ISSN : 2348-1927
Annals of Biological Sciences
Acute and Subacute Effects of Aqueous Extract of Picralima nitida Seeds on Dexamethasone Induced Insulin Resistance in Rats
1Department of Biological Sciences, Higher Teachers’ Training College, University of Yaoundé I, P.O Box 47, Yaoundé, Cameroon
2Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies (IMPM), P.O Box 13033, Yaoundé, Cameroon
- Corresponding Author:
- Nyunai N
- Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies
- E-mail: sakthika@apcmcollege.ac.in
Abstract
Background: Picralima nitida is a West African plant with varied applications in African folk medicine. Aqueous extract of seeds of Picralima nitida was used to evaluate its protective effect on dexamethasone-induced insulin-resistance in rats.
Methods and Findings: Male rats were divided into five groups for acute and subacute study. At the end of the acute study, Oral Glucose Tolerance Test was performed. For the subacute study, Groups 1 and 2 received distilled water for 11 days orally and from day 7, either saline or dexamethasone (8 mg/kg) intraperitoneally for the last 5 days. Groups 3, 4 and 5 were respectively pretreated with glibenclamide (10 mg/kg), P. nitida extract at the doses of 200 mg/kg or 400 mg/kg orally for 11 days. At the end of the experiment on day 12, blood was collected for biochemical analysis and the animals were subjected to a fasting glucose test and then thereafter sacrificed. The liver, pancreas, kidney, and adrenal were weighed and histological analysis performed. The Area Under the Curve (AUC) of the glucose tolerance test, performed 4 hours after the end of acute treatment at a dose of 400 mg/kg of the extract, was significantly reduced (p<0.01). In subacute treatment, the AUC of the aqueous extract of the seeds of Picralima nitida was significantly reduced at doses of 200 mg/kg and 400 mg/kg compared to rats treated with dexamethasone. Furthermore, there was a significant decrease in Total Cholesterol and LDL-Cholesterol with the extract treatment as well as with glibenclamide which decrease Total Cholesterol. Glibenclamide and the extract at 400 mg/kg prevented the drop in protein levels induced by dexamethasone. Adrenal atrophy was noted in all rats given dexamethasone, as well as increased liver and kidney weights. The pretreatment with the aqueous extract of the seeds of Picralima nitida prevented cytolysis and degeneration of hepatocyte, hypotrophy of the pancreatic islets and tubular and glomerular degeneration caused by dexamethasone.
Conclusions: This study reveals that the aqueous extract of the seeds of Picralima nitida has anti-hyperglycemic and antihypercholesterolemic properties that may justify its use in traditional medicine against diabetes abnormalities.
Keywords
Picralima nitida, Insulin resistance, Dexamethasone, Glycemia, H istology, Lipid profile
Introduction
Picralima nitida is distributed in Eastern Ivory Coast, Ghana, south Nigeria, south Cameroon, Gabon, Congo, the Democratic Republic of the Congo, Angola and Uganda. Generally in dense rain forests [1].
Picralima nitida (Akuamma) ordinarily referred to ‘Ebam’ in Ewondo dialect or ‘Nongmbak’ in Bassa dialect in Cameroon is a rainforest tree occurring in African wooded areas. P. nitida has numerous applications in African traditional medicine. Various parts of the plant which include the leaves, seeds, stem, bark, and roots are employed by herbalists for the remedy of fever, cardiovascular diseases, jaundice, gastro-intestinal issues and malaria [2]. Preparations from different parts of the plant are employed as crude drug or crude herbal extract as a remedy for various types of human diseases [3]. The seeds are widely utilized in West Africa particularly in Nigeria, Ivory Coast and Ghana as antipyretic, aphrodisiac agents, and for the treatment of malaria, pneumonia and different chest-conditions [2]. Herbalists have additionally made claims for the effectiveness of the coconut water extract of P. nitida seeds for the treatment of many diseases, such as diabetes [4,5]. In Cameroon, the bark is used against intestinal worms, kidney and spleen suffering, liver harm and malaria [1]. In the Ivory Coast, the P. nitida seeds have the notoriety of being extremely powerful, effective for breaks among the Bété clan, while in the Democratic Republic of the Congo (DRC) it is taken as decoction against pneumonia. At the same time, the root decoction is likewise utilized against yellow fever and even macerated in rum against pneumonia [1]. In Benin, the leaf decoction is used in water or taken via mouth for measles. In lemon juice, the seed powder is suspended to cure women with leucorrhea [1]. The extremely bitter bark is a remedy for venereal diseases in Gabon. In southeast Nigeria, Picralima nitida is employed as a medicinal drug for colds and as an aphrodisiac [6].
Indole alkaloids are the first set of alkaloids isolated from P. nitida; akuammine, pseudoakuammine, akuammidine, akuammicine, akuammigine, pseudoakuammigine, akuammiline, akuammenine and pseudoakuammicine [7,8]. After several supplementary compounds like picraline, desacethylpicraline, picraphylline and melinonine A and picracine [9-12], Ansa-Asamoah et al. [13] discovered a new alkaloid picratidine. Desacethylpicraline [11], desacethylakuammiline [12], akuammenine [14], pericine and apparicine (perkalline) [15] are other alkaloids found in this plant. Nwaogu [16] showed that the seeds of the plant are prosperous in amino acids, vitamins A and E, further zinc, iron, and manganese.
The extract of seed, fruit rind, and stem bark extracts showed great repressing activity against drug-resistant Plasmodium falciparum clones at doses of 1.23 μg/mL-32 μg/mL [17]. Kapadia et al. [18] verified the antimalarial activity of the seeds and demonstrated that the action of the crude phenolic alkaloid fraction at least in part resides in Akuammine. The chloroform extract of P. nitida seed showed activity at 50 μg/ml against Leishmania donovani [19]. The ethanolic and aqueous extracts of P. nitida leaf showed an increase in a larvicidal activity dependent on concentration and time [20]. The methanolic extract of fruit at a dose of 50 mg/kg created an antipyrexic of 38.7% on lipopolysaccharide-induced pyrexia in rabbits [21]. Akuammidine, akuammine, akuammicine and pseudoakuammigine exhibited analgesic activity [22]. Pseudoakuammigine was once additionally discovered to exhibit analgesic impact in vivo and antiinflammatory activity [3,16]. The antidiarrheal effect of the methanol fruit-rinds against Shigella dysenteriae type 1-induced diarrhoea was firm in vivo [23]. P. nitida fruit-rind extract effectively and considerably reduced in vivo Shigella dysenteriae type 1 density, the frequency and mass of diarrhoea stool after day-3 in diarrhoeic rats [23]. The ethanol extracts of the root and stem bark confirmed antimicrobial effect on E. coli, P. aeruginosa, Bacillus subtilis, S. aureus and Salmonella kintambo with the best activity against S. kintambo [24,25]. The methanol leaf extract of P. nitida at 300 mg/kg exhibited great antidiabetic activity with 38.48% glycaemia reduction [26]. Yessoufou et al. [27] point out that P. nitida extensively reduced glycaemia in diabetic pregnant rats from day 16 until the delivery on day 21. Additionally, the methanol extracts of the seed, pulp and fruit rind of P. nitida exhibited hypoglycaemic effect at 300 and 900 mg/kg in alloxan-induced diabetes in rats [28].
The purpose of this work was to assess the impact of Picralima nitida aqueous extract on insulin resistance induced by dexamethasone in rodents.
Materials and Methods
Plant biological material
Harvest
The fruits of Picralima nitida were harvested in a suburb of the Littoral Region (in the locality of Mandjo) in April 2018, in the semi-rainy season. The seeds were removed from the fruits, dried in the shade for three months, then were identified at the National Herbarium of Cameroon located in Yaoundé, and compared with plant sample N0 2136/SRFK.
Extraction
1030 g of dried seed powder of Picralima nitida was macerated in 2400 ml of distilled water for 24 hours [29]. After separation by decantation, the resulting solution was filtered using a Wattman No. 3 filter paper. The product obtained was lyophilized at the Food Technology Laboratory of the Institute of Medical Research and Medicinal Plants Studies (IMPM), Cameroon. This operation yielded 65.9 g of aqueous extract, a yield of 6.39%.
Experimental animals
The study was performed on male albino rats of the Wistar strain weighing between 160 g and 260 g. These rats were obtained at the Higher Teachers’ Training College (ENS) Animal House of the University of Yaoundé I (Cameroon). During this period, they were fed on standard rat diet and water ad libitum. They were housed at a controlled ambient temperature of 25 °C ± 2°C with 50% ± 10% relative humidity and with a 12 h light/12 h dark cycle.
Drugs and chemicals
Glibenclamide was obtained from Mahalakshmi chemicals, Hyderabad, Telangana state, India. Ketamine and Diazepam injections were procured from a local pharmacy with a labeled amount of 5 mg/ml. Other chemicals used were of analytical grade and used as received.
Acute effects of the aqueous extract of Picralima nitida seeds on dexamethasone-induced insulin resistance
Twelve hours fasted rats were randomly divided into five groups of five animals (n=5) each. Group I (Control): received gavage of vehicle 10 ml/kg and normal saline 1 ml/kg i.p., Group II (DEXA): received gavage of vehicle 10 ml/kg and treated with dexaméthasone 8 mg/kg i.p., Group III (GLIB+DEXA): received gavage of glibenclamide 10 mg/kg and dexaméthasone 8 mg/kg i.p., Group IV (PNE+DEXA): received gavage of Picralima nitida extract 200 mg/kg and dexaméthasone 8 mg/kg i.p., Group V (PNE+DEXA): received gavage of Picralima nitida extract 400 mg/kg and dexamethasone 8 mg/kg i.p. Normal saline or dexamethasone was administered 30 min after oral gavage of vehicle, glibenclamide or extract. After 4 h of dexamethasone injection, animals were subjected to OGTT. Blood glucose was measured at 30, 60 and 120 minutes after administration of glucose (2 g/kg).
Subacute study of the protective effects of the aqueous extract of Picralima nitida seeds on dexamethasone-induced insulin resistance
Experimental procedure
Rats were randomly divided into five groups of five animals (n=5) each and received different treatments once daily for 11 days. Group I (Control): received gavage of vehicle 10 ml/kg and normal saline 1 ml/kg i.p., Group II (DEXA): received gavage of vehicle 10 ml/kg and pre-standardized dose of dexamethasone 8 mg/kg i.p., Group III (GLIB+DEXA) received gavage of glibenclamide 10 mg/kg and dexamethasone 8 mg/kg i.p., Group IV (PNE+DEXA): received gavage of Picralima nitida extract 200 mg/kg and dexamethasone 8 mg/kg i.p. while Group V (PNE+DEXA) received gavage of Picralima nitida extract 400 mg/kg and dexamethasone 8 mg/kg i.p. Group 2, Group 3, Group 4 and Group 5 rats were treated with dexamethasone (8 mg/kg/I .p.) from 7th to 11th day during the study period. Dexamethasone was administered 30 min after oral gavage of vehicle or extract [31].
At the end of the experimental period, animals were fasted for 12 h and subjected to Oral Glucose Tolerance Test (OGTT). Blood samples were collected by retro-orbital plexus puncture under light ether anesthesia. Thereafter at the end of OGTT, the rats were anesthetized by intraperitoneal injection of urethane (1.5 g/kg) and then sacrificed. The blood samples were centrifuged at 2000 RPM for 20 minutes. The serum was used for further biochemical analysis. The pancreas, adrenal glands, kidney and liver of each rat were removed and weighed, and their tissues were dissected out and were fixed in formalin (10%) for further histopathological investigations, while adrenals were only photographed. Body weight of animals was recorded daily during the study period.
Estimation of biochemical parameters
Lipid profile (Total Cholesterol (TOT CHOL), High-Density Lipoproteins Cholesterol (HDL CHOL) and Serum Triglyceride (TG)) and serum protein were determined by commercial kits. Atherogenic Index (AI) was calculated by the following formula: AI=(TOT CHOL - HDL CHOL)/ HDL CHOL [31]. LDL-Cholesterol concentration was determined according to the formula of Friedwald [32].
Oral glucose tolerance test (OGTT)
The animals were fasted for 12 hours after the last treatment dose on the eleventh day, at the end of which the first blood glucose level was measured on blood taken from the retro-orbital plexus, using a capillary tube, by a CERA-CHEK brand glucometer. The rats were then subjected to an oral glucose overload (2 g/kg). Then the blood glucose levels were measured 30, 60 and 120 minutes after the administration of the glucose solution.
Histopathology examination
After macroscopic analysis, representative fragments of the liver left kidney and pancreas were fixed in a 10% solution of buffered formalin (pH 7.4) and enclosed in paraffin, following the technique of Mayer [33] with slight modifications and then observed under a light microscope. Five-micrometer sections were gotten and colored with Hematoxylin-Eosin (HE) for analysis of tissue changes, under an optical microscope (100 X).
Statistical analysis
The values were expressed as mean ± SEM. The data were analysed by using GraphPad Prism software version 5.03. The results were compared by using the ANOVA-One-Way test followed by the Dunnett test. The difference was considered statistically significant at p<0.05.
Results
Effect of aqueous extract of Picralima nitida seeds on glucose level during the acute studyEffect of aqueous extract of Picralima nitida seeds on glucose level during the acute study
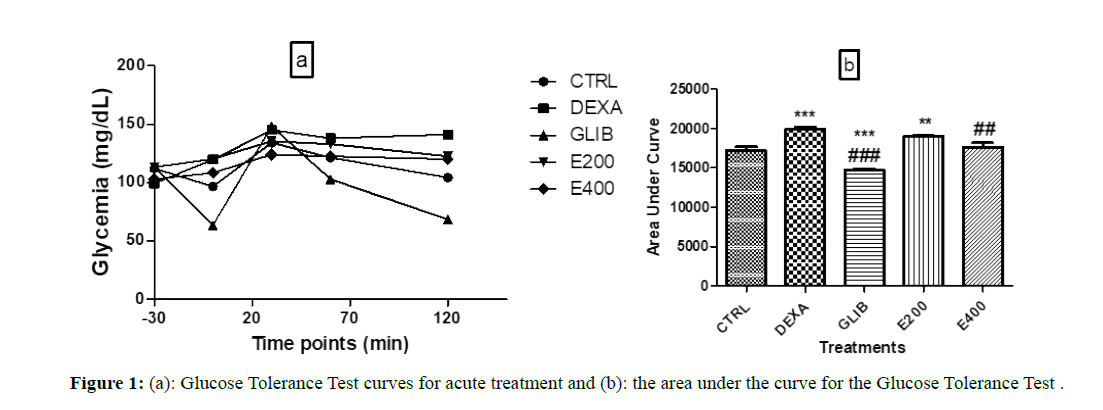
Figure 1a shows the average baseline blood glucose and the blood glucose variation curves during the glucose tolerance test in each group during acute treatment. It shows that the basic blood sugar levels (before the start of the various treatments) are between 103 mg/ dL and 120 mg/dL.
Figure 1: (a): Glucose Tolerance Test curves for acute treatment and (b): the area under the curve for the Glucose Tolerance Test .
Figure 1b shows the Areas Under the Curve (AUC) of the glucose tolerance test of each group during acute treatment. The AUC of the group treated with dexamethasone (DEXA) and that of the group pretreated with the extract at the dose 200 mg/kg (E200) are significantly higher than that of the control group (CTRL), (respectively 19879.6 ± 336.36; p<0.001 and 19034 ± 116.22; p<0.01 vs. 17208 ± 443.23). The group treated with glibenclamide (GLIB) has a lower AUC (14740.6 ± 186.78; p<0.001) compared to the control group (17208 ± 443.23). The AUC of the group pretreated with the extract at a dose of 400 mg/kg (E400) is significantly lower than that of the group treated with dexamethasone (17629.6 ± 546.66 vs.19879.6 ± 336.36; p<0.01).
Each value represents the mean ± SEM, n=5; ** p˂0.01; *** p˂0.001 is the significant difference in blood glucose compared to the group treated with distilled water and NaCl. ##p<0.01; ### p<0.001 is the significant difference in blood glucose compared to the group treated with distilled water and dexamethasone. CTRL: group of rats treated with distilled water and NaCl 9%; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: groups of rats treated with the extract at a dose of 200 mg/kg and 400 mg/kg, and with dexamethasone.
Effect of aqueous extract of Picralima nitida seeds on glucose level during the subacute study
Effect on blood glucose level
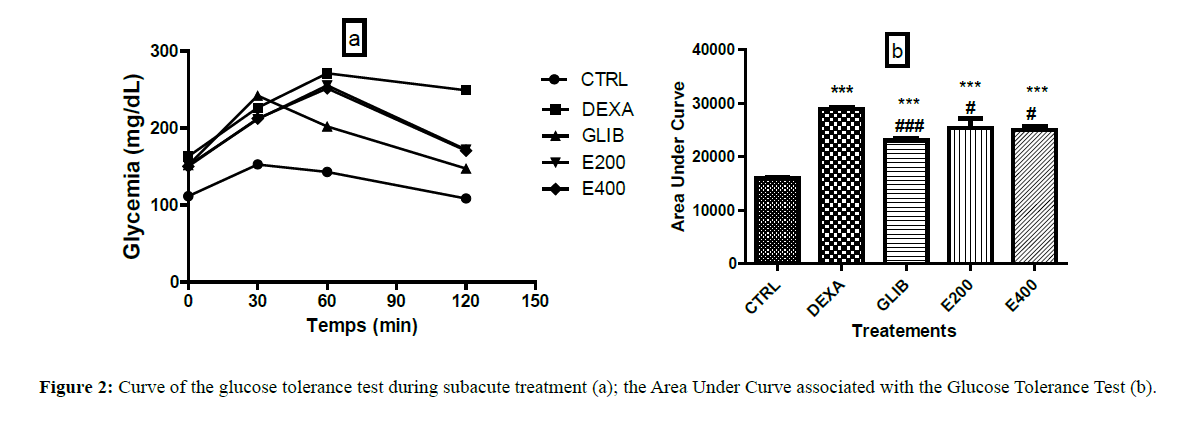
Figure 2a shows the basic blood glucose levels and the blood glucose variation curves during the glucose tolerance test in each group. It shows that the basic glycaemia (before glucose administration) is between 111 mg/dL and 163 mg/dL.
Figure 2: Curve of the glucose tolerance test during subacute treatment (a); the Area Under Curve associated with the Glucose Tolerance Test (b).
Figure 2b shows the AUCs of the glucose tolerance test for each group at the end of the treatment. Animals treated with dexamethasone (28910 ± 258.86), glibenclamide (23046.20 ± 349.79), and plant extract respectively 200 mg/kg (25282.60 ± 1845.43) and 400 mg/kg (25061 ± 731.11) showed significantly higher AUCs than those in control animals (15953 ± 187.35) (p<0.001).
Each value represents the mean ± SEM, n=5; *** p˂0.001 corresponds to the significant difference in blood glucose compared to the group treated with distilled water and NaCl. #p<0.05 and ### p<0.001 corresponds to the significant difference in blood glucose compared to the group treated with distilled water and dexamethasone. CTRL: group of rats treated with distilled water and 9% NaCl; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: groups of rats treated with the extract at a dose of 200 mg/kg and 400 mg/kg, and with dexamethasone respectively.
The AUCs of animals treated with glibenclamide and those pre-treated with extract (200 mg/kg and 400 mg/kg) when compared to that of animals treated with dexamethasone shows a significant decrease. The AUC of animals having received glibenclamide was 23046.20 ± 349.78 vs. 28910 ± 258.87 in dexamethasone group; p<0.001). For those who received the seeds extract of Picralima nitida the AUCs were 25282.60 ± 1845.43 and 25061 ± 731.11 respectively at 200 mg/kg and 400 mg/kg; p<0.05 as compared to the dexamethasonetreated rats (28910 ± 258.87).
Effect of aqueous extract of Picralima nitida seeds on body weight variation of rats
Bodyweight loss (p<0.001) was observed from Day 7 in all groups when compared with the control group. This loss of body weight increased until the end of the experiment respectively in the groups treated with glibenclamide (3.17 ± 3.72 to -15.15 ± 3.77; p<0.001) and in the group treated with the extract at 400 mg/kg of extract (from -7.00 ± 3.17 to -18.68 ± 3.70; p<0.001) (Table 1).
| Groups | |||||
|---|---|---|---|---|---|
| Day measurement | CTRL | DEXA | GLIB | E200 | E400 |
| Day 1 | 3.54 ± 3.02 | 6.97 ± 1.66 | 2.76 ± 1.92 | 5.52 ± 1.51 | 3.86 ± 2.40 |
| Day 3 | 16.26 ± 4.94 | 11.97 ± 2.63 | 5.03 ± 2.42 | 7.72 ± 2.42 | 1.61 ± 4.26 |
| Day 5 | 18.95 ± 4.25 | 15.43 ± 3.55 | 6.91 ± 3.16 | 8.60 ± 3.77 | 4.85 ± 3.90 |
| Day 7 | 22.00 ± 3.87 | 11.90 ± 4.69 | 3.17 ± 3.72* | 1.64 ± 3.61** | 1.67 ± 3.93** |
| Day 9 | 23.19 ± 3.61 | -0.03 ± 2.98*** | -7.66 ± 4.05*** | -6.53 ± 3.13*** | -7.00 ± 3.17*** |
| Day 11 | 17.95 ± 3.65 | -8.89 ± 2.50*** | -15.15 ± 3.77*** |
-12.64 ± 4.86*** | -18.68 ± 3.70*** |
| Each value represents the mean ± SEM, n=5; * p<0.05; ** p<0.0; *** p<0.001 corresponds to the significant difference in weight variations with respect to the group treated with distilled water and NaCl. CTRL: group of rats treated with distilled water and NaCl 9%; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: groups of rats treated with the extract at a dose of 200 mg/kg and 400 mg/kg, and with dexamethasone respectively | |||||
Table 1: Effect of different treatments on the bodyweight variation of rats.
Effect of aqueous extract of Picralima nitida seeds on the relative weight of some organs
The results show a significant increase in the relative weight of the kidneys of the dexamethasone-treated group (0.64 ± 0.01 to 0.86 ± 0.003; p<0.001), of the pretreated group with glibenclamide (0.64 ± 0.01 to 0.93 ± 0.04 ; p<0.001), and of the groups pretreated with the extract at 200 mg/kg (0.64 ± 0.01 to 0.78 ± 0.05; p<0.001) and 400 mg/kg (0.64 ± 0.01 to 0.87 ± 0.04; p<0.001) in comparison to the control group of rats. Similarly, a significant increase in relative liver weight was observed in the dexamethasone-treated group (2.31 ± 0.06 to 4.74 ± 0.14 ; p<0.001), in the glibenclamide-pretreated group (2.31 ± 0.06 to 4.71 ± 0.37 ; p<0.001), and in the pretreated groups at the extract dose of 200 mg/kg (2.31 ± 0.06 to 5.08 ± 0.35 ; p<0.001) and 400 mg/kg (2.31 ± 0.06 to 5.04 ± 0.28 ; p<0.001) when compared to the control group. The relative weight of the pancreas stayed unchanged in all pretreated rat groups when compared to the control group.
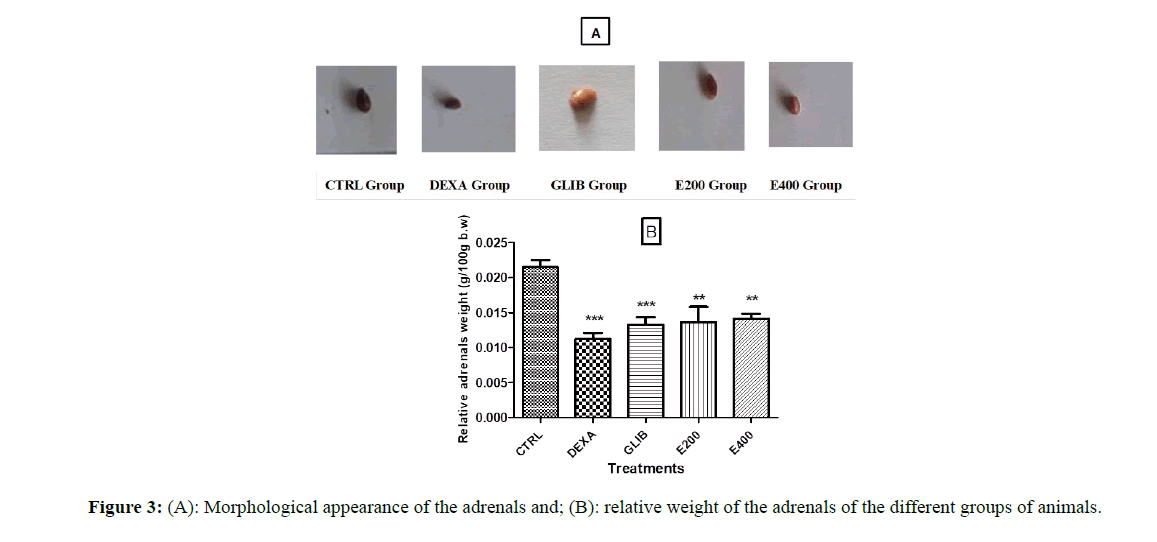
Figure 3A shows the effect of the different treatments on the external aspect (photography) of the adrenals. It presents an appearance of smaller adrenals in the group of rats treated with dexamethasone compared to the other groups (control and pretreated with glibenclamide and the extract of Picralima nitida.
Figure 3B shows the relative weight of the adrenals. It shows a significant reduction (p<0.001) in the relative weight of the adrenals in rats treated with dexamethasone (0.02 ± 0.00 to 0.01 ± 0.00; p<0.001), those pretreated with glibenclamide (0.02 ± 0.00 to 0.01 ± 0.00; p<0.001) and those pretreated with Picralima nitida seeds extract at 200 mg/kg (0.02 ± 0.00 to 0.01 ± 0.00; p<0.01) and at 400 mg/kg (0.02 ± 0.00 to 0.01 ± 0.00; p<0.01) when compared to the control group. This significant decrease is also observed in the group of rats pretreated with the extract at the dose of 200 mg/kg (p<0.05).
Each value represents the mean ± SEM, n=5;** p<0.01; *** p<0.001 correspond to the significant difference in the relative weight of the adrenals compared to the group treated with distilled water and NaCl, #p<0.05 corresponds to the significant difference in the relative weight of the adrenals compared to the group treated with distilled water and dexamethasone. CTRL: group of rats treated with distilled water and 9% NaCl; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: group of rats treated with the extract at a dose of 200 mg/kg or 400 mg/kg, and with dexamethasone.
Effect of aqueous extract of Picralima nitida seeds on biochemical parameters
Lipid parameters
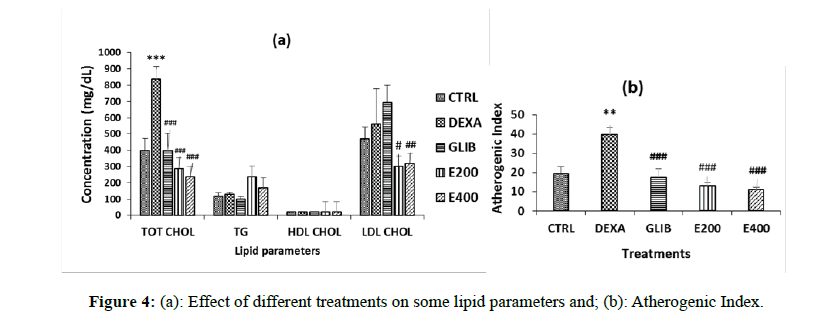
The lipid profile (Total Cholesterol, triglycerides, HDL-Cholesterol and LDL-Cholesterol) is shown in Figure 4.
A significant (p<0.05) increase in Total Cholesterol was observed in the dexamethasone-treated rats compared to the control group (400 ± 72.46 to 837.5 ± 78.06; p<0.001). In rats pretreated with plant extract, a significant decrease in total cholesterol was observed at doses of 200 mg/kg (400 ± 72.46 to 287.5 ± 33.07, p<0.001) and 400 mg/kg (400 ± 72.46 to 237.50 ± 18.54; p<0.001) compared with the group of rats treated with dexamethasone. A significant decrease in LDL-Cholesterol was observed in the pretreated rats with plant extracts at doses of 200 mg/kg ((559.33 ± 220.27 to 300.72 ± 35.89; p<0.05) and 400 mg/kg (559.33 ± 220.27 to 317.94 ± 23.69; p<0.01), compared to the group of rats treated with dexamethasone.
The Atherogenic Index decreases in all treated groups either with glibenclamide or with the extract (p<0.01) when compared to dexamethasone-treated rats.
Each value represents the mean ± SEM, n=5; *p<0.05 and ***p<0.001; corresponds to the significant difference of one parameter compared to the control group; #p<0.05, ## p<0.01 and ### p<0.001 correspond to the significant difference of a parameter with respect to the DEXA group. CTRL: group of rats treated with distilled water and NaCl 9%; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: groups of rats treated with the extract at a dose of 200 mg/kg or 400 mg/kg, and with dexamethasone.
Protein profile
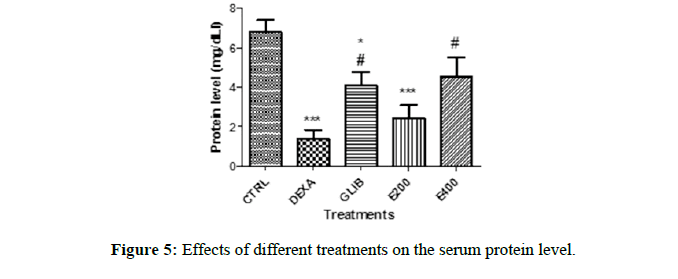
The protein profile in each group is shown in Figure 5. The comparison of the protein level of the control group with the other groups reveal a significant decrease in the level of protein in the group treated with dexamethasone (6.83 ± 0.59 to 1.39 ± 0.44; p<0.001), the pre-treated group with glibenclamide (6.83 ± 0.59 to 4.11 ± 0.67; p<0.05) and that pretreated with the extract at a dose of 200 mg/kg (6.83 ± 0.59 to 2.442 ± 0.65; p<0.001). However, the protein level in the group treated with glibenclamide and that treated with the extract (400 mg/kg) show a significant increase when compared to the dexamethasone group (1.39 ± 0.44 to 4.11 ± 0.67, p<0.05; 1.39 ± 0.44 to 4.52 ± 1.01, p<0.05) respectively).
Each value represents the mean ± SEM, n=5; *p<0.05 and ***p<0.001 corresponds to the significant difference in the protein profile of the rats compared to the control group. #p<0.05 corresponds to the significant difference of a parameter with respect to the DEXA group. CTRL: group of rats treated with distilled water and NaCl 9%; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: group of rats treated with the extract at a dose of 200 mg/kg and 400 mg/kg, and with dexamethasone.
Effect of aqueous extract of Picralima nitida seeds on liver, pancreas and kidney histology
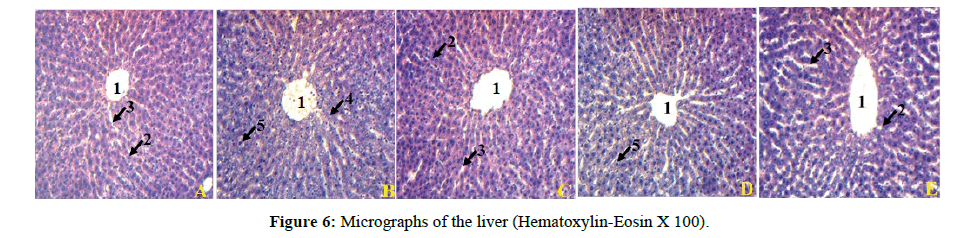
Histological analysis of the liver of rats treated with dexamethasone revealed the presence of hepatocyte degeneration and hepatic cytolysis compared to the livers of control rats. The liver of animals pretreated with glibenclamide and plant extract at a dose of 400 mg/ kg shows a histological improvement in the hepatic parenchyma compared to the group of rats treated with dexamethasone (Figure 6). histological improvement in the hepatic parenchyma compared to the group of rats treated with dexamethasone (Figure 6).
(A): rat liver treated with distilled water and NaCl; (B): rat liver treated with distilled water and dexamethasone; (C): rat liver treated with glibenclamide and dexamethasone; (D): rat liver treated with plant extract at 200 mg/kg and dexamethasone; (E): rat liver treated with plant extract at 400 mg/kg and dexamethasone; 1=Central vein, 2=Hepatocyte, 3=Sinusoid capillary, 4=Hepatocyte degeneration, 5=Hepatic cytolysis.
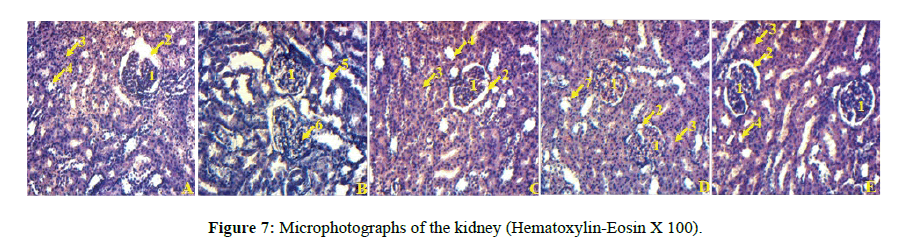
Histological analysis of the kidneys of rats treated with dexamethasone showed tubular and glomerular degeneration compared to those in the group of control rats. The kidneys of rats pretreated with the plant extract (200 mg/kg and 400 mg/kg) and glibenclamide show an improvement in the structure of the kidneys compared to those of rats treated only with dexamethasone (Figure 7).
A: rat kidney treated with distilled water and NaCl, B: rat kidney treated with distilled water and dexamethasone, C: rat kidney treated with glibenclamide and dexamethasone, D: rat kidney treated with plant extract at a dose of 200 mg/kg and dexamethasone, E: rat kidney treated with plant extract at a dose of 400 mg/kg and with dexamethasone; 1=Glomerulus, 2=Urinary space, 3=Proximal convoluted tubule, 4=Distal convoluted tubule 5=Tubular degeneration, 6=Glomerular degeneration, 7=Tubular clarification.
Histological analysis of the pancreas of rats treated only with dexamethasone showed hypotrophy of the pancreatic islets compared to the control rats. The rat group pretreated with glibenclamide and plant extract (200 mg/kg and 400 mg/kg) showed an improvement in the pancreatic islets compared to rats treated only with dexamethasone (Figure 8 and Table 2).
| Groups | |||||
|---|---|---|---|---|---|
| Organs (g/100 g bw) |
CTRL | DEXA | GLIB | E200 | E400 |
| Kidney | 0.64 ± 0.02 | 0.86 ± 0.04** | 0.94 ± 0.04*** | 0.78 ± 0.06 | 0.87 ± 0.04** |
| Pancreas | 0.47 ± 0.02 | 0.52 ± 0.03 | 0.58 ± 0.04 | 0.52 ± 0.07 | 0.47 ± 0.04 |
| Liver | 2.31 ± 0.07 | 4.74 ± 0.14*** | 4.71 ± 0.38*** | 5.08 ± 0.35*** | 5.05 ± 0.29*** |
| Each value represents the mean ± SEM, n=5; ** p <0.01; *** p<0.001 corresponds to the significant difference in the relative weight of the organs compared to the group treated with distilled water and NaCl. CTRL: group of rats treated with distilled water and NaCl 9‰; DEXA: group of rats treated with distilled water and dexamethasone; GLIB: group of rats treated with glibenclamide and dexamethasone; E200 and E400: groups of rats treated with the extract at a dose of 200 mg/kg and 400 mg/kg, and with dexamethasone | |||||
Table 2: Effect of different treatments on the relative weight of kidneys, pancreas, and liver.
(A): rat pancreas treated with distilled water and NaCl; (B): rat pancreas treated with distilled water and dexamethasone; (C): rat pancreas treated with glibenclamide and dexamethasone; (D): rat pancreas treated with plant extract at a dose of 200 mg/kg and dexamethasone; (E): rat pancreas treated with plant extract at a dose of 400 mg/kg and with dexamethasone. 1=islets of Langerhans; 2=pancreatic acini; 3=Hypotrophy of the pancreatic islets.
Discussion
The aim of this work was to assess the activity of the aqueous extract of P. nitida on the insulin resistance induced in the Wistar rat by dexamethasone in acute and subacute treatment.
Acute study shows that treatment with the extract significantly improves the glycemic profile of animals treated at a dose of 400 mg/ kg compared to those treated only with dexamethasone. This effect is more pronounced with the group treated with glibenclamide. However, there is an increase of the Area Under the Curve (AUC) of the groups treated respectively with dexamethasone and the extract at the dose 200 mg/kg. The AUC of the group of animals treated with glibenclamide, on the other hand, shows a significant decrease, both in comparison with the control group and in comparison with the group of animals treated with dexamethasone. Glibenclamide is an insulin secretor, it works by stimulating bêta cells to produce insulin, and its better effectiveness could be explained by this mode of action.
The dose of 400 mg/kg of the extract protects the rats from increasing AUC induced by dexamethasone. The value of the AUC, at a dose of 200 mg/kg shows that at this dose dexamethasone effects remain perceptible, despite the administration of the extract.
In the acute study, a reduction in the fasting blood glucose levels with seeds extract of Picralima nitida (400 mg/kg) in the dexamethasonemodel of hyperglycemia was similar to that of the rats treated with glibenclamide (10 mg/kg) when compared with dexamethasone control rats.
Dexamethasone has a half-life of more than 24 hours. It is an inhibitor of insulin secretion, the latter being stimulated by glucose and insulin-secreting drugs [34]. It is known that dexamethasone alters glucose homeostasis but the mechanism until now is not well understood, which is an indication that the extract might improve glucose metabolism trough pancreatic effects. The hypoglycemic effect can be caused by various mechanisms, either secretion of insulin, improvement of insulin receptors or both. It would, therefore, be necessary for future studies to determine the mechanism for lowering the glycemia of this extract, by measuring the level of insulin.
In the subacute study, dexamethasone-induced elevated blood glucose levels in all groups treated with the extract and glibenclamide respectively. However, glibenclamide and the extract at the doses used significantly lowered this rise in blood glucose. Several other studies have demonstrated the hypoglycemic effect of the seeds of P. nitida on different models of diabetic animals [29,35,36]. These may have been caused by the presence of several biologically active secondary metabolites although the P. nitida seed is known to be very rich in indole alkaloids highly implicated in the inhibition of phosphoenolpyruvate carboxykinase, an important enzyme in gluconeogenesis, thus producing hypoglycaemia [37].
Glucocorticoids in general increase blood glucose levels by various mechanisms; increased hepatic glucose production (gluconeogenesis), decreased peripheral glucose uptake into muscle and adipose tissue, breakdown of muscle and fat to provide additional substrates for glucose production [38]. Also, prolonged glucocorticoid exposure is associated with the development of severe insulin resistance and metabolic dysfunction [38]. The present study also confirms the same findings. The administration of dexamethasone provokes in rats an important increase of glycemia, which is in line with the earlier study of Prashant [39]. P. nitida aqueous seeds extract lowered glycemia of rats in a dose-dependent manner when compared to rats treated with dexamethasone only.
Several factors explain the increase of blood glucose subsequently to dexamethasone administration, like depletion of insulin sensitivity, reduced Langerhans islets, and elevated hepatic gluconeogenesis role [40]. According to the literature Picralima nitida plant seeds are helpful in the treatment of diabetes [4,5]. Our study supports the earlier findings that the aqueous extract of Picralima nitida seeds significantly decreased elevated blood glucose levels [28].
The decrease in body weight observed from the 7th day in all groups who received dexamethasone compared to the control group is in line with the previous work of [41] and that of [39]. Negative issues of glucocorticoid treatment are already reviewed, those containing insulin resistance, physiological disorders like increase of leptin, anorexia, and emaciation, associated with hyperglycemia and hypertriglyceridemia [42,43]. P. nitida seed extract treatment was unable to repress dexamethasone-induced weight loss.
On the other hand, an increase in the liver and kidney weight was noticed in all the groups treated with dexamethasone; reflecting an enlarged organ induced by dexamethasone may be due to either hepatic steatosis (fat accumulation) [44,45] or to elevated foreign substance metabolism (dexamethasone). Administration of certain xenobiotic compounds to rodents has long been recognized to induce hepatocellular hypertrophy and hyperplasia, thereby increasing liver mass in the absence of liver injury [46]. Kidney hypertrophy due to the accumulation of extracellular matrix proteins is directly related to a decline in kidney function perhaps due to structural changes of the glomerular basement membrane [47,48].
A significant decrease in the weight of the adrenal glands of all animals treated with dexamethasone was noted in comparison with the rats of the control group. Despite the reduced appearance of the adrenal morphology of the rats treated with the extract, the adrenal weights remained significantly unchanged when compared to the treated group only with dexamethasone. This drop-in adrenal weights in groups pretreated with dexamethasone can be explained by the fact that when glucocorticoids are used at supraphysiologic doses, glucocorticoid-induced Hypothalamic-Pituitary-Adrenal (HPA) axis suppression renders the adrenal acorns unable to generate sufficient cortisol if glucocorticoid treatment is abruptly stopped, resulting in atrophy of the adrenals.
Another biological consequence linked to the administration of dexamethasone is the increase of total cholesterol level, LDL Cholesterol, triglyceride and decreased HDL cholesterol [49]. This study shows that the seeds extract of P. nitida lowers the level of Total Cholesterol and LDL Cholesterol increased by the administration of dexamethasone. In addition, the atherogenic index which gives a general view of the lipid profile indicates a decreased drop in the groups of rats treated with the extract and glibenclamide respectively. This demonstrates the beneficial effect of the administration of Picralima nitida extract on the lipid profile. The same effect was observed with the hydroalcoholic extract of Allium elburzense which significantly reduced serum blood glucose, triglyceride, total cholesterol, low-density lipoprotein-cholesterol levels and increased the high-density lipoprotein-cholesterol level to the dyslipidemic control group [50].
In this study, the treatment with dexamethasone-induced a fall in proteins in all the groups, except that of the rats treated with the extract (400 mg/kg), in which this level is unchanged. Glibenclamide and the extract at 400 mg/kg respectively induced an increase in the protein level compared to the group treated only with dexamethasone. This study corroborates those of other authors who have shown that dexamethasone significantly induced muscle wasting and altered the balance between protein synthesis and degradation [51,52].
Histology revealed the degeneration and cytolysis of the liver cells of rats treated with dexamethasone. In this group, we also noticed the degeneration of the tubules and glomeruli in the kidneys. Additionally, there was a hypotrophy of the pancreatic islets. All these abnormalities were absent in the groups of rats treated with the extract and with glibenclamide respectively. Treatment with P. nitida seeds extract to dexamethasone-exposed rats produced significant improvement in liver, kidney and pancreas functions, showing the beneficial role of P. nitida to counteract the dexamethasone-induced dysfunctions. This result is similar to that of Onoh [53].
These findings also agree with Donald et al. [54], who showed the ameliorative effects of Picralima nitida seed against in vivo carbon tetrachloride-mediated hepatotoxicity in rats.
Conclusion
Dexamethasone treatment in insulin-resistant rats decreased appetite and body weight, induced glucolipid metabolic disturbances and appetite peptide expression in the hypothalamus through an indirect effect secondary to metabolic changes. Elucidation of the mechanism requires further study. The P. nitida seeds extract prevented also the development of hyperglycemia, hypercholesteremia in dexamethasone-induced insulin-resistant rats. So this extract could be a better choice and might be useful in the prevention of insulin resistance abnormalities associated with insulin resistance.
References
- Neuwinger, H.D., 1996. African ethnobotany: Poisons and drugs: chemistry, pharmacology, toxicology. Chapman and Hall, London, UK. p. 941
- Falodun, A., Nworgu, Z.A., and Ikponmwonsa, M.O., 2006. Phytochemical components of Picralima nitida Stapf and its effect on isolated nonpregnant rat uterus in oestrus. Pak J Pharm Sci, 19(3), pp. 256-258.
- Duwiejua, M., Woode, E., Obiri, D.D., 2002. Pseudo-akuammigine, an alkaloid from Picralima nitida seeds, has anti-inflammatory and analgesic actions in rats. J Ethnopharmacol, 81(1), pp. 73-79.
- N’guessan, K., Kouassi, K.E., and Kouadio, K., 2009. Ethnobotanical study of plants used in traditional medicine to treat diabetes, by Abbey and Krobou People of Agboville (Côte-d’Ivoire). Am J Sci Res, 4, pp. 45-58.
- Adegoke, B.M., and Oloyede, B.O., 2013. Antihyperglycemic and anti hyperproteinaemia activity of extracts of Picralima nitida seed and Tapinanthus bangwensis leaf on alloxan-induced diabetic rabbits. IJIAS, 3(4), pp. 1125-1131.
- Erharuyi, O., Falodun, A., and Langer, P., 2014. Medicinal uses phytochemistry and pharmacology of Picralima nitida (Apocynaceae) in tropical diseases: A review. Asian Pac J Trop Med, 7, pp. 1-8.
- Henry, T.A., and Sharp T.M., 1927. The alkaloids of Picralima klaineana. J Chem Soc, pp. 1950-1959.
- Henry, T.A., 1932. The alkaloids of Picralima klaineana. J Chem Soc, pp. 2759-2768.
- Thomas, A.F., 1954. D. Phil. Thesis, Oxford.
- Olivier, L., et al., 1962. Alcaloïdes du Picralima nitida Stapf: isolement d’un nouvel alcaloïde: la picraline. Ann Pharm Franc, 20, pp. 361-366
- Britten, A.Z., and Smith, G.F., 1963. Akuamma alkaloids. Part 6: The reactions of picraline. J Chem Soc., pp. 3850-3854.
- Ledouble., G., et al., 1964. Alcaloïdes du Picralima nitida. Etude des Feuilles et des racines. Isolement de deux alcaloïdes nouveaux: la picraphylline et la picracine. Ann Pharm Franc, 22, pp. 463-468.
- Ansa-Asamoah, R., et al. 1990. Picratidine, a new indole alkaloid from Picralima nitida. J Nat Prod, 53, pp. 975-977.
- Olivier, L., et al., 1965. Structure et configuration absolute de la picraline de la pseudoakuamigine, de l’akuammiine et de l’akuammiline. Bull Soc Chim France, pp. 868-876.
- Arens, H., et al., 1982. Detection of pericine, a new CNS-active indole, alkaloid from Picralima nitida cell suspension culture by opiate receptor binding studies. Planta Med, 46, pp. 210-214
- Nwaogu, L.A., 2016. Chemical profile of Picralima nitida seeds used in ethnomedicine used in West Africa. FUTOJNLS, 2(2), pp. 110- 122.
- Iwu, M.M., and Klayman, D.L., 1992. Evaluation of the in vitro antimalarial activity of Picralima nitida extracts. J Ethnopharmacol, 36, pp. 133-135.
- Kapadia, G.J., Angerhofer, C.K., and Ansa-Asamoah, R., 1993. Akuammine: an antimalarial indolemonoterpene alkaloid of Picralima nitida seeds. Planta Med, 59(6), pp. 565-566.
- Iwu, M.M., 1992. Evaluation of plant extracts for anti-leishmanial activity using a mechanism-based radiorespirometric microtechnique (RAM). Planta Med, 58, pp. 436-441.
- Ubulom, P.M.E., et al., 2012. Larvicidal and antifungal properties of Picralima nitida (Apocynaceae) leaf extracts. Eur J Med Plants, 2(2): 132-139.
- Ezeamuzie, I.C., et al., 1994. Anti-inflammatory, antipyretic and anti-malarial activities of a West African medicinal plant-Picralima nitida. Afr J Med Med Sci, 23(1), pp. 85-90.
- Menzies, J.R., et al., 1998. Opioid activity of alkaloids extracted from Picralima nitida (fam. Apocynaceae). Eur J Pharmacol, 350(1), pp. 101-108.
- Kouitcheu, L.B.M., Tamesse, J.L., and Kouam, J., 2013. The anti-shigellosis activity of the methanol extract of Picralima nitida on Shigella dysenteriae type I induced diarrhoea in rats. BMC, 13, p. 211.
- Nkere, CK., and Iroegbu, CU., 2005. Antibacterial screening of the root, seed and stem bark extract of Picralima nitida. Afri J Biotechnol, 4(6), pp. 522-526.
- Iroegbu, C.U., and Nkere, C.K., 2005. Evaluation of the antibacterial properties of Picralima nitida stem bark extracts. Intern J Mol Med Adv Sci, 1, pp. 182-189.
- Teugwa, C.M., et al., 2013. Antioxidant and antidiabetic profiles of two African medicinal plants: Picralima nitida (Apocynaceae) and Sonchus oleraceus (Asteraceae). BMC Complem Altern Med, 13, pp. 175.
- Yessoufou, A., et al., 2013. Anti-hyperglycemic effects of three medicinal plants in diabetic pregnancy: modulation of T cell proliferation. BMC Complement Altern Med, 13, p. 77.
- Inya-Agha, S.I., Ezea, S.C., and Odukoya, O.A., 2006. Evaluation of Picralima nitida: Hypoglycaemic activity, toxicity, and analytical standards. Intern J Pharmacol, 2(5), pp. 576-580.
- Aguwa, C.N., et al., 2001. Antidiabetic effect of Picralima nitida aqueous seed extract in experimental rabbit model. J Nat Remedies, 1(2), pp. 135-139.
- Nayak, I.M.N., 2017. Comparison of pioglitazone and metformin efficacy against glucocorticoid-induced atherosclerosis and hepatic steatosis in insulin-resistant rats. J Clin Diagn Res, 11(7), pp. FC06-FC10.
- Harnafi, H., et al., 2008. The hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum In triton WR-1339-induced hyperlipidemic mice. Food Chem, 108, pp. 205-212.
- Friedewald, W.T., Levy, R.I., and Fredrickson, D.S., 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem, 18(6), pp. 499-502.
- Mayer, P., 1896. Hematoxylin and eosin (H and E) staining protocol. Mitt Zool Stn Neapel, 12(303).
- Lambillotte, C., Gilon, P., and Henquin, J.C., 1997. Direct glucocorticoid inhibition of insulin secretion: An in vitro study of dexamethasone effects in mouse islets. J Clin Invest, 99, pp. 414-423.
- Igboasoiyi, A.C., et al., 2007. Screening of the seed of Picralima nitida for hypoglycaemic activity. Pak J Biol Sci, 10(5), pp. 828-830.
- Ajao, S.M., et al., 2009. Comparative study of the hypoglycemic effects of coconut water extract of Picralima nitida seeds (Apocynaceae) and Daonil in alloxan-induced diabetic albino rats. Afr J Biotechnol, 8(4), pp. 574-576.
- Webb-Leyden, J., 1966. Enzyme and metabolic inhibitors, Academic Press: New York, pp. 321-326.
- Robert, C., et al., 1999. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci, 96:513-523.
- Prashant, M., 2012. Effect of Psoralea corylifolia on dexamethasone-induced insulin resistance in mice. J King Saud Univ Sci, 24, pp. 251-255.
- Bhujbal, S.S., et al., 2012. Effect of Woodfordia. Fruticose on dexamethasone-induced insulin resistance in mice. Rev Bras Farmacogn, 22, pp. 611-616.
- Eason, J.M., 2000. Detrimental effects of short-term glucocorticoid use on the rat diaphragm. Phys Ther, 80, pp. 160-167.
- Kim, D.S., et al., 2002. Effect of chromium picolinate supplementation on insulin sensitivity, serum lipid, and body weight in dexamethasone-treated rats. Metabolism, 51, pp. 589-594.
- Shalam, M.D., Harish, M.S., and Farhana, S.A., 2006. Prevention of dexamethasone and fructose-induced insulin resistance in rats by SH-01D, a herbal preparation. Indian J Pharmacol, 38 (6), pp. 419-422.
- Moitra, J., et al., 1998. Life without white fat: a transgenic mouse. Genes Dev, 12(20), pp. 3168-3181.
- Poirier, H., 2005. Development of conjugated linoleic acid (CLA)-mediated lipoatrophic syndrome in the mouse. Biochimie, 8, pp. 73-79.
- Hall, A.P., et al., 2012. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes: conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol, 40, pp. 971-994.
- Hargrove, G.M., et al., 2000. Diabetes mellitus increases endothelin-1 gene transcription in rat kidney. Kidney Int, 58, pp. 1534-1545.
- Kumar, G.S., Shetty, A.K., and Salimath, P.V., 2008. Modulatory effect of bitter gourd (Momordica charantia Linn.) on alterations in kidney heparan sulfate in streptozotocin-induced diabetic rats. J Ethnopharmacol, 115, pp. 276-283.
- Dolatabadi, A, and Mahboubi, M., 2015. A study of the influence of dexamethasone on lipid profile and enzyme lactate dehydrogenase. J Med Life, 8(3), pp. 72-76.
- Safaeian, L., et al., 2018. The effects of hydroalcoholic extract of Allium elburzense Wendelbo bulb on dexamethasone-induced dyslipidemia, hyperglycemia, and oxidative stress in rats. Res Pharm Sci, 13(1), pp. 22-29.
- Clarke, B.A., et al., 2007. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab, 6, pp. 376-385,
- Waddell, D.S., et al., 2008. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab, 295(4), pp. E785-E797.
- Onoh, O.S., 2019. Chemoprotective Effect of methanol leaf extract of Picralima nitida on carbon tetrachloride-induced oxidative stress in albino rats (Master's Thesis, Federal University of Technology, Owerri).
- Donald, I.M., et al., 2016. Hepatoprotective potentials of Picralima nitida against in vivo carbon tetrachloride-mediated hepatotoxicity. J Phytopharmacol, 5(1), pp. 6-9.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences