias Mombin exhibits in vivo antioxidaant potency on the liver of wistar rats.
Figure 9: Relative response of inflammatory LV GSR biomarkers on liver cells of wistar rats at 30, 100 and 1000 mg/kg on epigallocatechin, epicatechin and stigmasterol stigmasterol phytosterol (synergy).
Figure 10: Relative response of inflammatory LV CAT biomarkers on liver cells of wistar rats at 30, 100 and 1000 mg /kg on epigallocatechin, epicatechin and stigmasterol phytosterol (synergy).
Figure 11: Relative response of anti-inflammatory LV GPX-1 biomarkers on liver cells of wistar rats at 30, 100 and 1000 mg /kg on epigallocatechin, epicatechin and stigmasterol phytosterol (synergy).
Discussion
In this research work, the properties of epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) derived from ethyl acetate stem bark extract of Spondias Mombin on wistar rats using molecular method of analysis were discussed. It was observed that epigallocatechin, epicatechin and stigmasterol phytosterol derived from Spondias Mombin extract has a wide degree of anti-nephrotoxicity, antiinflammatory and antioxidant properties. The molecular assessment of the stem bark extract of Spondias Mombin revealed that the extract has anti-inflammatory property and little significant toxic effect on the kidney and liver of experimental rats. Epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) isolated from ethyl acetate stem bark extract of Spondias Mombin reduced the basal nephrotoxicity, with significant effect on selective biomarkers varying concentration per weight of the experimental rats. The biomarkers are (Km-1 GapdH, IL-1B and IL alpha, LV-GSR, LVCAT and LV-GPX-1, KD IL Alpha, KD IL –IB , KD KIM -1) on wistar rats at 30, 100 and 1000 mg/kg concentration per weight.
Figures 5-7 shows the epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) isolated from ethyl acetate stem bark extract of Spondias Mombin has reluctance effect the basal Nephrotoxicity biomarker on wistar rats at 30, 100 and 1000 mg/kg concentration per weight. In Figure 4, KD-1L alpha, it was observed that, epigallocatechin, epicatechin and phytosterol (synergy) has a significant effect on the gene coding for inflammation at this 1000 mg/kg concentration and little effect on the KD-1L alpha gene coding for nephrotoxicity at 30 mg/kg concentration, the larger effect was establish at high concentration. Epigallocatechin, epicatechin and phytosterol (Synergy) has a little affect at 100 mg and 1000 mg/kg concentration in the Figure 4 while KD IL Alpha, KD IL– IB, KD KIM-1 has significant rapid effect at 100 mg and 1000 mg/kg concentration. This establish the tremendous effect of Epigallocatechin, epicatechin and phytosterol (Synergy) on selected biomarkers, this show that the derived compound from Spondias Mombin is not toxic to the kidney of the experimental rat.
Figures 8-11 described epigallocatechin, epicatechin and stigmasterol phytosterol (Synergy), derived from Stem bark extract of Spondias Mombin which exhibits in vivo antioxidant potency on the liver of wistar rats. The selected biomarkers are GSR, CAT and GPX-1. Epigallocatechin, epicatechin and phytosterol (Synergy) has a deleterious effect on the biomarkers, which signifies the antioxidant potentials of the derived compound on the selected biomarkers. It has been shown that epigallocatechin, epicatechin and phytosterol (synergy) completely shut down the LV-GSR biomarker at 1000 mg/kg concentration compared to control experimental rat is unaffected. This is to say that the epigallocatechin, epicatechin and phytosterol (synergy) has no deleterious effect on the liver cell and it is also good antioxidant because of its effect on the selected biomarkers of reducing free radical in human system.
Epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) has the greater effect on the amplified gene which is responsible for the reduction of oxidative stress, nephrotoxicity and inflammation. Figure 3 demonstrates reduction in the basal nephrotoxicity effect on selected biomarkers which are KD IL Alpha, KD IL –IB, KD KIM -1, and also to demonstrate the anti-inflammation potency of the derived compound from Spondias Mombin extract which can be seen in Figure 3. The pathway of nephrotoxicity describe the PAMP and DAMPs as stated in Figure 3, TLR4 and ILI-R were amplified to cause cell injury, NFKappa8 was activated and trans-located, NF-Kappa 8 bind with PRO-IL-1B and KIM-I, and more NFKappa 8 were produced and activate the destruction of kidney cell. The epigallocatechin, epicatechin and phytosterol (Synergy) binds with the amplified gene and its pathway and completely reduce its harmful effect which is demonstrated in Figures 4-7.
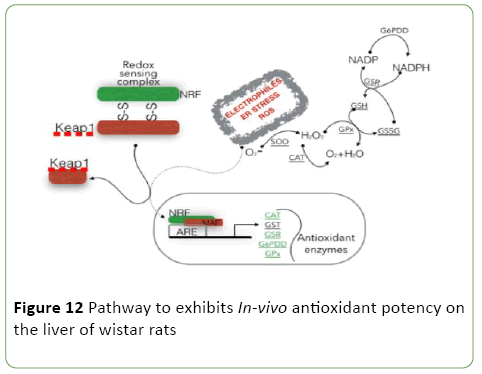
Figure 12, describes the antioxidant pathway of epigallocatechin, epicatechin and stigmasterol phytosterol (synergy). It was observed that isolated compound from Spondias Mombin extract binds with redox sensor complex (NRF) AND KEAP 1 in the liver cells of the wistar rat, to reduce the oxidative stress of electrophiles endoplasmic reticulum stress from ROS. There by making the derived compound from Spondias Mombin extract binding to the tri-carboxylic acid pathway in the liver cell. The epigallocatechin, epicatechin and phytosterol (synergy) shot down the oxidative amplified enzymes CAT- GST-GSR-G6POD- GPX (Figure 12).
Natural antioxidant is used for defense mechanisms in the human system, scavengers reactive species. They can be classified into different groups according to their properties. Endogeneous antioxidant which include glutathione, alpha lipoic, co-enzymes Q, ferritin, Uric acid, Bilirubin, Metallothiomecin, Carnitine, Melatonin enzyme, Superoxide dismutase (SOD), Catalase (CAT), Glutaperooxidase (GPXs), Thioredoxin (TRX), Peroxiredoxins (PKXs) (Figure 3). It should be noted that PRXs and GPXs are ubiquitous family of antioxidant enzymes (PRX I-V) (GPX I-V). They also control cytokine induced peroxide level and mediate signal transduction in mammalian cells. GPXs are unlisted as one of the biomarkers in the slope of this project work. Somigliana E et al. [24] reported that there are other important factors or fact in natural antioxidant that co-exist in a delicate balance with oxidative inputs.
Antioxidant inhibits free radicals chain oxidation reaction, resulting in oxidation of fatty acids, edible fats. This efficiency as scavengers of oxygen free radicals in the cell and tissues is negligible as compound with natural antioxidant enzymes. Antioxidant prevent oxidation of other chemical, they protect the key cell component by neutralizing the damaging effect of free radical (Figure 12) which are natural byproduct of cell metabolism [25-27]. Halliwell B reported the second event that occur in the cell, they reported that oxidative stress (OS) induced by reactive oxygen (ROS) can be described as a dynamic imbalance in the body and the level of antioxidant to scavenge them and to protect the body against their deleterious activity [28]. Both the event occur instantaneously and one serves as the watch dog for the other but in other to reduce such naturally occurring effect in the body, compound like epigallocatechin, epicatechin and phytosterol (synergy) are needed in the human system which can be seen in the Spondias Mombin tree in abundant quantity. The importance of the isolated compound (Epigallocatechin, epicatechin and phytosterol (synergy) has play a very important role in the antioxidant imbalance, which has been demonstrated in Figures 3 and 12. LV-GSR, LV-CAT, LV GPX-1 where the compound completely shut down the biomarkers gene during the bicistronic event in the cells of the test animal. Epigallocatechin, epicatechin and phytosterol (synergy) is not toxic as demonstrated in Figure 3. KD IL alpha, KD IL-IB and KD KIM-1 where the expressed gene reduces as the concentration increases from 30 mg/ml to 1000 mg/kg concentration.
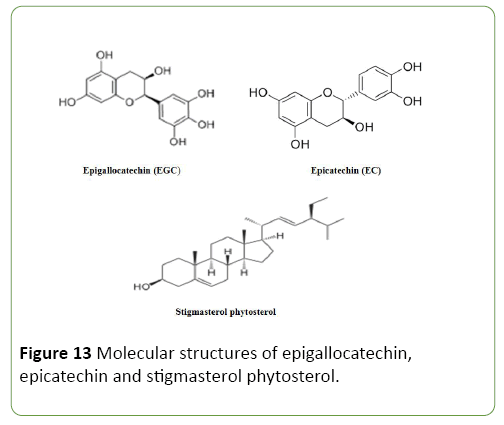
In this context, isolated compound epigallocatechin, epicatechin and phytosterol (synergy) (Figure 13) has proved to be a potentially active antioxidant and anti-inflammatory elixir which can serve as a good protector that can extend the human life span when added to its food or drinking water in regular bases, reported the deleterious mechanism of the second event in the cell. Free radicals are formed when oxygen is metabolized or formed in the body, chemical species which possess an unpaired electron on the outer (valence) shell of the molecules. The excessive amount of ROS may be harmful because they can initiate biomolecular oxidation which lead to cell injury and death, this create oxidative stress which results to numerous diseases and disorder such as aging, cancer, therosclerosis cirrhosis, cataracts and even death [29,30] describe the mechanism of this deleterious event in the cell as seen in Figures 8 and 9. It was observed that free radicals are highly reactive and can react with proteins, lipid, carbohydrates and DNA, this free radical attack the nearest stable molecules, stealing its electron when the attacked molecules losses its electron, it become a free radical itself beginning a chain reaction finally resulting in the destruction of a living cell.
Epicatechin have been proven to have diverse benefits to human health. It reduces the risks of diabetes mellitus and cardiovascular diseases this shows the basic important aspect of Spondias Mombin tree, which have pharmalogical effects such as anti hyporlipidemic, anti-inflammatory, antioxidative effect, anticarcinogenic and cytoprotective [6].
Epicatechin decreases the susceptibility of low density lipoprotein to oxidation which prevents the initiation of artherosclerosis, HIV protein (Tat) and gp120 is known to cause neurotoxicity in human via mechanisms that activate macrophages and glial cells and finally, oxidative stress is demonstrated in Figures 6, 7 and 12. To suggest that epicatechin are neuro-protective by blocking the neurotoxic effects of the HIV protein which cause oxidative stress [31]. However, it should be mentioned that Catechins have an antiproliferative effect on tumor cells as well as inhibiting metastasis and it ability to modulate antioxidant enzymes in vivo. It has been shown to increase the activity of super oxide dismutase and catalase. It also suppresses lipid peroxidation of tumor cells [32]. It must be clearly stated that stigmasterol phytosterol have been shown to lower/reduce blood cholesterol and this lowering may reduce the risk of coronary heart disease. Phytosterols are under preliminary research for their potential to inhibit lung, stomach, ovarian and breast cancers as well as colon and prostate cancers. The synergestic approach in the use of the three isolated compound in the reduction of antioxidant and anti-inflammatory activity in human system should never be over emphasis, which should be encouraged.
Plant supplement diet should be encouraged because it is from this supplement that man can have a full dose such a compound (epigallocatechin, epicatechin and stigmasterol phytosterol (synergy) which is describe during the course of this research work, it should be reiterated that discouraging populace from Natural food and herbal supplements nutritionally antioxidant deficiency will resurfaces and it will lead to oxidative stress. Lamai RS [32] reported that lipid peroxidation is an oxidative deterioration of polyunsaturated lipids and it involves ROS and transitional metals ions. Food in diet lacking a minimum quantity of antioxidant will lead to cell injury leading to the yielding of a wide range of cytotoxic effects, most of which are aldehyde and hydroxynormal [31].
Conclusion
Epigallocatechin, epicatechin and stigmasterol phytosterol (Synergy) isolated from Stem bark extract of Spondias Mombin is an importance compound that should be encouraged and used in drug prospecting, it also shows the efficacy of the isolated compound, its importance and mechanism of action. The compound isolated from stem bark of Spondias mombim extract has proven itself to be natures extraordinary nephrotoxicity, anti-Inflammatory, antioxidant agent and it has also been proved during the course of this project work that Spondias Mombin is a potent medicinal plant that its uses and application should be encouraged. More time and attention should be spent on developing it as a sustainable drug for prophylaxis and treatment of complications and diseases.
References
- Song JM, Lee KH, Seong BL (2005) Antiviral effect of catechins in green tea on influenza virus. Antivir Res 68: 66-74.
- Fester K, Kutchan TM (2009) Introduction to the different classes of natural products. In: Osbourn A,Lanzotti V (eds.). Plant-derived natural products: Synthesis, function and application. Springer, Germany.
- Nath S, Bachani M, Harshavardhana D, Steiner JP (2012) Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol 18: 445-455.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, et al. (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47: 3954-3962.
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, et al. ( 2003)Epigallocatechingallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy ClinImmunol 112: 951-957.
- Lin YT, Wu YH, Tseng CK, Lin CK, Chen WC, et al. (2013) Green tea phenolic epicatechins inhibit Hepatitis C virus replication via cyclooxygenase-2 and attenuate virus-induced inflammation. PLoS One 8: 54466.
- Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, et al. (2014) Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One 9: 87161.
- Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ (2011) Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechingallate in human corneal epithelial cells. Mol Vis 17: 533-542
- Yamamoto MM, Tachibana H ( 2012) Anti-allergic action of O- methylated EGCG in green tea cultivar benifuuki. J Food Drug Anal 20: 313-317.
- Sharangi A (2009) Medical and therapeutic potentialities of tea (Camellia sinensis L.)–A review. Food Res Int 42: 529-535.
- Kim HS, Quon MJ, Kim JA (2014) New insights into the mechanisms of polyphenols beyond antioxidant properties:Lessons from the green tea polyphenol, epigallocatechin 3- gallate. Redox Biol2: 187-195.
- WesterterpPlantenga MS (2010) Green tea catechins, caffeine and body-weight regulation. PhysiolBehav 100: 42-46.
- Babu PV, Liu D, Gilbert ER ( 2013) Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem 24: 1777-1789.
- Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, et al. (1993) Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res 21: 289-299.
- Lin-Chao S, Bremer H (1986) Effect of the bacterial growth rate on replication control of plasmid pBR322 in Escherichia coli. Mol Gener Geneti 203: 143-149.
- Jahn CE, Charkowski AO, Willis DK (2008) Evaluation of isolation methods and RNA integrity for bacterial RNA quantitation. J Microbiol Meth 75: 318-324.
- Butterfield DA, Hardas SS, Lange MB (2010) Oxidative modified glyceraldehyde-3-phos-phate dehydrogenase (GAPDH) and Alzheimer’s disease: Many pathways to neurodegeneration. J Alzheimers Dis 20: 369-393.
- Anders MW (2005) Formation and toxicity of anesthetic degradation products. Annu Rev Pharmacol Toxicol 45: 147-176.
- Miller SCM, LiPuma JJ, Parke JL (2011) Culture-based and non-growth-dependent detection of the Burkholderiacepacia in soil environments. App Environ Microbiol 68: 3750-3758.
- Heuk SH, Schlums I, SérézalGE, Martini SC, Chiang N, et al. (2017) CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immun 46: 287-300.
- Walley JW, Coughlan S, Hudson ME, Covington MF, Kaspi R, et al. (2007) Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PLoS Genet 3: 172.
- Pinho C, Rocha S, Carvalho BM, Lopes S, Mourão S, et al. (2010) New primers for the amplification and sequencing of nuclear loci in a taxonomically wide set of reptiles and amphibians. Cons Genet Res 2: 181-185.
- Judy B, Haan D, Stefanovic N,Nikolic-Paterson D, Lyndee L, et al. (2005) Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet Amer J RenPhysiol 289: 544-551.
- Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, et al. (2005) Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. GyneOncol101: 331-341.
- Yoshida T, Oka S, Masutani H, Nakamura H, Yodoi J (2003) The role of thioredoxin in the aging process: involvement of oxidative stress. Antioxi Redox Signal 5: 563-570.
- Koltover VK (2010) Antioxidant biomedicine: From free radical chemistry to systems biology mechanisms. RussiChem Bull 59: 37-42.
- Shirwaikar A, Kuppusamy R, Isaac SR (2006) In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull 29: 1906-1910.
- Halliwell B (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies. Arch Biochem Biophy 476: 107-112.
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, et al. (2003) Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity [ORACFL]) of plasma and other biological and food samples. J Agric Food Chem 51: 3273-3279.
- Nath K, Saini S, Sharma YK (2005) Chromium in tannery industry effluent and its effect on plant metabolism and growth. J Environ Biol 26: 197-204.
- Tsuchiya H, Tanaka T, Nagayama M (2008) Antiproliferative effects associated with membrane lipid interaction of green tea catechins. J Health Sci 54: 576-580.
- Lamai RS (2009) Preliminary phytochemicalandantibacterialscreening oftheethanolicstem bark extract of Phyllanthusmuellerianus. Project submitted to the Department of Pharmacognosy and Drug Development. Ahmadu Bello University Zaria, Nigera.pp:34-35.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences