Animal Venom Derived Toxins are Novel Analgesics for Treatment of Arthritis
Upadhyay RK*
Department of Zoology, DDU Gorakhpur University, Gorakhpur, UP, India
- *Corresponding Author:
- Ravi Kant Upadhyay
Department of Zoology
DDU Gorakhpur University
Gorakhpur, UttarPradesh, India
Tel: 9838448495
E-mail: rkupadhya@yahoo.com
Received Date: February 04, 2018; Accepted Date: March 12, 2018; Published Date: March 19, 2018
Citation: Upadhyay RK (2018) Animal Venom Derived Toxins are Novel Analgesics for Treatment of Arthritis. J Mol Sci. 2:6.
Abstract
Present review article explains use of animal venom derived toxins as analgesics of the treatment of chronic pain and inflammation occurs in arthritis. It is a progressive degenerative joint disease that put major impact on joint function and quality of life. Patients face prolonged inappropriate inflammatory responses and bone erosion. Longer persistent chronic pain is a complex and debilitating condition associated with a large personal, mental, physical and socioeconomic burden. However, for mitigation of inflammation and sever pain in joints synthetic analgesics are used to provide quick relief from pain but they impose many long term side effects. Venom toxins showed high affinity to voltage gated channels, and pain receptors. These are strong inhibitors of ion channels which enable them as potential therapeutic agents for the treatment of pain. Present article emphasizes development of a new class of analgesic agents in form of venom derived toxins for the treatment of arthritis.
Keywords
Analgesics; Venom toxins; Ion channels; Channel inhibitors; Pain; Inflammation
Introduction
Arthritis is chronic inflammatory disease in which patient sense severe localized and constant joint pain, aching, stiffness and swelling. The pain increases with the daily wear and tear of joint, muscle strains caused by forceful movements against stiff painful joints and fatigue. More than 200 rheumatic diseases or severe disorders are known [1,2]. Most of them are related to tissues, joints and other connective tissues [3,4]. The most common form of arthritis is osteoarthritis while its other common rheumatic conditions are gout, fibromygalia and rheumatoid arthritis [4]. Osteoarthritis (OA) is a progressive degenerative joint disease that has a major impact on joints function and body movement that affect quality of life. Rheumatoid arthritis is another form of arthritis which is characterized by prolonged inappropriate inflammation, and pain in skeletal-muscular joint [5]. Chronic pain, accompanies inflammation and joint deformation (RA) [6]. Bone erosion is a central feature of rheumatoid arthritis. It begins in the joints with the inflammation of the synovium. Rheumatoid arthritis affect people of all ages and disease may occur at any age, it usually begins after age of 40. This is most common form of arthritis [7] that affects both of the largest and the smaller joints of the body, including the hands, wrists, feet, back, hip, and knee. This disease typically affects the weight-bearing joints, such as the back, spine, and pelvis. Chronic pain is a complex and debilitating condition associated with a large personal and socioeconomic burden.
Arthritis patients also show other co-morbidities such as heart diseases, chronic respiratory diseases, diabetes, and stroke. High blood pressure, physical laxity or inactivity, high-cholesterol, obesity and smoking are important risk factors. Gout is also pain related disease that is caused by deposition of uric acid crystals in the joint with severe inflammation. In the early stages, the gouty arthritis usually occurs in one joint, but with time, it spreads in many joints and patient become quite crippling. Gouty arthritis becomes highly painful and potentially debilitating in absence of proper treatment [8]. The joints in gout often become swollen due to deposition in uric acid in joints and muscles. At this stage, for decreasing the production of uric acid few drugs such as allopurinol, febuxostat are provided to increase uric acid elimination from the body through the kidneys (e.g., probenecid), which is referred as refractory chronic gout or RCG [9]. Zicotinide is a peptide drug and has been approved for the treatment of severe chronic pain in patients only when administered by intrathecal route. Peptide toxins from venomous animals are natural resources with diverse biological functions and possess multiple therapeutic potential against many diseases. Present review article emphasizes use of animal toxins for pain control and examines the possible analgesic mechanisms of these molecules.
Pathophysiology
Pain is a multidimensional sensory experience, and involves multiple mechanisms especially in generation of pathophysiological nociceptive pain. Agents that decrease pains are referred to as analgesics, pain relieving agents also are called antinociceptives. A number of classes of drugs are used to relieve pain. NSAID are useful for mild to moderate pain. Local anesthetics inhibit pain transmission by inhibiting ion of voltage-regulated sodium channels. These agents often are highly toxic when used in concentrations sufficient to relive chronic or acute pain in ambulatory patients. Sever acute or chronic pain generally is treated most effectively with narcotic analgesic. So morphine and its derivatives are unique among all other analgesics in reducing pain.
Arthritis is caused in part by the production of pro-inflammatory cytokines and receptor activator of nuclear factor kappa B ligand (RANKL), a cell surface protein present in Th17 cells and osteoblasts [10]. More than 80% of rheumatoid arthritis patients contain rheumatoid factor that is an antibody circulates in the blood. Bone continuously undergoes desorption, become hollow and fragile. Bone resorbing osteoclasts activity is increased by osteoblasts through the RANK/RANKL mechanism [11,7]. (Arthritis Drugs) This adaptive immune response is initiated in part by CD4+ T helper (Th) cells, specifically Th17 cells [7,10] Th17 cells are present in higher quantities at the site of bone destruction in joints and produce inflammatory cytokines associated with inflammation, such as interleukin-17 (IL-17) [11]. Due to production of inflammatory cytokines, local activation of NF-kappaB and the subsequent expression of NF-kappaB-regulated genes mediate joint inflammation and destruction.
Reasons
Due to changing lifestyle and mobility pressure, there is a sharp increase in number of arthritis cases. By year 2030, more than 25% of world population will be affected by any form of arthritis. Age, gender and certain genes are among non modifiable factors which are responsible for arthritis. Age is important risk factor as the risk of developing most types of arthritis increases with age. Arthritis is mostly seen much more in middle age women who feel severe pain in joints like finger joints, wrists, knees and elbows. Overweight and obesity are among some modifiable risk factors. Excess of weight is risky for progression of knee osteoarthritis; it is also responsible for joint injuries. After severity of pain synovial fluid get microbial infection that increases joint pain and inflammation that potentially cause the development of various arthritis related disorders. This is another severe form of arthritis that starts with sudden onset of chills, fever and joint pain. The condition is caused by bacteria elsewhere in the body. Infectious arthritis must be rapidly diagnosed and treated promptly to prevent irreversible joint damage [7].
Majority of adult population is reported to have a form of arthritis i.e. rheumatoid arthritis, gout, lupus or fibromyalgia. The main risk factors for osteoarthritis include prior joint trauma, obesity, and a sedentary lifestyle. It also occurs as a result of injury. Unlike the wear-and-tear damage of osteoarthritis, rheumatoid arthritis affects the lining of your joints, causing a painful swelling that can eventually result in bone erosion and joint deformity. In Rheumatoid arthritis body's own immune system starts to attack body tissues and severe damage occurs to the joint lining and cartilage which eventually results in erosion of two opposing bones. In beginning disease remains undifferentiated and does not detected but as soon as pain increases it appears [12]. Certain rheumatic conditions also involve the immune system and various internal organs of the body [13]. This disease is symmetrical and appears on both sides of the body. It can lead to severe deformity if no treatment is done. In children, the disorder appears due to genetic reasons and prevail skin rash, fever, pain and disability in limbs and knee joints that limits the daily activities. But there is no cure available for rheumatoid arthritis. Only slow and continuous exercise provides relief in joint pain.
Therapeutics
In present time clinicians use various therapeutic methods for management of severe pain of joints, and mitigation of inflammation. For relieving neuropathic pain synthetic analgesics are used to provide quick relief. These drugs act in various ways on the peripheral and central nervous systems and temporarily decrease the pain sensation, sometimes found less effective. Common analgesics which are provided to relieve pain and inflammation are paracetamol and non-steroidal anti-inflammatory drugs such as salicylates and few opioid drugs such as morphine and oxycodone. Opioid analgesics agonize opioid receptors μ Ò¡ δ which are G-protein coupled receptors. This lead to a series of events which ultimately block neuronal pain transmission by inhibition of activation of voltage gated Ca+2 channels which depresses NT release. Opioid analgesics increase K+ conductance outside the membrane and cause hype polarization of cell, reducing its excitability, and do inhibition of adenyl cylase. All these analgesics are either anti-depressant or anticonvulsant agents which damage neurons and generate intense side effects on liver, kidney, vascular tissues and nerve function. These exhibit limited efficacy in many patients and impose dose-limiting side effects that hinder their clinical use. There is a need to identify mechanisms and molecular components responsible for pain, its control. There must be quenching search of new targets and designing novel analgesic drugs by using animal venom derived toxins [14].
Venom-derived toxins as analgesics
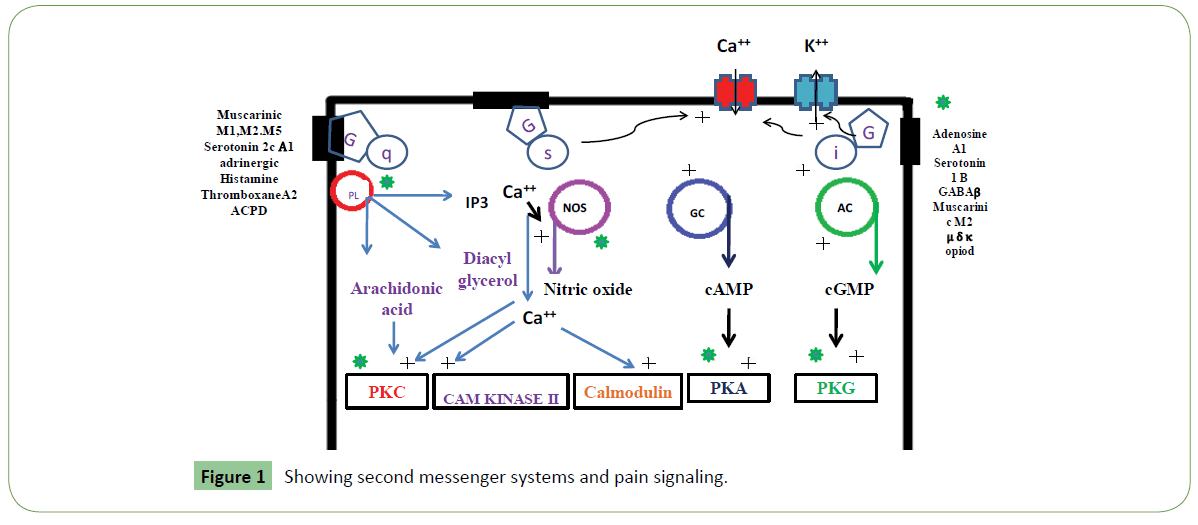
Venom-derived toxins from various venomous species are mixtures of different bio-molecules including peptides. Some venom- toxins bind with pain sensitive receptors, ion channels and potentially block essential components of the pain signaling system (Table 1 and Figure 1). Because different animal groups possess varied toxin structure that binds like a different category of channel inhibitor. These show much therapeutic potential and provide relive from chronic pain and inflammation [15]. Animal toxins behave as analgesics and show recovery in induced arthritis and save from potentially devastating effects of inflammation. Hence, these can eventually use as a new class of anti-inflammatory agents. These could also use to probe mechanisms underlying pain signaling, channelopathies and receptor expression [16]. From literature and clinical investigations it is much clear that animal venom derived toxins display better analgesics potency and fewer side effects than existing therapeutic drugs. Because of high selectivity and specificity of animal toxins to pain receptors, and they act as potential channel blockers these could be used as potential candidates for the development of new analgesic drugs [14].
| Toxin | Animal species | Action on ion channels |
|---|---|---|
| Heterodotxoins | Heterodera venatoria brown huntsman spider or laya) and | Blocks potassium channels |
| Saxitoxin | Dinoflagellates | It blocks voltage-dependent sodium channels. |
| Conotoxin | Cone snails | Paralyze and hunt prey |
| Iberiotoxin | Buthus tamulus Eastern Indian scorpion | blocks potassium channels. |
| Dendrotoxin | Mamba snake | blocks potassium channels |
| Tetrodotoxin | Puffer fish | It blocks sodium channels |
| Polaymine toxins | wasps | open-channels blockers of ionotropic glutamate (iGlu) receptors. voltage dependent channels blockers of calcium ions permeable AMPARs |
| Philanthotoxin | semi-irreversible blocker of ion-channels | |
| Pompilidotoxins | solitary wasps Anoplius samariensis (alpha-PMTX) and Batozonellus maculifrons (beta-PMTX) | voltage-gated sodium channel isoform-specific effects, facilitate synaptic transmission in the lobster neuromuscular junction, slowly did sodium channel inactivation. PMTXs |
| ω-conotoxin | MVIIA (ziconotide) blocks the voltage-gated calcium 2.2 (CaV 2.2) channels | Mediates the release of neurotransmitters and Proinflammatory mediators from peripheral nociceptor nerve terminals |
| conotoxin | voltage-gated calcium channels. | Bind to α9α10-nAChRs receptor |
| Huwentoxin-IV | (HwTx-IV) 1 voltage-gated sodium channel | Potent antagonist of hNav1.7. Nav1.7 is a involved in the generation and conduction of neuropathic and nociceptive pain signals |
Table 1: Some common animal toxins as ion channel blockers.
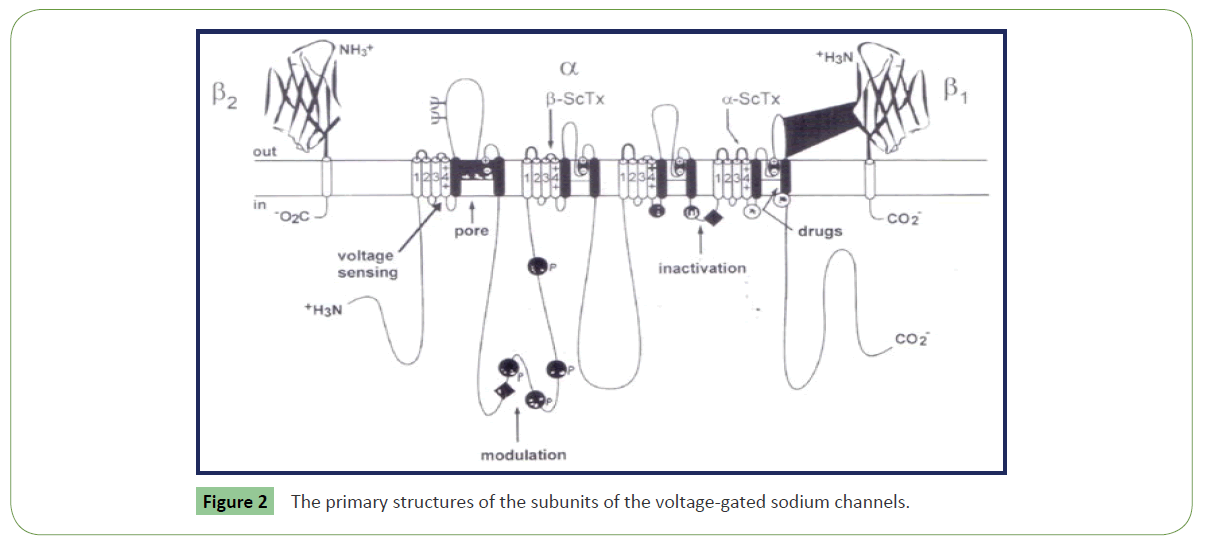
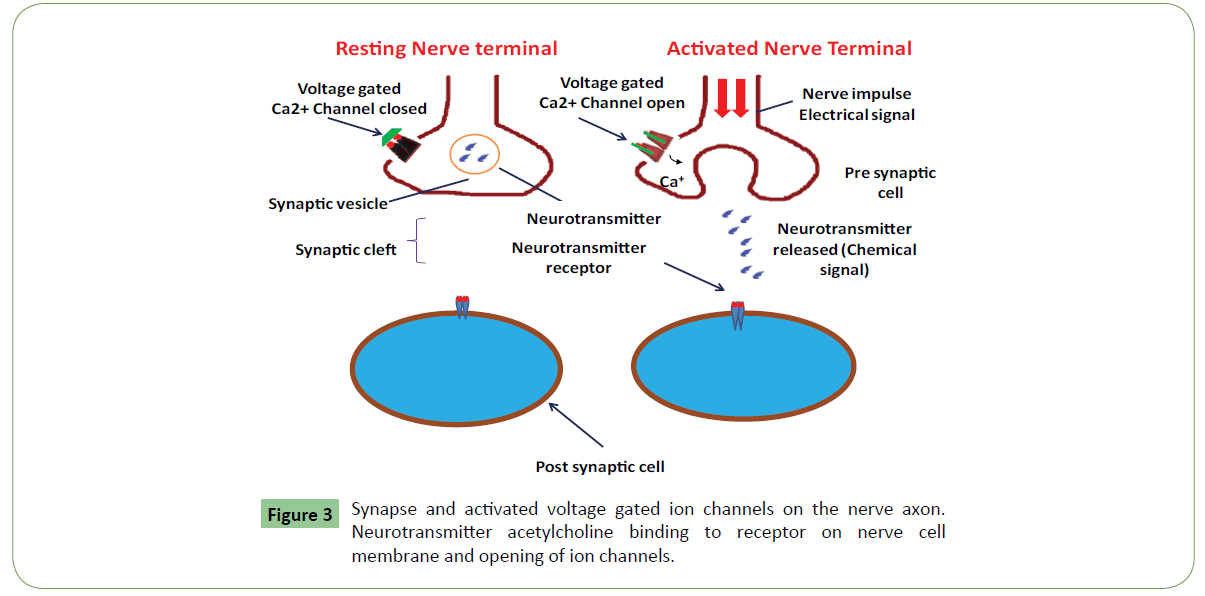
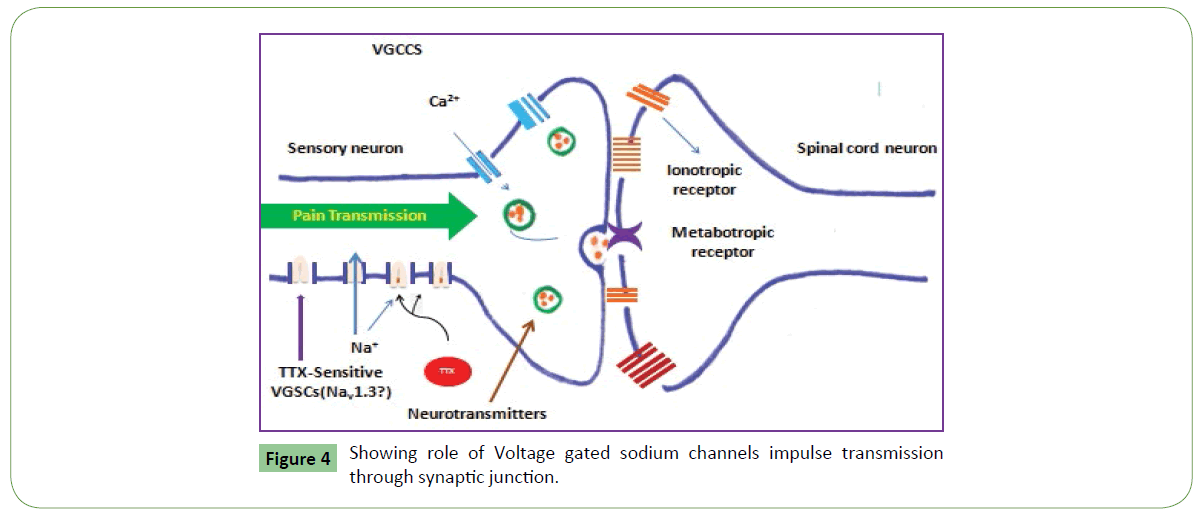
Clinical and experimental data cleared that voltage-gated sodium channels (VGSCs) play a key role in the pathogenesis of neuropathic pain [17,18] (Figure 2). Animal venoms are important source of potent and selective peptide molecules. These toxins from animal venom selectively target Nav1.3, 1.7, 1.8, and 1.9, and act as potential analgesic candidates [14,19]. These strongly interact with GPCRs and can be used in treatment of pain. Venom toxins selectively target voltage-gated and ligand-gated ion channels to inhibit neuronal excitability and blunt synaptic transmission of pain signals (Table 1 and Figure 3). These also bind to G-protein coupled receptors (GPCRs) expressed on the cell surface and act to transduce extracellular signals and regulate physiological processes related to pain [17,18]. They act as channel inhibitors of pain sensing neurons and high affinity to voltage gated pores on membrane surface. These voltage-gated sodium channels (NaV channels) are essential for the initiation and propagation of action potentials that critically influence person’s ability to respond to a diverse range of stimuli [18]. These also regulate neuronal excitability by governing action potential (AP) generation and propagation. These Abnormal functions of NaV channels generate many human disorders, including chronic neuropathic pain. Both functional properties and expression pattern of NaV channel subtypes will help to uncover their specific roles in acute and chronic pain. These could be targeted for revealing pain by using selective inhibitors. Chloride channels are gated by the inhibitory GABA receptor. GABA receptor mediates the effects of gamma-amino butyric acid (GABA), the major inhibitory neurotransmitter in the brain. GABA receptor found throughout the CNS. Most abundant fast inhibitory ligand-gated ion channel found in the mammalian brain located in the post synaptic membrane (Figure 4). Venom toxins can be used for development of analgesic therapeutic agents [18].
Peptide toxins (biotoxins) from venomous animals are used in pain treatments. These biotoxins produce analgesia via GPCR modulation [19]. Ion channels are narrow, water-filled tunnels which are located within the membrane of all cells and also found in many intracellular organelles. These allow only selected ions of a certain size and/or charge to pass into and out of cells and show selective permeability. These are pore forming integral membrane proteins which establish a resting membrane potential, assist in shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane. These are "multi-subunit" assemblies which regulate the flow of ions across membranes, and cell volume. Calcium, sodium and potassium ions control essential functions inside cell. Sodium and potassium ions are used to maintain nerve function calcium ions assists to regulate the contraction of muscle cells.
There are various categories of ion channels. Most of the voltage-gated ion channels, contain pore-forming subunit(s) α subunit, and β, γ auxiliary subunits. These channel pores are selective for specific species of ion, such as sodium or potassium. Gated ion channels open and close because of some sort of stimulus, when they open, they change the permeability of the cell membrane. However, some channels are permeable to the passage of more than one type of ion, typically sharing a common charge: positive (cations) or negative (anions). Ions often move through the segments of the channel pore in single file nearly as quickly as the ions move through free solution. First category of channels is voltage-gated sodium which open and close in response to membrane potential. There are nine members of this channel family which are responsible for action potential initiation and propagation in excitable cells, including nerve impulse, muscle, and neuroendocrine cells. These are also expressed at low levels in non-excitable cells. Upon inhibition of sodium channel transmission of nerve impulses in neurons does not take place that results in transient paralysis and display loss of nerve function. The pore-forming α subunits are very large and contain up to 4,000 amino acids. These consist of four homologous repeat domains (I-IV) each comprising six transmembrane segments (S1- S6) for a total of 24 trans-membrane segments. The members of this family also co-assemble with auxiliary β subunits, each spanning the membrane once. Both α and β subunits are extensively glycosylated. Tetrodotoxin is a sodium channel blockers. Sodium channels are founding members of ion channel super family. Inhibition of sodium channels stops transmission of nerve impulses in neurons and it impose paralysis due to loss of nerve function.
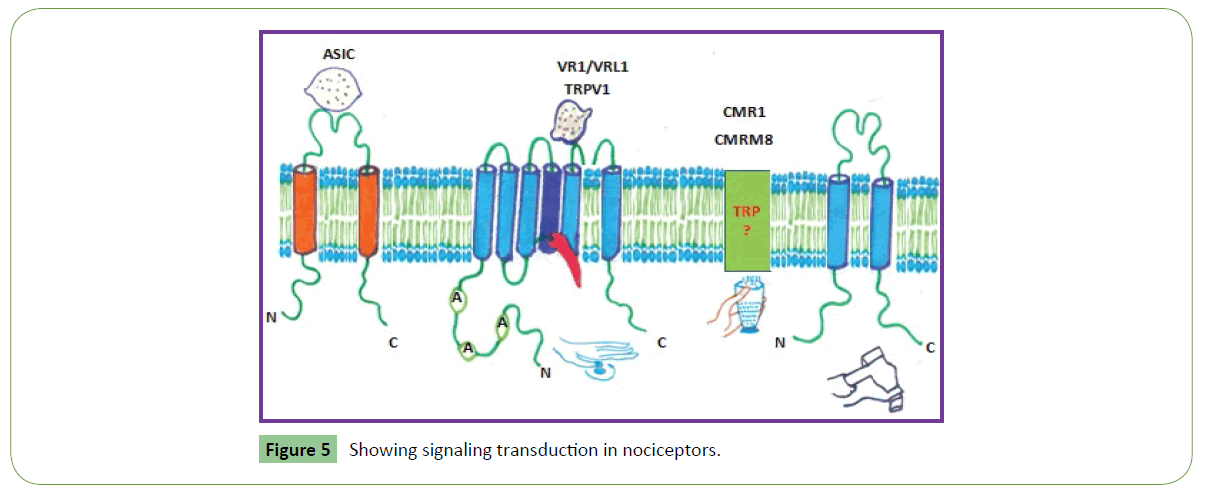
Second category of channels is voltage-gated calcium channels which have 10 members. These play important role in both linking muscle excitation with contraction as well as neuronal excitation with transmitter release. The α subunits have an overall structural resemblance to those of the sodium channels and are equally large. More specifically, voltage-gated calcium channel 2.2 (Cav2) does modulation of mechano- and thermosensitive spinal neuronal responses in osteoarthritis [20]. It also plays an important role in spinal nociceptive transmission; thereby blocking Cav2.2 activity represents an attractive opportunity for OA pain treatment. Similarly, ω-conotoxin MVIIA (ziconotide) specifically blocks the voltage-gated calcium 2.2 (CaV 2.2) channels, which mediates the release of neurotransmitters and proinflammatory mediators from peripheral nociceptor nerve terminals. It is successfully used in humans to alleviate long persistent pain, because it blocks CaV 2.2. It also suppresses peripheral arthritic pain by mediating calcium influx and signaling in nociceptor sensory neurons [20]. Thus, Nav subtypes (Nav1.3, 1.7, 1.8, and 1.9) assist in nociceptive transduction, and are attractive targets for analgesic drug discovery [14] (Table 2 and Figure 5). Both voltage-gated calcium channels (VGCC) and TRPV1 receptors play important role in pain transmission and modulation (Figure 5). Interaction between Phα1β toxin (a VGCC blocker) and SB366791 (selective TRPV1 antagonist) shows analgesic effect in acute pain induced by capsaicin. Phα1β toxin potentiates in 15 fold the antinociceptive action of the TRPV1 blocker SB366791 [21].
| Class | Channel | Pathophysiology |
|---|---|---|
| Voltage-gated sodium | Nav1.1,1.2,1.3 Nav1.7 |
Migraine, upregulated in nerve injury, neuropathic pain, Erythromelagia, Chronic inflammatory, acute and neuropathic pain, Paraoxysmal extreme pain, congenital indifference to pain, acute and neuropathic pain |
| Voltage gated potassium | Kv1.3,Kv1.4, Kv4.2,KChIP2.2 KCNQ2,KCNQ3 KCNQ2/KCNQ4 |
Neuropathic pain |
| Voltage gated calcium | Cav2,1/β4/α2&1 Cav2.2/ β3/ α2δ1 Cav 32. |
Familial hemiplegic, migraine, inflammation pain, inflammatory, neuropathic pain Neuropathic pain, cold allodynia, chemical irritation |
| Hyper-polarization gated | HCN1 | Neuropathic pain |
| Calcium activated potassium | BK (kCa1.1) 1K(Kca3.1) |
Morphine induced hyper algesia, neuropathic pain |
| Transient receptor potential | TRPA1 TRPV/TRPV4 TRPC4 TRPM4,TRPM8 |
Impaired nociception, hereditary episodic pain, inflammatory pain, chemical irritation Thermal hyperalagesia, inflammatory, neuropathic pain Visceral pain Cold allodynia, inflammatory neuropathic pain |
| P2X receptors | P2X1,X2,X3,X4,X7 | Nociception, inflammatory pain |
| Ligand gated | NMDA receptors GABAA, a3by2 nAChRa7 |
Neurodegenerative disease, migraine, neuropathic pain Inflammatory pain |
| Acid sensing ion channel | ASIC1a,2a,3 | Inflammatory pain |
| G-protein coupled receptors | GPCRs) expressed on the cell surface | Transduce extracellular signals and regulate physiological processes related to pain |
| voltage-gated sodium channels | NaV channels, Nav1.3, 1.7, 1.8, and 1.9 | Nociceptive transduction, nitiation and propagation of action potentials |
| voltage-gated calcium channel 2.2 | (CaV2) | Modulation of mechano- and thermosensitive spinal neuronal responses, nociceptive transmission; blocking Cav2.2 activity, pain treatment. |
| spider Ceratogyrus darling | Cd1a Cav2.2 | Inhibits two anti-nociceptive targets Inactivate gating, associated with the Cav2.2 α-subunit pore, and altering the activation of Nav1.7. |
| Cav2 antagonist | TROX-1 | Analgesic effect primarily via Cav2.2 blockade. |
| short cationic peptides, kinins and polyamines | Complex mixture | Slow down pain, inflammation in neurodegenerative diseases |
| Antitumor-analgesic peptide | AGAP | Analgesic activity |
| Gated channels | PLA2s peptide | hHdrolytic activity, nociceptive and antinociceptive actions |
| Gated channels | AMP-activated protein kinase activators | Preventing sodium (Na+) channel phosphorylation, decrease sensory neuron excitability |
Table 2: Ion channels classes involved in arthritic pain and inflammation.
Voltage-gated potassium channels (KV) are known mainly for their role in repolarizing the cell membrane following action potentials. There are 40 members of this family which is further divided into 12 subfamilies. Large α subunits have six transmembrane segments, homologous to a single domain of the sodium channels. Correspondingly, they assemble as tetramers to produce a functioning channel. Another category of voltage-gated proton channels open with depolarization, but in a strongly pH-sensitive manner. These channels open only when the electrochemical gradient is outward, such that their opening will only allow protons to leave cells. These assist in acid extrusion from cells. These also assist during respiratory burst managed by phagocytes e.g. eosinophils, neutrophils and macrophages.
Also known as ionotropic receptors, this group of channels open in response to specific ligand molecules binding to the extracellular domain of the receptor protein. Ligand binding causes a conformational change in the structure of the channel protein that ultimately leads to the opening of the channel gate and subsequent ion flux across the plasma membrane. Examples of such channels include the cation-permeable "nicotinic" Acetylcholine receptor, ionotropic glutamate-gated receptors, acid sensing ion channels (ASICs), ATP-gated P2X receptors, and the anion-permeable γ -aminobutyric acid-gated GABAA receptor [22]. Ion channels are activated by second messengers which are categorized in on the basis of ligand attached. Gating also includes activation and inactivation by second messengers from the inside of the cell membrane ions rather than from outside the cell, as in the case for ligands.
Spider venom
Spider venoms are rich sources of peptidergic ion channel modulators which bear high therapeutic potential. The spider venom peptide Huwentoxin-IV (HwTx-IV) 1 is a potent antagonist of hNav1.7. Nav1.7 is a voltage-gated sodium channel involved in the generation and conduction of neuropathic and nociceptive pain signals [23]. Spider venoms toxins are modulators of ion channels involved in pain transmission. Cd1a, a novel peptide from the venom of the spider Ceratogyrus darlingi inhibits two anti-nociceptive targets. It interferes with Cav2.2 inactivate gating, associated with the Cav2.2 α-subunit pore, and altering the activation gating of Nav1.7. Cd1a remains inactive at some of the Nav and Cav channels expressed in skeletal and cardiac muscles and nodes of Ranvier. It has no side effects and an efficacious low dose provides relief from peripheral pain [24]. Engineered spider peptide toxin HwTx-IV binds to lipid membranes and shows an increased inhibitory potency at human voltage-gated sodium channel sub type hNaV1.7. [25]. Similarly, HwTx-IV analogue shows an increase in ability to bind to lipid membranes that would improve its inhibitory potency at hNaV1.7. [25]. TROX-1 acts as a state-selective Cav2 antagonist and exert analgesic effect primarily via Cav2.2 blockade. TROX-1 improves electrophysiology block Cav2.2, channels which sense and mediate OA pain. TROX-1 is an improved therapeutic chronic debilitating pain soothing agent (Table 2). The scorpion venom-derived peptide HsTX1 and its analog HsTX1[R14A] are potent Kv1.3 blockers. HsTX1[R14A] is selective for Kv1.3 over closely-related Kv1 channels [26].
Insect venom
Insect venom is a rich source of therapeutically important toxins peptides and other bioactive molecules. These display anti-inflammatory or anti-nociceptive properties. Peptides isolated from bee venom (BV) exhibits strong anti-inflammatory, anti-bacterial, antimutagenic, radioprotective, anti-nociceptiveimmunity promoting, hepatocyte protective and anti-cancer activity. The variety of proteins and peptides isolated from honey bee venom and wasp venom. These include melittin, adiapin, apamine, bradykinin, cardiopep, mast cell degranulating peptide, mastoparan, phospholipase A2 and secapin. It contains short cationic peptides, kinins and polyamines [27] that can be used in the treatment of pain, inflammatory disease, and neurodegenerative diseases such as epilepsy and aversion. Mastoparan a cationic peptide and its analogs show promising analgesic potential [27]. Pelagia noctiluca (P. noctiluca) venom toxins are potent anti-inflammatory agents that can be used for the treatment of anti-inflammatory diseases [28]. Similarly, samsum ant venom (SAV) is a powerful antioxidant and shows anti-inflammatory activity [29]. It restores the normal biochemical and oxidative stability by improving the TNF-α/NF-κB mediated inflammation in CCL4-treated rats. DBV decreases rheumatoid factor, pain perception parameters, C-reactive protein, erythrocytes sedimentation rate, urinary hydroxyproline, serum transaminase level, and serum nitric oxide level [30]. Both IL-6, TNF-α level was found to be decrease by ADBV treatment in collagen induced arthritis model [30].
Snake venom
Cobrotoxin (CTX) is an active component of the venom from Naja atra [31] shows beneficial effects on rheumatoid arthritis. It inhibits NF-κB pathway partly contributes to the anti-inflammatory properties of CTX. Similarly, human phospholipase A2 (hPLA2) of the IIA group (HGIIA) catalyzes the hydrolysis of membrane phospholipids, producing arachidonic acid and originating potent inflammatory mediators [32]. Similarly, hydrostatin-TL1 (H-TL1) isolated from snake venom Hydrophis cyanocinctus antagonize TNF-α and shows significant anti-inflammatory activity in vitro and in vivo. H-TL1 is a potential peptide for the development of new agents to treat TNF-α- associated inflammatory diseases [33] (Table 2). sPLA2s from snake venoms shows nociceptive and antinociceptive actions [29,34]. This secreted PLA2s shows hydrolytic activity on phospholipids and modulates inflammation and pain. Hence, by uncovering molecular targets it can be used to control inflammation and neuro-degeneration [34] Moreover, interactions of human PLA2 HGIIA and two svPLA2s, Bothrops toxin II and Crotoxin B (BthTX-II and CB, molecules inhibit metabolic enzymes and are potential anti-inflammatory agents [32]. Anti-TNF-α biologics are effective therapies for various inflammatory diseases such as inflammatory bowel disease (IBD) and sepsis [33]. (TNF)-α is a pleiotropic cytokine that shows intense pro-inflammatory and immunomodulatory properties.
Scorpion venom
A polypeptide toxin peptide isolated from Heterometrus laoticus scorpion venom shows analgesic and anti-inflammatory and acts on Kv1.3 potassium channel [35]. St20 is a cloned peptide that has a putative 23-residue signal peptide, followed by a presumed 34-residue mature peptide including 8 cysteines [36]. St20 from the non-buthidae scorpion Scorpiops tibetanus and target potassium channel. Similarly, Krait venom possesses neurotoxins, membrane toxins, cardiotoxins, finger toxins, metalloproteinases, cholinesterases, L-amino acid oxidases and serine proteases which showed therapeutic potential against arthritis, inflammation and blood coagulation disorder [37]. It binds to pain receptor in the nociceptive pathway, transient receptor potential vanilloid 1 (TRPV1) of the TRP superfamily which is a target for several toxins. TRPV1 is involved in thermoregulation, inflammation, and acute nociception. antinociceptive, anti-inflammatory and antiarthritic activities of Bungarus fasciatus venom (BFV) in experimental animal models [38].
Some conotoxins act as analgesics, interacting with ion channels receptors in nerves so the ion channel cannot open. Conotoxin GeXIVA interacts with α9α10-nAChRs receptor and is a good analgesic [39,40]. It blocks ion channels and distort supply of ions from entering a neighboring nerve fiber. Conotoxins showed non-canonical coupling of GABAB receptors to voltage-gated calcium channels Antitumor-analgesic peptide (AGAP), purified from Buthus martensii Karsch, possesses analgesic activities. Trp38 is a conserved aromatic residue of AGAP, might play an important role in mediating AGAP activities. AGAP mutant (W38G) retains the analgesic efficacy; it is less toxic to skeletal and cardiac muscles [41]. Similarly, HYP-17 significantly relieves the central neuropathic pain induced by spinal cord injury (SCI), and inhibited c-Fos expression in lumbar (L) 4-L5 spinal segments. HYP-17 has potential analgesic activities against nociceptive, inflammatory and neuropathic pain [42].
Ligand-gated ion channels
In many ion channels, passage through the pore is governed by a "gate", which may be opened or closed in response to chemical or electrical signals, temperature, or mechanical force. Ligand gated open or close in response to ligand such as Ach binding to receptor protein. Receptor proteins are usually glycoproteins. E.g. acetylcholine binds to acetylcholine receptor on a Na+ channel. Channels opens, Na+ enters the cell. These are associated with transport of specific ions in or out of the cell. These are highly selective in types of ion transported. Ions are transported across electrochemical gradient. Transient receptor potential (TRP) channels are ligand-gated ion channels that detect physical and chemical stimuli and promote painful sensations via nociceptor activation. These have physiological role in the mechanisms controlling several physiological responses like temperature and mechanical sensations, response to painful stimuli, taste, and pheromones. These channels are expressed in pain sensing neurons and primary afferent nociceptors (Figure 5). They function as transducers for mechanical, chemical, and thermal stimuli into inward currents, an essential first step for provoking pain sensations pain [43]. Phα1β peptide isolated from the venom of the armed spider Phoneutria nigriventer, produces analgesia by blocking the TRPA1 channel and could be used for pain treatment [43]. Phα1β and CTK 01512-2 selectively target TRPA1, but not other TRP channels [44]. AMP-activated protein kinase (AMPK) activators decrease sensory neuron excitability, potentially by preventing sodium (Na+) channel phosphorylation by kinases such as ERK or via modulation of translation regulation pathways [45]. Protease inhibitors found in animal venom bind to protease enzymes and prevent their activity. These also inhibit fibrin activity in arthritic joints and induce chronic arthritis in patients. Because of transient receptor potential and inhibition of voltage-gated sodium/calcium channels, venom components mainly several enzymes (kinases, lipases, amine oxidases, and matrix metalloproteinases), and induction of synthesis of cytokines/chemokines, transcription factors, nerve growth factor, and modulation of several G protein-coupled receptors (cannabinoids, purinoceptors, and neuropeptides) animal toxins could be formulated as new analgesic drugs with fewer side effects (Table 2) [46].
Conclusion
The high selectivity and specificity of animal toxins have enabled their use as potential therapeutics in the treatment of pain and inflammation. Due to their channel blocking nature, they disrupt sensation that assists in pain relieving. However, role of toxins in defining the contribution of NaV channels in acute and chronic pain states their potential to be used as analgesic. Further, identification of mechanisms and molecular components responsible for pain generation will assist in pain control it also led to the selection of new targets for designing novel analgesic drugs. Due to reported massive side effects of opioids on body organs there is a great need to develop anti-inflammatory and analgesic agents with novel mechanisms of action with lesser side effects. Practically, main problem is unparallel findings and unrelated facts due to large translational gap between the preclinical experimental data and the clinical results. These finding also vary in different animal models during trial, researches face difficulties with the investigational techniques particularly for pain, as well as species specific differences in the mechanisms. But these could be resolved by advancement of technology mainly molecular high-throughput screens and automated electrophysiology tools. Besides, medication, early diagnosis, daily exercise, proper treatment and healthy dietary habits can reduce the disease spectrum and chronic inflammatory pain. No doubt animal toxins are good candidates for the development of new analgesic drugs.
References
- David Brownstein (2001) Overcoming arthritis. Medical alternative press 4173, Fieldbrook Rd. West Bloomfield, MI 48323.
- George Tilden (2009) Arthritis: The Cure: St Nartin’S Griffin, New York.
- Jessica K, Black ND (2006) The Anti-inflammatory Diet and Recipe Book, Hunter House Publishers, Turner Publishing Company, New York.
- Darlington G, Gamlin L (1998) Diet and Arthritis: A comprehensive guide to controlling arthritis through diet, Random House Publishers, India.
- Won HY, Lee JA, Park ZS, Song JS, Kim HY, et al. (1998) Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS ONE 6: e18168.
- Baddack U, Frahm S, Antolin-Fontes B, Grobe J, Lipp M, et al. (2015) Suppression of peripheral pain by blockade of voltage-gated calcium 2.2 channels in nociceptors induces Rankl and impairs recovery from inflammatory arthritis in a mouse model. Arthritis Rheumatol 67: 1657-1667.
- Reid MC, Shengelia R, Parker SJ (1998) Pharmacologic management of osteoarthritis-related pain in older adults. The Ame J Nursing 112: S38–43.
- Zheng Z, Sun Y, Liu Z, Zhang M, Li C, et al. (2015) The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des Devel Ther 9: 4931-4942.
- Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, et al. (2000) Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 12: 1092-1099.
- Roberto Pacifici (2016) The role of IL-17 and TH17 cells in the bone catabolic activity of PTH. Front Immunol 7: 57.
- Adamopoulos IE, Bowman EP (2008) Immune regulation of bone loss by Th17 cells. Arthritis Res Ther 10: 225.
- Severe Arthritis Disease Facts (2010) Arthritis Foundation 1355 Peachtree St NE Suite 600 Atlanta, GA.
- Becker, Michael A (2005) Arthritis and allied conditions: A textbook of Rheumatology edition 15. Lippincot Williams & Wilkins. 2303–2339.
- Cury Y, Picolo G (2006) Animal toxins as analgesics--an overview. Drug News Perspect 19: 381-392.
- Singh S, Nair V, Gupta YK (2011) Linseed oil: an investigation of its antiarthritic activity in experimental models. Phytother Res 26: 246-252.
- Gazerani P, Cairns BE (2014) Venom-based biotoxins as potential analgesics. Expert Rev Neurother 14: 1261-1274.
- Cardoso FC, Lewis RJ (2017) Sodium channels and pain: from toxins to therapies. Br J Pharmacol.
- Daniel JT, Clark RJ (2017) G-Protein Coupled Receptors Targeted by Analgesic Venom Peptides. Toxins (Basel) 9: E372.
- Wu Y, Ma H, Zhang F, Zhang C, Zou X, et al. (2018) Selective voltage-gated sodium channel peptide toxins from animal venom: pharmacological probes and analgesic drug development ACS Chem Neurosci 9: 187-197.
- Rahman W, Patel R, Dickenson AH (2015) Electrophysiological evidence for voltage-gated calcium channel 2 (Cav2) modulation of mechano- and thermosensitive spinal neuronal responses in a rat model of osteoarthritis. Neuroscience 305:76-85.
- Palhares MR, Silva JF, Rezende MJS, Santos DC, Silva-Junior CA, et al. (2017) Synergistic antinociceptive effect of a calcium channel blocker and a TRPV1 blocker in an acute pain model in mice. Life Sci 182: 122-128.
- Hanukoglu I (2017) ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters. The FEBS J 284: 525-545.
- Revell JD, Lund PE, Linley JE, Metcalfe J, Burmeister N, et al. (2013) Potency optimization of Huwentoxin-IV on hNav1.7: a neurotoxin TTX-S sodium-channel antagonist from the venom of the Chinese bird-eating spider Selenocosmia huwena. Peptides. 44: 40-46.
- Sousa SR, Wingerd JS, Brust A, Bladen C, Ragnarsson L, et al. (2017) Discovery and mode of action of a novel analgesic β-toxin from the African spider Ceratogyrus darlingi. PLoS One 12: e0182848.
- Akello JA, Lawrence N, Deplazes E, Cheneval O, Chen RM, et al. (2017) Spider peptide toxin HwTx-IV engineered to bind to lipid membranes has an increased inhibitory potency at human voltage-gated sodium channel hNaV1.7 Biochimica et Biophysica Acta (BBA) - Biomembranes 1859: 835-844.
- Tanner MR, Tajhya RB, Huq R, Gehrmann EJ, Rodarte KE, et al. (2017) Prolonged immunomodulation in inflammatory arthritis using the selective v1.3 channel blocker HsTX1[R14A] and its PEGylated analog. Clin Immunol 180: 45-57.
- Dongol Y, Dhananjaya BL, Shrestha RK, Aryal G (2016) Wasp venom toxins as a potential therapeutic agent. Protein Pept Lett 23: 688-698.
- Ayed Y, Sghaier RM, Laouini D, Bacha H (2016) Evaluation of anti-proliferative and anti-inflammatory activities of Pelagia noctiluca venom in Lipopolysaccharide/Interferon-γ stimulated RAW264.7 macrophages. Biomed Pharmacother 84: 1986-1991.
- Al-Tamimi J, Alhazza IM, Al-Khalifa M, Metwalli A, Rady A, et al. (2016) Potential effects of samsum ant, Brachyponera sennaarensis, venom on TNF-α/NF-κB mediated inflammation in CCL4-toxicity in vivo. Lipids Health Dis 15:198.
- Nipate SS, Hurali PB, Ghaisas MM (2015) Evaluation of anti-inflammatory, anti-nociceptive, and anti-arthritic activities of Indian Apis dorsata bee venom in experimental animals: biochemical, histological, and radiological assessment. Immunopharmacol Immunotoxicol 37: 171-184.
- Zhu Q, Huang J, Wang SZ, Qin ZH, Lin F (2016) Cobrotoxin extracted from Naja atra venom relieves arthritis symptoms through anti-inflammation and immunosuppression effects in rat arthritis model. J Ethnopharmacol 194: 1087-1095.
- Sales TA, Marcussi S, da Cunha EFF, Kuca K, Ramalho TC (2017) can inhibitors of snake venom phospholipases A₂ lead to new insights into anti-inflammatorytherapy in humans? a theoretical study. Toxins (Basel) 9.
- Wang N, Huang Y, Li A, Jiang H, Wang J, et al. (2016) Hydrostatin-TL1, an anti-inflammatory active peptide from the venom gland of Hydrophis cyanocinctus in the south china sea. Int J Mol Sci 17.
- Zambelli VO, Picolo G, Fernandes CAH, Fontes MRM, Cury Y (2017) secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins 9: 406.
- Hoang AN, Vo HD, Vo NP, Kudryashova KS, Nekrasova OV, et al. (2014) Vietnamese Heterometrus laoticus scorpion venom: evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 77: 40-48.
- Xiao M, Ding L, Yang W, Chai L, Sun Y, et al. (2017) St20, a new venomous animal derived natural peptide with immunosuppressive and anti-inflammatory activities. Toxicon 127: 37-43.
- Gomes A, Saha PP, Bhattacharya S, Ghosh S, Gomes A (2017) Therapeutic potential of krait venom. Toxicon 131: 48-53.
- Ghosh S, Saha PP, Dasgupta SC, Gomes A (2016) Antinociceptive, anti-inflammatory and antiarthritic activities of Bungarus fasciatus venom in experimental animal models. Indian J Exp Biol 54: 569-576.
- Mohammadi SA, Christie MJ (2015) Conotoxin interactions with α9α10-nAChRs: Is the α9α10-nicotinic acetylcholine receptor an important therapeutic target for pain management? Toxins (Basel) 7: 3916-3932.
- Wu X, Huang YH, Kaas Q, Harvey PJ, Wang CK, et al. (2017) Backbone cyclization of analgesic conotoxin GeXIVA facilitates direct folding of the ribbon isomer. J Biol Chem 292: 17101-17112.
- Xu Y, Meng X, Hou X, Sun J, Kong X, et al. (2017) A mutant of the Buthus martensii Karsch antitumor-analgesic peptide exhibits reduced inhibition to hNav1.4 and hNav1.5 channels while retaining analgesic activity. J Biol Chem 292: 18270-18280.
- Lee JY, Kam YL, Oh J, Kim DH, Choi JS, et al. (2017) HYP-17, a novel voltage-gated sodium channel blocker, relieves inflammatory and neuropathic pain in rats. Pharmacol Biochem Behav 153: 116-129.
- Tonello R, Fusi C, Materazzi S, Marone IM, De Logu F, et al. (2017) The peptide Phα1β, from spider venom, acts as a TRPA1 channel antagonist with antinociceptive effects in mice. Br J Pharmacol 174: 57-69.
- Marwaha L, Bansal Y, Singh R, Saroj P, Bhandari R, et al. (2016) TRP channels: potential drug target for neuropathic pain Inflammopharmacology 24: 305-317.
- Asiedu MN, Han C, Dib-Hajj SD, Waxman SG, Price TJ, et al. (2017) The AMPK activator A769662 blocks voltage-gated sodium channels: discovery of a novel pharmacophore with potential utility for analgesic development. PLoS One 12: e0169882.
- Botz B, Bölcskei K, Helyes Z (2017) Challenges to develop novel anti-inflammatory and analgesic drugs. Wiley Interdiscip Rev Nanomed Nanobiotechnol 9.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences