ISSN : 2393-8854
Global Journal of Research and Review
A Review on Synthesis and Biological Evaluation of Plants Based Metallic Nanoparticles
Shama Parveen*, Garima Sharma, and S. B. Sharma
Department of Chemistry, Motherhood University, Haridwar, 247661 Uttrakhand, India
- *Corresponding Author:
- Parveen S
Department of Chemistry,
Motherhood University,
Haridwar, 247661 Uttrakhand,
India;
E-mail: shamahussainktw@gmail.com
Received Date: January 25, 2021;Accepted Date: February 08, 2021;Published Date: February 15, 2021
Citation: Parveen S, Sharma G, Sharma SB (2021) A Review on Synthesis and Biological Evaluation of Plants Based Metallic Nanoparticles. Glob J Res Rev Vol.8 No.S1: 002.
Abstract
Nanotechnology is a field of research and innovation concerned with building ’things’-generally, materials and devices on the scale of atoms and molecules. Nanotechnology has become very popular in the past few years. The development of synthesis of metallic nanoparticles has become a major focus of researchers. Metallic nanoparticles have become increasingly used because of their advantages including high stability and loding capacity. Nanoparticles can be synthesized chemically or biologically approaches. One of the most considered methods is the production of metal nanoparticles using organisms. Among these organisms, the plants seem to be the best candidates and are suitable for the large-scale synthesis of nanoparticles. Metallic nanoparticles that have immense application are industries of different type namely gold, silver, and copper, iron oxide and titanium magnetic, etc. the size of the nanoparticles are in the range of 1 to 100 nm. Characterization of synthesized nanoparticles is accomplished through UV spectroscopy, X-ray, FTIR, SEM, TEM, XRD, DLS and EDX. This review based on the recent scientific publications to synthesize metallic nanoparticles along with various methods for silver, copper and titanium metallic nanoparticles by using plants also the review explore the recent development in antibacterial, anticancer and biosensing application of metallic nanoparticles reported by the researchers during the last decades.
Keywords
Silver; Copper; Titanium oxide nanoparticles; Evaluation of biological activities
Introduction
This review is concerned with the synthesis of metallic nanoparticles using plant extracts and their biological evaluation. Plant based synthesis is useful not only because of its reduced environmental impact compared with some of the physicochemical production methods, but also because it can be used to produce large quantities of nanoparticles that are free of contamination and have a well-defined size and morphology. Plant based synthesis can actually provide Nanoparticles (NPs) of a better defined morphology and size than some of the physicochemical methods of production [1-11].
The ability of plant extracts to reduce metal ions has been known since the early 1900s, although the nature of the reducing agents involved was not well understood. In view of its simplicity, the use of plant extract for reducing metal salts to nanoparticles has attracted considerable attention within the last few years. Plant extract mediated synthesis of metallic nanoparticles is an increasing focus of attention because of the enhanced biological properties of plant mediated nanoparticles [12-32]. Nanoparticles are already used in numerous applications including in catalysis, biosensors, antimicrobial, photo thermal therapy and pharmaceutical production.
Characterization Techniques
Several techniques are employed for the physico-chemical and morphological characterization of the produced metallic nanoparticles. A detailed overview of the determined charactersticsand attribute of each morphological and physico- chemical chracterization technique is presented in Table 1 [33-45].
| Morphological and physico-chemical chracterization technique | Determined characterstics and attribute |
|---|---|
| Ultraviolet-visible spectroscopy (UV-vis) | Concentration and shape of NPs |
| Fourier Transform Infrared spectroscopy (FTIR) | Nature of bonds and functional groups |
| X-ray diffraction (XRD | Size and crystallinity of NPs |
| Scanning Electron Microscopy (SEM) | Shape, size and structure of nano-formulations |
| Field Emission Scanning Electron Microscopy (FESEM) | Structural and morphological characteristics |
| Transmission Electron Microscopy (TEM) | Shape, size and structure of nano-formulations |
| Particle Size Analysis (PSA) | Size distribution of solid or liquid particulate materials |
| Malvern Zetasizer (MZS) | Size, zeta potential, and protein mobility |
| Energy-dispersive X-ray spectroscopy (EDX/EDS) | Composition of NPs |
| Nanoparticle Tracking Analysis (NTA) | Particle size, concentration, and fluorescent properties |
| Nanoparticle Tracking Analysis (NTA) | Particle size, concentration, and fluorescent properties |
| Small-angle X-ray scattering (SAXS) | Shape and size conformation |
| X-ray Reflectometry (XRR) | Thickness, density, and roughness |
| X-ray fluorescence spectroscopy (XRF) | Chemical composition and concentration |
| Brunauer-Emmett-Teller analysis (BET) | Specific surface area |
| Selected Area Electron Diffraction (SAED) | Shape, size and structure of nano-formulations |
| Atomic Force Microscopy (AFM) | Particle size and surface characterization |
| Atomic Force Microscopy (AFM) | Particle size and surface characterization |
| Particle size and surface characterization | Amount of metal present in metallic nano-formulations |
Table 1: Determined characterstics and attribute of each morphological and physico-chemical chracterization technique [1].
Synthesis of nanoparticle
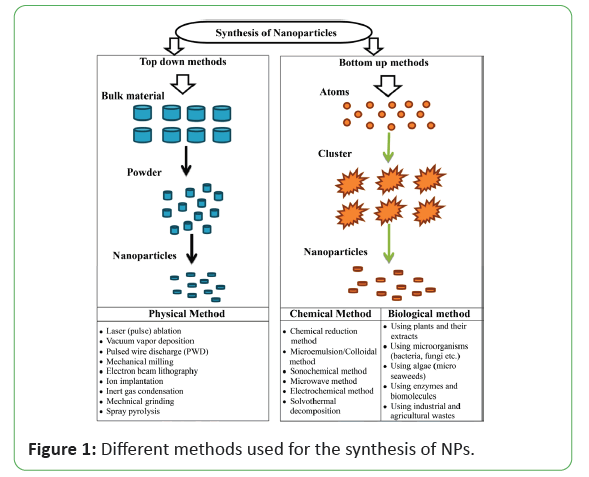
There are different physical and chemical methods for successfully synthesizing NPs. One can categorize all these methods into two main approaches that can apply to any research in the field of nanoscale science: (1) the top-bottom and (2) the bottom-top approach shown in Figure 1.
Each of which has specific characterization and application. The use of environmentally benign materials like plant extract bacteria, fungi and enzymes for the synthesis of metallic nanoparticles offer numerous benefits of eco-friendliness and compatibility for pharmaceutical and other biomedical applications as they do not use toxic chemicals for the synthesis protocol. In general, metallic nanoparticles are produced by two methods, i.e. "bottom-up" (buildup of material from the bottom: atom by atom, or molecule by molecule or cluster by cluster) and "top-down" (slicing or successive cutting of bulk material to get nano-sized particle). The "bottom-up" approach is usually a superior choice for the nanoparticles preparation involving a homogeneous system wherein catalysts (for instance reducing agent and enzymes) synthesize nanostructures that are controlled by the catalyst itself [46-55]. However, the "top-down" approach generally works with the material in its bulk form, and the size reduction to the nanoscale is achieved by specialized ablations, for instance thermal decomposition, mechanical grinding, etching, cutting, and sputtering. The main demerit of the top-down approach is the surface structural defects. Such defects have a significant impact on the physical features and surface chemistry of metallic nanoparticles of the biological methods of synthesis, the methods based on microorganisms have been widely reported [56-61]. Microbial synthesis is of course readily scalable, environmentally benign and compatible with the use of the product for medical applications, but production of microorganisms is often more expensive than the production of plant extracts. Plant mediated nanoparticle synthesis using whole plant extract or by living plant were also reported in literature has been studied [62-69].
Use of plant extracts in nanoparticle synthesis
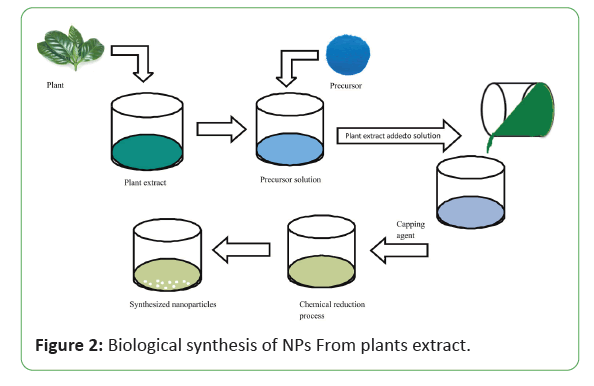
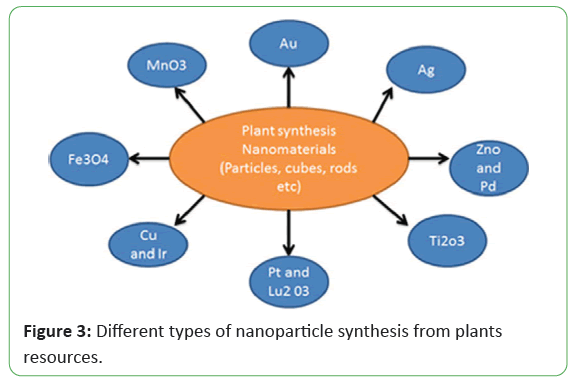
In these days, the emphasis has been shifting toward the synthesis of NPs by using plants and plant extracts to gain the advantages of this method shown in Figure 2. Many scientists used different plant extracts to prepare different type of NPs in Figure 3 with different sizes and shapes depending upon the synthesis conditions.
Synthesis of Ag-NPs from plant extract
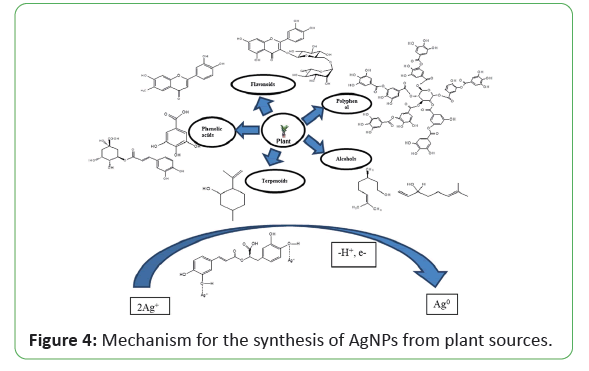
Illustrated that the first approach of using plants for the synthesis of metallic NPs was done by using Alfalfa sprouts, which was the first description about the synthesis of Ag-NPs using living plant system. A plant mediated nanoparticles synthesis using whole plant extract or by the living plants were also studies in the literature studied that Acorus calamus extract can be used as capping agent for the synthesis of Ag-NPs to evaluate its oxidation state, anticancer, and antibacterial effect. The spherical Ag-NPs have been synthesized using the extract of Abutilon indicum and also studied their high antimicrobial activity against S. typhi, E. coli, S. aureus, and B. subtilis microorganism by illustrated that Ag-NPs are synthesized by peanut shell extract and their characteristics and antifungal activity is compared with commercial Ag-NPs. UV-Vis spectra, XRD peaks, and FTIR confirmed that synthesized and commercial NPs are similar and HRTEM results indicate that NPs were mostly spherical and oval in shape with an average diameter up to 10-50 nm. In another method, spherical Ag- NPs were also synthesized by using the fruit extract of Malus domestica as capping agent with an average diameter of 20 nm. The formation of NPs is analyzed by UV–Vis spectroscopy, distinctive phases and morphology are confirmed by using XRD and TEM, and FTIR is used to identify the biomolecules which are responsible for reduction and stabilization of NPs reported that Ag-NPs are synthesized using the extracts of Boerhaavia diffusa, XRD and TEM results showed an average size 25 nm having face-centered cubic geometry with spherical shape. These NPs were used for antibacterial action against three fish bacteria, namely Pseudomonas fluorescens, Aeromonas hydrophila, and Flavobacterium reprted Ag-NPs with an average size of 15-22 nm by using reishi mushroom (Ganoderma lucidum) extract reported that Origanum vulgare L. plant extract can be used as capping agents for the synthesis of AgNPs to evaluate its oxidation state. Synthesized Ag-NPs characterized by various microscopic and spectroscopic techniques and results indicated the formation of crystalline face-centered cubic. It is found that green synthesis using plant and plant extracts appears to be faster than other microorganisms, such as bacteria and fungi [70-85]. The use of plant and plant extracts in green synthesis has drawn attention because of its rapid growth, providing single step technique, economical protocol, non-pathogenic, and eco-friendly for nanoparticles synthesis. The different parts of plant such as stem, root, fruit, seed, callus, peel, leaves and flower are used to syntheses of metallic nanoparticles in various shapes and sizes by biological approaches (Figure 4).
Different sizes and shapes of silver, copper and titanium oxide nanoparticles derived from plants resources. Biosynthesis reaction can be altered by wide range of metal concentration and amount of plant extract in the reaction medium; it may transform the shape and size of the nanoparticles [86-88]. Other plant species which has been used for the synthesis of Ag-NPs shown in Table 2.
| Plant name | Plant part | Size (n.m.) | Shape | Reference |
|---|---|---|---|---|
| Tephrosia tinctonia | Stem | 73 | Spherical | [7] |
| Grewia flavis cences | Leaf | 50-70 | Hexagonal | [8] |
| Skimmia laureola | Leaf | 46 | Spherical | [9] |
| Clerodendrum serratum | Leaf | 35 | Spherical | [10] |
| Averrhoa carambola | Leaf | 14 | spherical | [11] |
| Crataegus microphylla | Fruit | 30-50 | Spherical | [12] |
| Reishi mushroom | Plant extract | 15-22 | Spherical | [13] |
| Gymnema sylvestre | Leaf | 20-30 | Spherical | [14] |
| Cucurbita pepo | Leaves | 5-100 | Spherical | [15] |
| Tragopogon collinus | Leaf | 7 | Nano crystals | [16] |
| Musa paradisicia | Leaf | 20-30 | Spherical | [18] |
| Tribulus terristris | Fruit | 16-28 | Spherical | [19] |
| Ziziphus jujube | Leaf | 20-30 | Crystalline | [20] |
| Allium ampeloprasum | Leaf | 2 and 43 | Quasi-spherical, spherical, ellipsoidal, hexagonal and irregular. | [21] |
| Enhydra fluctuans | Plant extract | 100-400 | Spherical | [22] |
| Gliricidia sepium | Plant extract | Oct-50 | Spherical | [23] |
| Eucalyptus citriodora | Plant extract | -20 | Spherical | [24] |
| Gliricidia sepium | Plant extract | Oct-50 | Spherical | [25] |
| Ficus benghalensis | Leaf | -16 | Spherical | [26] |
| Pistacia atlantica | Seed | Oct-50 | Spherical | [27] |
| Ocimum tenuiflorum, | Plant extract | 28 | Irregular | [28] |
| Solanum tricobatum, | Plant extract | 26.5 | Irregular | [29] |
| Ziziphora tenuior | Extract | 38 | Cubic | [30] |
| Fiscus carica | Leaves | 13 | Spherical | [31] |
| Cymbopogan citratus | Leaves | 32 | Spherical | [32] |
| Acalpia indica | Leaves | 0.5 | Spherical | [33] |
| Premnna herbacea | Leaves | 0-30 | Spherical | [34] |
| Calotropus procera | Plant | 19-45 | Spherical | [35] |
| Centella asiatica | Leaves | 30-50 | Spherical | [36] |
| Argyreia nervosa | Seed | 20-50 | Spherical | [37] |
| Psorelia corylilifolia | Seeds | 100-110 | Spherical | [38] |
| Brassica lappa | Leaves | 16.4 | Spherical | [39] |
| Melia dubia | Leaves | 35 | Spherical | [40] |
| Memeceylon edule | Leaves | 20-25 | Triangular, circular, hexagonal | [41] |
| Datura metal | Leaves | 16-40 | Quasi linear | [42] |
Table 2: Plant mediated synthesis of silver nanoparticles.
Synthesis of Cu-NPs from plants extract
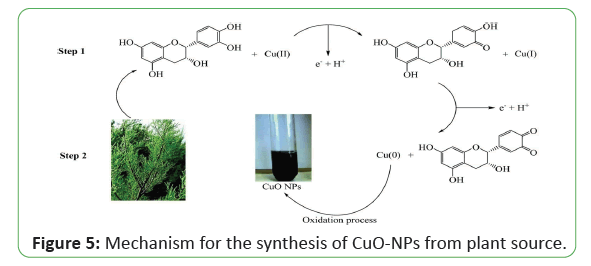
Plant-based polyphenols are considered to be the largest groups of natural antioxidants with extraordinary potential as drugs, nutraceuticals, and food additives. The underlying principle in the green synthesis approaches is that the phytochemicals present in the plant parts serve the twin role of a natural reductant besides being a nanoparticle stabilizer (Figure 5). According to reports, highly stabilized NPs may be quickly synthesized from plant extracts rather than microbebased synthesis. Therefore, the plant extract could be an efficient approach for reducing NPs early material plus stabilized that. For example, an interesting study by S. Renganathan and his coworkers studied that Cu-NPs were synthesized using leaf extract from Capparis zeylanica as a reducing agent in aqueous CuSO4 solution. The NPs were fabricated for 12 h which were cubical in shape with size range 50-100 nm. The antimicrobial study of the Cu-NPs was established using both gram positive and gram negative pathogens (as Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa) reported the green approach of CuO-NPs synthesis using Calotropis procera which belongs to the asclepiadaceous family. These NPs are widely used in many applications such as catalysis because of their narrow band gap, used in photo catalytic properties (Figure 6). In another study, reported the green synthesis of copper NPs using Punica granatum peels extract. Punica granatum fresh peels are obtained and washed for several times. After peels dried, they turned into powder and mixed with sterile distilled water and boiled until the color of solution change to yellow. To synthesize CuO-NPs, copper acetate powder was dissolved in the water and stirred with magnetic stirrer. After that, P. granatum extract was added to the solution at the first step, the color of solution turned to green and then it turned to brown which shows the formation of monodispersed Cu-NPs. and reported the green synthesis of copper NPs from fresh leaves of Abutilon indicum. The fresh leaves of Abutilon indicum were collected and washed gently to remove dust particles as well as dried and shaded parts. Next, the leaves were pulverized and seived using a 200 nm mesh sieve to be used as fuel. To synthesize CuO NPs, Copper (II) nitrate trihydrate was mixed with Abutilon indicum extract in double-distilled water. The solution was homogenized for 2-5 min with constant stirring using a magnetic stirrer. Next, combustion reaction was performed on the mixture using a pre-heated muffle furnace at the temperature 400 ± 5 C to produce CuO-NPs. The resultant mixture was filtered to remove the ash contents of the plant extracts. The solution was washed with distilled water, followed by methanol to remove impurities. In another methods M. M. S. R. Subbaiya studied that Hibicus rosa-sinensis leaf extract can be used to reduce the CuNO3 solution, then the solution was placed in a dark room for 48 h and spherical Cu-NPs were prepared. Cu-NPs showed good antimicrobial activity against clinically important pathogens like Bacillus subtilis and E. coli. It is also illustrated that the synthesized Cu-NPs were acting as an effective. Haneefa and co-authors synthesized Cu-NPs using lemon solution as a reducer and curcumin as an additive under certain conditions. Experimental outcomes revealed that size of Cu-NPs was observed in the range of 60-100 nm with nearly spherical shape. Antimicrobial activity of Cu-NPs is more excellent as compared to standard drug against both bacterial and fungus species like S. aureus, B. subtilis and Candida albicans. Similar inhibition response was practically noted against E. coli, S. bacillus (bacterial species) and Cochliobolus lunata, Aspergillus niger (fungus species). From this discussion it can be summarized that the synthesized Cu-NPs are prominent in antibacterial and antifungal studies which may be found in various applications of medicine used papaya extract to synthesize Cu-NPs at 50-60° C under constant stirring for 1 h. It has been concluded that the green synthesized Cu-NPs are nearly spherical and crystalline. The average particles size of Cu-NPs is around 20 nm. Shende and his coworkers reported Cu-NPs synthesis with an average size of 20 nm by using Citron juice (Citrus medica Linn.). The synthesized Cu-NPs showed a significant inhibitory response against E. coli followed by Klebsiella pneumoniae, Pseudomonas aeruginosa, Propioni bacterium acnes and Salmonella typhi. Regarding pathogenic fungi testes, Fusarium culmorum was observed to be the most responsive followed by Fusarium oxysporum and Fusarium graminearum. Suresh studied the green synthesis of tea decoction stabilized Cu-NPs at room temperature which are stable in air with respect to oxidation for about 25 days because of the thin layer of tea decoction molecules surrounded by the NPs. The average particle size is found to be around 11 nm. Further Subhankari and Nayak reported the production of Cu-NPs using aqueous extract of Syzygium aromaticum (Cloves). Copper sulfate was reduced with aqueous solution of clove extracts in 1 h and 5–40 nm spherical Cu-NPs were obtained. Kulkarni studied that the leaf extract of Ocimum sanctum can make a reduction of Cu cations into Cu-NPs within 8–10 min. So this technique can be applied for rapid and eco-friendly synthesis of Cu-NPs. Sampath and his fellows synthesized bud-shaped Cu-NPs using a green reduction method where polyvinyl pyrrolidone (PVP), L-Ascorbic Acid (AA) and isonicotinic acid hydrazide (INH) are used as a capping agent, antioxidant agent and reducing agent, respectively and water as a solvent at 60-700 C (pH-7) in the presence of air and the average particle size was observed as 6.95 nm. Antibacterial study of these Cu-NPs was analyzed by measuring response against Gram negative (E. coli) and Gram positive (S. aureus) bacteria. In another study by it was observed that datura meta leaf extract can reduce CuSO4 solution into Cu-NPs with size 5 nm in 8-10 min. These NPs have excellent antimicrobial response compared to standard Chloramphenicol, hence used as antimicrobial agent. Kundu studied the synthesis of Cu-NPs using Citrus sinensis (orange) extract in CuSO4. The mixture was then incubated in a rotary shaker at 55 C for about 3 h. Manikandan and Sathiyabama synthesized Cu-NPs by adding acidic chitosan solution to CuSO4 solution with stirring for 12 h at 70 C. Transmission Electron Microscopy (TEM) showed the homogenous spherical size of NPs in the range of 20-30 nm and showed the antibacterial response against gram negative as well as gram positive bacteria. However, antibacterial response was more prominent against the gram negative bacteria which may be based on the variation in composition of cell wall. Recently biological synthesis of Copper oxide nanoparticles (CuO-NPs) was performed using S. acuta leaf extract CuO-NPs were synthesized and characterized using UV-vis, FTIR, SEM and TEM analyses. Synthesized CuO-NPs was tested against Gram negative (Escherichia coli and Proteus vulgaris) and Gram positive (Staphylococcus aureus) pathogens, which showed zones of inhibition at different concentrations. It is also investigated that these CuO-NPs showed effective photocatalytic activity against commercial dyes. The synthesis of spherical copper nanoparticles using zingiber officinale, piper nigrum and piper longum extract has been studied [89-110]. The synthesized copper nanoparticles were characterized using UV-Visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), x-ray diffraction (XRD), field emission gun scanning electron microscopy (FEG-SEM) with EDS and high-resolution transmission electron microscopy (HR- TEM). The size of these synthesized nanoparticles was found 15- 30 nm. These nanoparticles show antimicrobial activity against micro organisms like against Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 737), Pseudomonas aeruginosa (MTCC 1681) and Escherichia coli (MTCC 1687). In another methods Developed copper nanoparticles Cu-NPs from copper salt (CuCl) solution by using Camelia sinensis leaf extract. These synthesized nanoparticles characterized by using FTIR, SEM, TEM, EDX analysis and average size of syntesized nanoparticles 60 ± 6 nm. CuO-NPs was evaluated by using bromophenol blue (BPB) dye under sunlight irradiation. The maximum photo degradation of BPB dye was up to 83.7%. Uv-vis spectral analysis of Cu-NPs proved them as an efficient photo catalyst in dye degradation reported that face-centered cubic and spherical copper nanoparticle were synthesize by using Uncaria gambir Roxb. Leaves extract. These nanoparticles were characterized by UV- Visible Spectrophotometry, X-ray Diffraction, Fourier transform, Infrared, and Transmission Electron Microscopy analysis. X-ray Diffraction pattern performed three sharp peaks specifically referred to face-centered cubic structured of metallic copper. Transmission Electron Microscopy revelated that the size of nanaoparticles in diameter of 2.5-15 nm. The formation of copper oxide nanoparticles (CuO-NPs) by Ailanthus altissima leaf aqueous extract was reported these synthesized copper oxide nanoparticles were characterized by UV-vis, SEM, TEM, FT-IR analysis tools. CuO-NPs nanoparticles were well crystalline in nature with particle shape spherical and average particle size 20 nm and antimicrobial activity of these nanoparticles was determined by disk diffusion method against some selected species of bacteria, demonstrated a significant inhibitory activity against S. aureus followed by E. coli [111-123].
Many researcher has been reported the usage of different plant extracts to prepare copper and copper oxide nanoparticles such as Nerium oleander Leaf aqueous extract, peel extract of Punica granatum, fruit extract of Ziziphus spina-christi, Rosa canina fruit extract, fruit extract of Syzygium alternifolium (Wt.) Walp and Asparagus adscendens Roxb. root and leaf extract, some of which might be not cost effective or not easily available and other plants which has been used for the synthesis of copper nanoparticles shown in Table 3.
| Plant name | Plant part | Size (n.m.) | Shape | Reference |
|---|---|---|---|---|
| Calotropis procera L. | Latex | 15+1.7 |
Polydispers and spherical | [56] |
| Psidiumguajava L. | Leaves | 13.13-0.19 |
Spherical | [57] |
| Aloe vera F. | Fruit | 40 |
Spherical | [58] |
| Capparis zylanica | Leaves | 50-100 |
Cubical | [59] |
| O. sanctum | Leaves | 77 |
FCC | [60] |
| Datura metal | Leaves | 15-20 |
Spherical, hexagonal etc. | [61] |
| Citrus reticulata | Peel | Oct-40 |
Spherical | [62] |
| Citrus medica | Extract | Oct-60 |
FCC | [63] |
| Eclipta prostrata | Leaf | 23-57 |
FCC | [64] |
| Punica grantatum | Plant extract | 56-59 |
Spherical | [65] |
| Tilia | Aqueous extract | 4.7-17.4 |
Hemispherical | [66] |
| Uncaria gambis ROXB | Leaf | 2.5-15 |
Spherical | [67] |
| Lemon grass | Leaf | 5.67-9.10 |
Spherical | [68] |
| Ginger officinale | Mixing leaf extract | 15-30 |
Crystalline | [69] |
| Piper longer |
|
|||
| Piper nigrum |
|
|||
| Solanum lycopersicum | Extract | 40-70 |
Crystalline | [70] |
| Rhuscoriaria L. | Fruit | 22-27 |
Crystalline | [71] |
| Curcuma langa | Extract | May-20 |
Crystalline | [72] |
| Catharanthus roseus | Leaf | ___ |
Crystalline | [73] |
| Ailanthus altissima | Leaf | 20 |
Crystalline | [74] |
| Eringium caucasicum | Aqueous extract | 40 |
Crystalline | [75] |
| Enicostemma axillary (Lim.) | Leaf | 30 |
Crystalline | [76] |
| Gloriosa superb L. | Leaf | 05-Oct |
Monoclinic | [77] |
| Arevalanata | Leaves | 40-100 |
Spherical | [78] |
| Saraca indica | Leaf | 40-70 |
Spherical | [79] |
| Carica papaya | Leaves | 140 |
Rod shape | [80] |
| Osmium basilicum | Plant extract | 70 |
Spherical | [81] |
| Euphorbia esula L. | Leaves | 40 |
Face centered cubic | [82] |
| Cassia fistula | Fruit | 20-000 |
Amorphous | [83] |
| Aqueous ginkgo biloba | Leaf extract | 15-20 |
Spherical | [84] |
| Aqueous broccoli | Extract | 4.8 |
Spherical | [85] |
| Punica granatum | Seed extract | 40-80 |
Spherical | [86] |
| Plantago asiatica | Leaf extract | Jul-35 |
Spherical | [87] |
| Ixora coccinea | Leaf | 167.1 |
Spherical | [88] |
| Tridax procumbens | Leaf | 85-118 |
Spherical and monodispersed | [89] |
| Pineapple | Leaf extract | 30-50 |
Cubic | [90] |
| Tridax procumbens | Leaves | 87 |
Spherical | [91] |
| Triumfettarotundifolia | Plant extract | 12.46 |
Like triangle | [92] |
| Syzygium aromaticum | Bud | 12 |
Spherical | [93] |
| Acanthophyllum Laxiusculum schiman-czeika | Roots | 20-25 |
Spherical | [94] |
| Ageratina altissima[L.]R.M.king and H.Rob. | Leaves | 60-100 |
Spherical | [95] |
| Annona squamosa L. | Peel | 23 |
Polydispersed | [96] |
| Calotropis gigintae L. Dryand | Flower | 10 |
Spherical | [97] |
| Catharanthus | Leaves | 25 |
Cluster | [98] |
| Senna auriculata (L.) roxb. | Leaves | 38 |
Spherical | [99] |
| Cicer aerentium L. | Seed | 14 |
Spherical | [100] |
| Cinamomum tamla (Buch-Ham.) T.Nees | Leaves | Aug-20 |
Tetragonal | [101] |
| Citrus sinensis (L.) osbeck | Peel | 19 |
Tetragonal | [101] |
| Curcuma longa L. | Wp | 120 |
Polydisperse | [102] |
| Cynodon dactylon (L.) pers. | Leaves | 13-34 |
Hexagonal | [103] |
| Eclipta prostrate (L.) L. | Leaves | 36-68 |
Spherical | [104] |
| Ephorbia prostrate aition | Leaves | 83.22 |
Polydisperse | [105] |
| Hibiscus rosasinensis | Flower extract | 24.89 |
Crystalline | [106] |
| Jatropha curcas L. | Latex | 20-100 |
Spherical | [107] |
| Morinda citrifolia L. | Leaves | 15-19 |
Spherical | [108] |
| Moringa olifera lam. | Leaves | 100 |
Spherical | [109] |
| Nyctanthes arbor – tristis L. | Leaves | 100 |
Spherical | [111] |
| Ocimum basilicum L. | Leaves | 50 |
Hexagonal | [101] |
| Piper betle | Leaves | 7 |
Spherical | [112] |
| Psidium juajava L. | Leaves | 32.58 |
Spherical | [113] |
| Dandelion | Pollen | ___ |
Rod | [114] |
| Trigonella foenum –graecum L. | Leaves | 20-90 |
Spherical | [115] |
Table 3: Plant mediated synthesis of copper oxide nanoparticles.
Synthesis of titanium dioxide nanoparticles from plant extract
Nowadays the biosynthesis of nanoparticles has been used in a wide range by a synthetic route using plants. Plant based of Metallic Nanoparticles (MNPs) titanium dioxide nanoparticles (Tio2-NPs) synthesis is cost effective, eco-friendly and energy efficient. Researchers have exploited the potential of biological resources for the synthesis of Tio2-NP a simple precursor salt is mixed with biological extract; the metabolites present in the extract can then reduce and stabilize the bulk metal into elemental form following various mechanical steps. This biosynthetic approach offers many advantages and has emerged as a simple, safe and feasible substitute to chemical and physical methods apart from these, biological approach can effectively catalyze the synthesis process at any scale and condition. Moreover, NPs with controlled size and shape can also be produced. Owing to these benefits, numerous researchers have intended to explore diverse species for their potential to synthesize Tio2-NPs).
In the biological species, plants are considered as one of the most suitable candidates for the synthesis of NPs as they are cost effective, safe and easily available. A diverse array of compounds in plants (phenolic acids, alkaloids, proteins and among them enzymes, as well as carbohydrates) regulate through reduction and stabilization processes the synthesis of NPs. Numerous plants species have been used for synthesis of various shapes of Tio2- NPs show in Table 4. The reaction mixtures start vigorously when a precursor Tio2 salt is adul terated with plant extract; color change indicates the first sign of synthesis that can then be confirmed after wards by spectroscopic techniques. Several color indicators have been reported ranging from light green to dark green in the formation of Tio2-NPs Dobrucka. Majority of green synthesis studies have been conducted on leaves extracts, as it is a rich source of metabolites. Spherical Tio2-NPs resulted when Annona squamosa L. was added to the aqueous solution of Tio2 salt at room temperature.
| Plant name | Part | Size (n. m.) | Shape | Reference |
|---|---|---|---|---|
| Acanthophyllum Laxiusculum schiman-czeika | Roots | 20-25 | Spherical | [94] |
| Ageratina altissima[L.]R.M.king and H.Rob. | Leaves | 60-100 | Spherical | [95] |
| Annona squamosa L. | Peel | 23 | Polydispersed | [96] |
| Calotropis gigintae L. Dryand | Flower | 10 | Spherical | [97] |
| Catharanthus | Leaves | 25 | Cluster | [98] |
| Senna auriculata (L.) roxb. | Leaves | 38 | Spherical | [99] |
| Cicer aerentium L. | Seed | 14 | Spherical | [100] |
| Cinamomum tamla (Buch-Ham.) T.Nees | Leaves | Aug-20 | Tetragonal | [101] |
| Citrus sinensis (L.) osbeck | Peel | 19 | Tetragonal | [101] |
| Curcuma longa L. | Wp | 120 | Polydisperse | [102] |
| Cynodon dactylon (L.) pers. | Leaves | 13-34 | Hexagonal | [103] |
| Eclipta prostrate (L.) L. | Leaves | 36-68 | Spherical | [104] |
| Ephorbia prostrate aition | Leaves | 83.22 | Polydisperse | [105] |
| Hibiscus rosasinensis | Flower | 24.89 | Crystalline | [106] |
| Jatropha curcas L. | ||||
| Morinda citrifolia L. | Leaves | 15-19 | Spherical | [108] |
| Moringa olifera lam. | Leaves | 100 | Spherical | [109] |
| Nyctanthes arbor – tristis L. | Leaves | 100 | Spherical | [111] |
| Ocimum basilicum L. | Leaves | 50 | Hexagonal | [101] |
| Piper betle | Leaves | 7 | Spherical | [112] |
| Psidium juajava L. | Leaves | 32.58 | Spherical | [113] |
| Dandelion | Pollen | ----___ | Rod | [114] |
| Trigonella foenum –graecum L. | Leaves | 20-90 | Spherical | [115] |
Table 4: Detailed view of plant names, parts and their size in nm.
Furthermore, a wide propitious plant extracts are left to be explored for the synthesis of Tio2-NPs. Subhapriya and Gomathi has been studied the biosynthesis of Tio2 nanoparticles (Tio2- NPs) was attained by a chemical and bio-synthesized method by using the aqueous leaf extract of Trigonella foenum-graecum (TF-Tio2-NPs). FTIR, UV, XRD, HR-TEM, and HR-SEM methods was used for characterization of Tio2-NPs. The X-ray diffraction displayed the existence of TF-Tio2-NPs which is confirmed by the incidence of peaks at 25-28 corresponds to anatase form. HR-SEM perceptions revealed that synthesized Tio2-NPs were spherical in shape and the size of individual nanoparticles as well as a few aggregates was found to be 20-90 nm antimicrobial activities of biosynthesized nanoparticles (TF-Tio2-NPs) were examined using Kirby-Bauer method. The TF-Tio2 nanoparticles showed significant antimicrobial activity against all the tested microorganisms. In another methods studied that Tio2-NPs were synthesized by using a plant extract of H. the lbiecea and Ananos seneglensi and characterization studied was done by UV-vis spectroscopy, Scanning Electron Microscopy (SEM), X-Ray diffraction (XRD) and Fourier Transmission infrared spectroscopy (FTIR). SEM revealed that the synthesized Tio2-NPs are spherical and crystalline in nature with average sizes of 40 and 50 nm for H. thelbiecea and Ananos respectively. Both two plants showed good antimicrobial activity against clinically important pathogens. Antimicrobial study of Tio2-NPs shows that 20 μg/ml Tio2-NPs is effective for complete inactivation of Gram positive, Gram negative as well as fungal cultures [124-133]. Studied that herbal plant extract of Cassica fistula can be used as a capping agent for the synthesis of titanium doxide nanoparticles (Tio2- NPs). Spherical synthesized titanium doxide nanoparticles were characterized by SEM, TEM, FTIR, AFM, TGA. These nanoparticles showed antibacterial activity against Escherichia coli and staphylococcus aureus.
Evaluation of Biological Activity of Silver, Copper and Titanium Dioxide Nanoparticles
It has been reported that materials properties influenced by the structure of the materials. Metallic nanoparticles have the most favorable antibacterial properties because of their large surface area to volume ratio, and it is the foremost interest of researchers because of microbial resistance, which is growing against metal ion, resistant strains development and antibiotics. Amongst all noble metal nanoparticles, silver nanoparticles attained a lot of interests because of its best conductivity, chemical stability, anti-viral, antifungal and antibacterial activities which can be consolidated in the form of complex fibers, superconducting materials, electronic components, and cosmetic products. The scientific community has much interest in the synthesis of silver nanoparticles because of multiple types of applications. Silver nanoparticles are efficiently used in cancer diagnostics and treatment. It is the best way to synthesize nanoparticles as it avoids hazardous chemicals and gives natural agents for capping of silver nanoparticles to reduce the cost of microorganisms. Hence, this review compiled literature about the production of silver, copper and titanium oxide nanoparticles having the best biological activities. The biomedical application of synthesized NPs is significantly becoming more important due to their antibacterial, antifungal, anti-cancer, and biosensor.
Evaluation of antimicrobial activity
Antimicrobials are typically liquids. Antimicrobial liquids kill or inhibit the growth of microorganisms such as bacteria, fungi and protozoans. Antimicrobial drugs (e.g. penicillin) are selective and kill microbes (microbiocidal) or prevent their growth (microbiostatic). Disinfectants are non-selective antimicrobial substances (e.g. bleach) and are used on non living objects or the outside of the body. With the emergence and increase of microbial organisms resistant to multiple antibiotics and the continuing emphasis on health-care costs, many researchers have tried to develop new effective antimicrobial reagents free of resistance and cost. The most important problem caused by the chemical antimicrobial agents is multidrug resistance. According to in vitro antimicrobial studies, the metallic nanoparticles effectively obstruct the several microbial species. The antimicrobial effectiveness of the metallic nanoparticles depends upon two important parameters: (a) material employed for the synthesis of the nanoparticles and (b) their particle size. Over the time, microbial resistance to antimicrobial drugs has become gradually raised and is therefore a considerable threat to public health. For instance, antimicrobial drug resistant bacteria contain methicillin-resistant, sulfonamide-resistant, penicillin- resistant, and vancomycin-resistant properties. Antibiotics face many current challenges such as combatting multidrug-resistant mutants and biofilms. The effectiveness of antibiotic is likely to decrease rapidly because of the drug resistance capabilities of microbes. Hence, even when bacteria aretreated with large doses of antibiotics, diseases will persist in living beings. Biofilms are also an important way of providing multidrug resistance against heavy doses of antibiotics. Drug resistance occurs mainly in infectious diseases such as lung infection and gingivitis.
Evaluation of antimicrobial activity of silver nanoparticles
The fight against infections is as old as civilization. Silver, for instance had already been recognized in ancient Greece and Rome for its infection-fighting properties and it has a long and intriguing history as an antibiotic in human health care. Modern day pharmaceutical companies developed powerful antibiotics which also happen to be much more profitable than just plain old silver. Silver nanoparticles are the most admired inorganic nanoparticles, and they are utilized as efficient antimicrobial, antifungal, antiviral, and antiinflammatory agents. According to a literature survey, the antimicrobial potential of silver nanoparticles can be described in the following ways: (1) denaturation of the bacterial outer membrane (2) generation of pits/gaps in the bacterial cell membrane leading to fragmentation of the cell membrane, and interactions between Ag NPs and disulfide or sulfhydryl groups of enzymes disrupt metabolic processes; this step leads to cell death. The shape-dependent antimicrobial activity was also examined. According to truncated triangular nanoparticles are highly reactive in nature because their high-atom-density surfaces have enhanced antimicrobial activity explained the antibacterial activity of In situ synthesis of nanosilver on cotton using Tollen's reagent. Two bacteria, Staphylococcus aureus, and Escherichia coli were used to test the antibacterial activity of silver nanoparticles. Acalypha indica (Euphorbiaceae) leaf extracts have produced silver nanoparticles (20-30 nm) within 30 min. These nanoparticles had excellent antimicrobial activity against water borne pathogens E. coli and V. cholera (minimum inhibitory concentration (MIC)=10 mg ml- 1). Furthermore, spherical silver nanoparticles (40-50 nm) were produced using leaf extract of Euphorbia hirta (E. K. Elumalai). These nanoparticles had potential and effective antibacterial property against Bacillus cereus and S. aureus. In another study, silver nanoparticles (with an average size of 57 nm) were produced when 10 ml of Moringa oleifera leaf extract was mixed to 90 ml of 1 m Maqueous of AgNO3 and was heated at 60-80°C for 20 min. The formed nanoparticles had considerable antimicrobial activity against pathogenic microorganisms, including S. aureus, Candida tropicalis, K. pneumoniae, and C. krusei. It has been reported that cotton fibers loaded with biosynthesized silver nanoparticles (~20 nm) using natural extracts of Eucalyptus citriodora (neelagiri) and Ficus bengalensis (marri) had excellent antibacterial activity against gram-negative E. coli bacteria. These fibers have potential for utilizatio ninburn/wound dressings as well as in the fabrication of antibacterial textiles and finishings. Raundra Garcinia mangostana (mangosteen) leaf extract could be used as reducing agent in order to synthesize silver nanoparticles. The aqueous silver ions when exposed to leaf extract were reduced and resulted in silver nanoparticles with an average size of 35 nm.These nanoparticles had high effective antimicrobial activity against E. coli and S. aureus, Veerasamy). It was reported that Ocimum sanctum (tulsi) leaf extract could reduce silver ions into crystalline silver nanoparticles (4-30 nm) with in 8 min of reaction time. These nanoparticles were stable due to the presence of proteins which may act as capping agent. Ocimum sanctum leaves contain ascorbic acid which may play a role in bio-reduction of silver ions into metallic nanoparticles. Bio- synthesized silver nanoparticles have shown strong antimicrobial activity against both gram-negative (E.coli) and gram-positive (S. aureus) microorganisms. Singhal Furthermore, biosynthesis of silver nanoparticles by Cacumen platycladi extract was investigated. Reducing sugars and flavonoids in the extract were mainly responsible for the bioreduction of the silver ions and their reductive capability promoted at 90°C, leading to the formation of silver nanoparticles (18.4 ± 4.6 nm) with narrow size distribution. The nanoparticles had significant antibacterial activity against E. coli and S. aureus elucidated that Ag-NPs exhibited destabilization of the outer membrane and rupture of the plasma membrane, thereby causing depletion of intracellular ATP. Silver has a greater affinity to react with sulfur or phosphorus-containing biomolecules in the cell. Thus, sulfur-containing proteins in the membrane or inside the cells and phosphorus-containing elements like DNA are likely to be the preferential sites for silver Nanoparticle binding. In another Jasmine Kaur and Kulbhushan reported that Ag-NPs control bacterial growth in a variety of applications including dental work, catheters and burn wounds and anti-microbial activity of silver nanoparticles was evaluated against E.coli and S.typhii by colony counting method. Metabolic activity was also determined by MTT assay utilized aqueous Raphanus sativus root extract as a reducing and capping agent for the synthesis of silver nanomaterials for the first time and utilized the supernatant of the fungus strain Penicillium aculeatum Su1 to synthesize extracellular Ag-NPs and Jalal et al. studied the extracellular green synthesis of Ag-NPs using the supernatant of Candida glabrata isolated from oropharyngeal mucosa of human immuno deficiency virus (HIV) patients and evaluated them for antibacterial and antifungal potential against human pathogenic bacteria and fungi reported the biosynthesis of Ag-NPs using yeast strains synthesized Ag-NPs utilizing the culture supernatant of phenol degraded broth as the reducing agent and Ishida et al. studied the synthesis and antifungal activity of Ag-NPs synthesized utilizing the aqueous extract of the fungus Fusarium oxysporum. In another investigated antibacterial activity of copper nanoparticles was studied against micro organisms like against Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 737), Pseudomonas aeruginosa (MTCC 1681) and Escherichia coli (MTCC 1687) Kamrun and Shahin reported antimicrobial activity using cinamomum tamla leaf extract and its antimicrobial activity. Potential antimicrobial application against clinically isolated multidrug-resistant bacterial strains was reported by das.
Evaluation of antimicrobial activity copper oxide nanoparticles
Copper has broad-spectrum antimicrobial properties. This could be exploited for therapeutic benefit but, equally, exposures could yield undesirable activity against symbiotic bacteria in the environment, the skin or the intestine (i.e. the microbiome). So understanding copper-bacteria interaction is important. How much the nanostructuring of copper based materials governs their antimicrobial properties is not fully understood. Certainly this nanostructuring strategy may be used to tailor ‘slow release’ properties for copper ions or to allow stable aqueous formulations of copper at high concentrations without issues with hydrolysis and precipitation. However, this does not address whether particles themselves have a microbial impact beyond or in addition to released soluble copper. On the one hand, by comparison to silver, one might assume that it is only soluble copper, not particles, that are antimicrobial. On the other hand, increased activities of copper-based nanoparticles, above and beyond just copper ion content, have been indicated. Indeed, the issue of whether nanoparticles have specific activity against bacteria, remains unclear but important. For example, the European Food Safety Authority (EFSA) requests that, for new oral nanoparticles that are relevant to food, tests are carried out in terms of microbiome effects for safety assessment (EFSA, 2018).
In trying to determine whether copper-based particles, or the copper ions that they release in an aqueous environment, are responsible for antimicrobial activity, a number of aspects must be carefully considered. Firstly, in standard bacterial assays, total exposure to particulate or soluble (solubilised) copper will be determined by an interaction between time and rates of particle dissolution. Secondly, the chemistry of copper is complicated. At typical biological (near–neutral) pHs, particles may form when ‘soluble’ salts are added to a solution phase, due to oxo-hydroxide formation, polymerisation, cross linking and precipitation. Finally, particles, whether inadvertently formed or specifically added to a fluid phase, may agglomerate and/or aggregate. As such, studies investigating exactly what bacteria experience in terms of exposure to copper-based particles, and thus what chemical form is active, are lacking Synthesized CuNPs demonstrated a significant inhibitory activity by Kirby-Bauer disk diffusion method against Escherichia coli followed by Klebsiella pneumoniae, Pseudomonas aeruginosa, Propioni bacterium acnes and Salmonella typhi. In another study by Yoon reported that Cu-NPs showed efficient antibacterial activity as compared to the silver NPs by utilizing single representative strains of E. coli and B. subtilis for Ag and Cu-NPs. Scientists have also suggested the utilization of silver and copper particles as efficient disinfectants for wastewater produced from clinics containing infectious microorganisms.Y.S.and Zain reported that when Cu and Ag-NPs were fused together to make bi-metallic NPs, their antibacterial effects were improved where size played a key role in bactericidal effect (N.M.) CuO-NPs showed excellent antimicrobial activity against various bacterial strains (Escherichiacoli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterococcus faecalis, Shigella flexneri, Salmonella typhimurium, Proteusvulgaris, and Staphylococcusaureus). Moreover, E.coli and E.faecalis exhibited the highests ensitivity to CuO-NPs while K. pneumonia was the least sensitive and reported that the antibacterial activity of CuO nanoparticles was tested against bacterial strains K. pneumonia, S. Typhimurium, and E. aerogenes using the agar diffusion method. The minimum inhibitory concentration of these three gram-negative bacterial strains K. pneumonia, S. Typhimurium, and E. aero genes were found to be 0.55, 0.15, and 0.30 μg/ ml, respectively. Further antimicrobial properties of copper nanoparticles were investigated Radhakrishnan et al. using Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. The bactericidal effects of copper nanoparticles were studied based on the diameter of the inhibition zone in disk diffusion tests of nanoparticles dispersed in batch cultures. The copper nanoparticles showed excellent activity against Escherichia coli and Staphylococcus aureus, with excellent inhibition zones of 14 mm and 10 mm, respectively. Bacterial sensitivity to nanoparticles was found to vary depending on the microbial species and synthesized copper oxide nanoparticles (CuO-NPs) using Ailanthus alissima leaf aqueous extract were studied for antimicrobial activity against pathogenic bacteria by disc diffusion method. Chloromphenical was used as a control antimicrobial agent. These copper oxide nanoparticles showed inhibition zone against Gram negative Escerichia coli and Gram positive Staphyococcus aureus bacteria. It was observed that an increase in CuO-NPs concentration increases the MZI of E. coli and S. aureus bacteria. CuO NPs showed excellent antimicrobial activity against various bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterococcus faecalis, Shigella fexneri, Salmonella typhimurium, Proteus vulgaris, and Staphylococcus aureus). Moreover, E. coli and E. faecalis exhibited the highest sensitivity to CuO NPs while K. pneumonia was the least sensitive. CuO NPs showed excellent antimicrobial activity against various bacterial strains (Escherichia coli. Pseudomonas aeruginosa, Klebsiella pneumonia, Enterococcus faecalis, Shigella Salmonella typhimurium, Proteus vulgaris, and Staphylococcus aureus). Moreover, E. coli and E. faecalis exhibited the highest sensitivity to CuO NPs while K. pneumonia was the least sensitive. CuO NPs showed excellent antimicrobial activity against various bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterococcus faecalis, Shigella flexneri, Salmonella typhimurium, Proteus vulgaris, and Staphylococcus aureus). Moreover, E. coli and E. faecalis exhibited the highest sensitivity to CuO NPs while K. pneumonia was the least sensitive.
Evaluation of antimicrobial activity of titanium dioxide nanoparticles
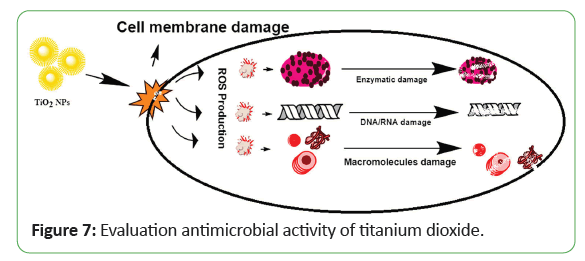
In literature, different metallic NPs have been used against various strains of bacteria. Tio2-NPs also exhibited a value-added property of antimicrobial function. In previous studies, researchers reported that Tio2-NPs exhibited significant toxicity effects against both Gram-positive and Gram-negative bacteria, as well as algae, zebrafish, and mice. The usefulness of the antibacterial effect of Tio2-NPs was used in the disinfection of water contaminated with Escherichia coli. The already published studies on bacterial activity have demonstrated that Tio2-NPs can kill bacteria even at low concentrations, in which the photocatalytic process of Tio2-NPs causes fatal damage to microorganisms. Only a few studies on the antifungal effect of NPs were found in scientific publications. Nevertheless, researchers only focused on the silver (Ag) and zinc (Zn) NPs and their antifungal activity against fungal species, Candida albicans (C. albicans). Both NPs exhibited a strong antifungal effect toward the C. albicans in a concentration- dependent manner. By comparison, zinc is less potent than silver NPs in reducing C. albicans growth with a dose of 0.1 mg/mL and a dose of 0.4,3.3, respectively. To date, access to the antifungal activity of most manufactured NPs remains extremely limited. Hence, in this paper, the antifungal activity of Tio2-NPs against C. albicans, a prevalent human pathogen, was investigated using a trypan blue exclusion as say, which is a simple quantification method. The study covers the effect of different forms (anatase versus rutile) of Tio2, their exposure times, and dosage concentrations on C. albicans culture. Nadeem and colleagues. Similarly, Tio2-NPs also exhibit eco-friendly biocidal properties, which are attributed to their strong oxidizing potential. These NPs have been used against a wide range of infectious microbes including various bacterial strains, endospores, fungi, algae, protozoa, viruses, microbial toxins and prions. Tio2-NPs trigger the onset of Reactive Oxygen Species (ROS) when confronted with microbial cells Jayaseelan and colleagues (2013). These ROS kill microbes by disrupting cell wall’s integrity mainly by phospholipids oxidation, which results in reduced adhesion and distorted ionic balance. Inside the cytosol, it inhibits the respiratory cytosolic enzymes and modifying macromolecules structures, producing substantial effects on cellular integrityand gene expression. Furthermore, it also decreases the phosphate uptake and cellular communication across the cell Jayaseelan and colleagues (2013). A possible mechanism of action is described in Figure 7. Both green synthesized and chemically derived Tio2- NPs kill microbes in same fashion but the biologically derived NPs show better antibacterial activity. Their excellent antimicrobial potential is attributed to the capping agents provided by the plant extracts Kumar and colleagues (2014). Morphology of NPs, membrane biochemistry and type of bacteria significantly affect antibacterial activity.
Green synthesized Tio2-NPs can efficiently inhibit both gram- positive and gram-negative bacteria, but due to comparative structural complexity of gram-negative bacterial cell wall, it is more reactiveagainst gram-positive bacteria Marimuthu and colleagues. The antimicrobial activity of biomediated Tio2-NPs can be enhanced if irradiated with UV and fluorescent light Jayaseelan and colleagues ,Roopan and colleagues. Tio2-NPs Nano composites also exhibit enhanced antileshmanial activity, when green synthesized Tio2-NPs were applied to Leishmanial cultures; lower cell viability, stunted growth and DNA fragmentation was observed Kubacka and colleagues. When compared to standard antibiotic disk Tio2-NPs showed better antimicrobial activity Santhoshkumar and colleagues. Thus with such improved antimicrobial activity it considerably diminishes the occurrences for the development of antibiotic confrontation of pathogenic stains. Jesline. John reported antimicrobial activity of zinc and titanium discovers antibacterial activity and UV protection of the in situ synthesized tiatanium oxide nanoparticles on cotton fabrics.
Evaluation of anticancer activity
At well over 500,000 lives lost per year, cancer is the second most common cause of death in the United States. Most patients diagnosed with cancer undergo chemotherapy treatment; however, some cancer cells are known for their high resistance against chemotherapy drugs, so called Multi-Drug Resistance (MDR) 2,3. MDR often leads to relapse in cancer patients that have undergone chemotherapy treatment. In addition to the complications associated with MDR, chemotherapy treatments lead to mild to severe side effects in over 80% of patients, including nausea, hair loss, fatigue, increased risk of infection and anemia. These side effects limit the dosages at which chemotherapy drugs may be used a higher dose would more effectively kill chemotherapy drug-resistant cancer cells, but at the cost of greater unintended damages to the body. A strategy to overcome this dose limiting issue is localized high-concentration delivery of chemotherapy drugs to sites of cancerous growth.
Evaluation of anticancer activity of silver nanoparticles
In a work done by synthesized chitosan-coated silver nano- triangles (ChitAg-NPs) were used as a photothermal agents against a line of human nonsmall lung cancer cells (NCI-H460). In another work, AgNPs (10 nm) were synthesized using Sargassum vulgare and its ability to kill cancerous human myeloblastic leukemic cells HL60 and cervical cancer cells HeLa was tested and have been found to be active against plasmodial pathogens and cancer cells. Biosynthesized AgNPs have also been found to inhibit the brain cancer cell line HNGC2. These spherical AgNPs ranged in size from 20-80 nm, and the IC50 values were dose- dependent. Biosynthesized AgNPs have also shown notable inhibitory activity against the cervical cancer cell lines Siha and HeLa. Growth of the Siha cancer cell line was inhibited by 2-18 nm triangular and hexagonal AgNPs, with an IC50 of ≤ 4.25 μg/ mL. In contrast, spherically shaped Ag-NPs, with sizes ranging between 5-120 nm, achieved inhibitory effects on HeLa cancer cell lines. The IC50 values varied depending on the preparation method of the AgNPs and also depended upon the plant extracts used. The bio synthesized AgNPs, inhibited four colon cancer cell lines (e.g., COLO 205, HCT 15, HCT-116 and HT29 cells), with IC50 values ranging from 5.5-100 μg/mL. The particles were substantially spherical in shape, with sizes ranging from 7.39-80 nm. Available data regarding these Ag-NPs in the context of brain, cervical and colon cancers. Cuboidal and spherical bio Ag-NPs with sizes ranging from 59-94 nm, prepared from different plant extracts, inhibited the A431 cell line, an epidermoid carcinoma, with IC50 values ranging from 78.580-83.57 μg/mL. Inhibition by bio-extract-derived spherical Ag-NPs with sizes ranging from 5-50 nm was also observed against the AGS cell line, a gastric carcinoma, with an IC50 of 21.05 μg/mL. The H1299 and HL-60 leukemia cell lines were inhibited by 22 nm spherical bio-Ag- NPs with an IC50 value of 5.33 μg/mL and in a dose-dependent manner, respectively. The data related to epidermoid carcinoma, laryngeal carcinoma, gastronomic carcinoma, hepatic cancer, intestinal cancer and kidney cancer.
According to a literature review, bio-extracts have been successfully used as reducing agents to prepare Ag-NPs that can block the activities of the A549 lung cancer cell line. The size of those spherically shaped NPs ranged from 13–136 nm, and the inhibitory actions were observed to follow a dose-dependent relationship, with various IC50 and LD50 values as mentioned below. The B16F10 melanoma cell line was inhibited by the 20- 228 nm spherical bio-Ag-NPs, with an IC50 value of 7.6 ± 0.8 μg/mL, following a dose-dependent relationship. The 100-120 nm flower-shaped AgNPs exhibited notable repressive actions against the KB oral cancer cell line, where the IC50 was 0.6 μg/ mL. The 15-32 nm spherical bio-Ag-NPs impeded the PA1 ovarian cancer cell line, with an IC50 value of 7.5 μg/mL an dina dose- dependent manner. In hibitory activities were observed against the Bx PC3 pancreatic cancer cellline by spherical bio-AgNPs with sizes ranging from 8-22 nm, and the IC50 was 38.9 μg/mL.
Evaluation of anticancer activity of copper oxide nanoparticles
Studies report that the biosynthesis of copper oxide nanoparticles from different plant extracts, such as that of ficus religiosa or acalipha indica. These NPs showed cytotoxic effects on A 549 human lung cancer cells and MCF-7 breast cancer cells, respectively. The mechanism of cytotoxicity was demonstrated to be through the induction of apoptosis with inhanced ROS generation. The green synthesis of these NPs has been proposed as realible, simple, nontoxic and ecofriendly method. Copper oxide nanoparticles from have many industrial applications, but recent studies have reported the antifungal and bacteriostatic properties of copper NPs polymer composites in vitro studies demonstrated that cuprous oxide NPS selectivily induce that apoptosis of tumor cells in vitro. Recent studies on the anticancer potential and cytotoxic activity of “plant based’’ synthesized copper NPs from Sargassum polycystum brown seaweed, utilizing copper sulfate (CuSO4) as the precursor, indicated that NP concentrations of 100 μg. ml−1 can efficiently provoke inhibition of the growth of MCF-7 breast cancer cells at percentages higher than 93% and with an IC50 value of 61.25 μg. Ml-1 Studies on the biosynthesis of copper NPs utilizing plants including Nerium oleander and Magnolia Kobus, have previously been reported.
Evaluation of biosensing activity
A biosensor is a device that combines a sensitive biological recognition component and a physical transducer to detect analytes of interest. Analysis results are displayed through transforming the biological reaction into a measurable signal, which can be used for qualitative and quantitative determinations. The biological recognition component part of biosensors usually includes nucleic acids, enzymes, antibodies, receptors, microorganisms, cells, tissues, and even some biomimetic structures. Physical transducers vary significantly with the source of the quantifiable signal, and utilize mostly optical and electrochemical systems.
Evaluation of biosensing activity of copper nanoparticles
The dispersion of metal nanoparticles displays intense colors due to the Surface Plasmon Resonance (SPR) absorption. The surface of metals like Cu can be treated as free electron systems called plasma, containing equal numbers of positive ions (fixed in position) and conduction electrons (free and highly mobile). Under the irradiation of an electromagnetic wave, the free electrons are driven by the electric field to oscillate coherently at a plasma frequency relative to positive ions. The metals rich in free electrons revealed this property so, it mostly used as a plasmonic material and a number of metals are supporting SPR for at least part of the UV-Vis-NIR region. These free electrons provide the negative real permittivity that is an essential property of any plasmonic material. However, metals are suffering from large losses, especially in the Uv-Vis. spectral ranges, arising in part from inter-band electronic transitions.
Evaluation of biosensing activity of titanium dioxide nanoparticlesn
Titanium dioxide was used as the substrate on the sensing surface, because Tio2-based semiconductor metal-oxide material has attracted substantial interest due to their low cost and production flexibility. The bio-compatibility of Tio2 nanoparticles in the development of biosensor promises better sensing performance and enhancing the electron-transfer kinetics. To increase the surface-to-volumeratio and enhance sensitivity, enabling the semiconducting conductance to be easily modulated by the target DNA for metal-oxide nanostructures have been proposed.
Conclusion
Biosynthesis of metal nanoparticles using plant derivatives is extremely studied in the last two decades. The plant metabolites induce the production of metallic nanoparticles in eco friendly manner. The use of plant extracts for making metallic nanoparticles is simple, convenient, and inexpensive, easily scaled up, less energy-intensive, eco-friendly, minimize the usage of unsafe materials and maximize the efficiency of the process.
References
- Gopinath V, MubarakAli D, Priyadarshini S, Priyadharsshini NM, Thajuddin N, et al. (2012) Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf B Biointerfaces 96: 69-74.
- Awwad A, Amer M (2020) Biosynthesis of copper oxide nanoparticles using Ailanthus altissima leaf extract and antibacterial activity. Chem Int 6: 210-217.
- Kesharwani J, Yoon KY, Hwang J, Rai M (2009) Phytofabrication of silver nanoparticles by leaf extract of Datura metel: Hypothetical mechanism involved in synthesis. J Bionanosci 3: 39-44.
- Nasrollahzadeh M, Sajadi SM (2015) Green synthesis of copper nanoparticles using Ginkgo biloba L leaf extract and their catalytic activity for the Huisgen [3+2] cycloaddition of azides and alkynes at room temperature. J Colloid Interface Sci 457: 141-147.
- Li J, Sun F, Gu K, Wu T, Zhai W, et al. (2011) Preparation of spindly CuO micro-particles for photodegradation of dye pollutants under a halogen tungsten lamp. Applied Catalysis A: General 406: 51-58.
- Khani R, Roostaei B, Bagherzade G, Moudi M (2018) Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina-christi (L.) willd: Application for adsorption of triphenylmethane dye and antibacterial assay. J Mol Liq 255: 541-549.
- Nakkala JR, Mata R, Gupta AK, Rani SS (2014) Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur J Med Chem 85: 784-794.
- Kumar PSM, Francis AP, Devasena T (2014) Biosynthesized and chemically synthesized titania nanoparticles: Comparative analysis of antibacterial activity. J Environ Nanotechnol 3: 73-81.
- Aygun A, Ozdemir S, Gulcan M, Cellat K, Sen F (2020) Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J Pharm Biomed Anal 178: 112970.
- Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13: 2638-2650.
- Ghidan AY, Al-Antary TM, Awwad A (2016) Green synthesis of copper oxide nanoparticles using punica granatum peels extract: Effect on green peach Aphid. Environ Nanotechnol Monit Manag 6: 95-98.
- Gopinath M, Subbaiya R, Selvam MM, Suresh D (2014) Synthesis of copper nanoparticles from nerium oleander leaf aqueous extract and its antibacterial activity. Int J Curr Microbiol App Sci 3: 814-818.
- Parikh P, Zala D, Makwana BA (2014) Biosynthesis of copper nanoparticles and their antimicrobial activity. OALib Journal 1200067: 1-15.
- Elisma N, Labanni A, Emriadi E, Rilda Y (2019). Green synthesis of copper nanoparticles using uncaria gambir roxb leaf extract and its characterization. Rasayan J Chem 12: 1752-1756.
- Din MI, Arshad F, Hussain Z, Mukhtar M (2017) Green adeptness in the synthesis and stabilization of copper nanoparticles catalytic, antibacterial, cytotoxic and antioxident activities. Nanoscience Res Lett 12: 638.
- Jain D, Daima HK, Kachhwaha S, Kothari SL (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Digest Journal of Nanomaterials and Biostructures 4: 557-563.
- Saifuddin N, Wong C, Yasumira A (2009) Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J Chem 6: 61-70.
- Rajaram K, Aiswarya DC, Sureshkumar P (2015) Green synthesis of silver nanoparticle using tephrosia tinctoria and its antidiabetic activity. Materials Letters 138: 251-254.
- Sana SS, Badineni VR, Arla SK, Boya VKN (2015) Eco-friendly synthesis of silver nanoparticles using leaf extract of grewia flaviscences and study of their antimicrobial activity. Materials Letters 145: 347-350.
- Ahmed MJ, Murtaza G, Mehmood A, Bhatti TM (2015) Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: Characterization and antibacterial activity. Materials Letters 153: 10-13.
- Patil AB, Mengane SK (2016) Green synthesis of silver nano particles from clerodendrum serratum and antimicrobial activity against human pathogens. J Bionanosci 10: 491-494.
- Das S, Chakraborty T (2018) A review on green synthesis of silver nanoparticle and zinc oxide nanoparticle from different plants extract and their antibacterial activity against multi-drug resistant bacteria. J Innov Pharm Biol Sci 5: 63-73.
- Mortazavi-Derazkola S, Ebrahimzadeh MA, Amiri O, Goli HR, Rafiei A, et al. (2020) Facile green synthesis and characterization of Crataegus microphylla extract-capped silver nanoparticles (CME@Ag-NPs) and its potential antibacterial and anticancer activities against AGS and MCF-7 human cancer cells. J Alloys Compd 820: 153186.
- Gomathi M, Prakasam A, Rajkumar PV, Rajeshkumar S, Chandrasekaran R, et al. (2020) Green synthesis of silver nanoparticles using gymnema sylvestre leaf extract and evaluation of its antibacterial activity. S Afr J Chem Eng 32: 1-4.
- Kumar D, Arora S, Kumar A, Danish M, Bahukhandi KD, et al. (2020) Green synthesis of silver nanoparticles using cucurbita pepo leaves extract and its antimicrobial and antioxidant activities. Advances in Water Pollution Monitoring and Control 115-125 pp.
- Seifipour R, Nozari M, Pishkar L (2020) Green synthesis of silver nanoparticles using tragopogon collinus leaf extract and study of their antibacterial effects. J Inorg Organomet Polym Mater 30: 2926-2936.
- Chartarrayawadee W, Charoensin P, Saenma J, Rin T, Khamai P, et al. (2020) Green synthesis and stabilization of silver nanoparticles using lysimachia foenum-graecum hance extract and their antibacterial activity. Green Proces Synth 9: 107-118.
- Shodhganga (2018) Indian electronic theses & dissertations. Shodh ganga a resevoir of Indian theses.
- Gavade NL, Kadam AN, Suwarnkar MB, Ghodake VP, Garadkar KM (2015) Biogenic synthesis of multi-applicative silver nanoparticles by using ziziphus jujuba leaf extract. Spectrochim Acta A Mol Biomol Spectrosc 136: 953-960.
- Roy N, Barik A (2010) Surface optical modes in GaN nanowires. Int J Nanotechnol Appl 4: 95-101.
- Gavade NL, Kadam AN, Suwarnkar MB, Ghodake VP, Garadkar KM (2015) Biogenic synthesis of multi-applicative silver nanoparticles by using ziziphus jujuba leaf extract. Spectrochim Acta A Mol Biomol Spectrosc 136: 953-960.
- Ravindra S, Mohan YM, Reddy NN, Raju KM (2010) Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloids and surfaces a: physicochemical and engineering aspects 367: 31-40.
- Raut RW, Jaya RL, Niranjan SK, Vijay DM, Sahebrao BK (2009) Phytosynthesis of silver nanoparticle using gliricidia sepium (jacq.). Curr Nanosci 5: 117-122.
- Saxena A, Tripathi RM, Zafar F, Singh P (2012) Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Materials Letters 67: 91-94.
- Sadeghi B, Rostami A, Momeni SS (2015) Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 134: 326-332.
- Logeswari P, Silambarasan S, Abraham J (2015) Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc 19: 311-317.
- Sadeghi B, Gholamhoseinpoor F (2015) A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 134: 310-315.
- Ulug B, Turkdemir MH, Cicek A, Mete A (2015) Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim Acta A Mol Biomol Spectrosc 135: 153-161.
- Geetha N, Geetha TS, Manonmani P, Thiyagarajan M (2014) Green synthesis of silver nanoparticles using Cymbopogan Citratus (Dc) Stapf. Extract and its antibacterial activity. Aus J Basic Appl Sci 8: 324-331.
- Kumarasamyraja D, Jeganathan NS (2013) Green synthesis of silver nanoparticles using aqueous extract of acalypha indica and its antimicrobial activity. Int J Pharma Bio Sci. 4: 479-476.
- Kumar S, Daimary RM, Swargiary M, Brahma A, Kumar S, et al. (2013) Biosynthesis of silver nanoparticles using Premna herbacea leaf extract and evaluation of its antimicrobial activity against bacteria causing dysentery. Int J Pharm Biol Sci 4: 378-384.
- Gondwal M, Pant GJN (2013) Biological evaluation and green synthesis of silver nanoparticles using aqueous extract of Calotropis procera. Int J Pharm Biol Sci 4: 635-643.
- Rout A, Jena PK, Paridaand UK, Bindhani BK (2013) Green synthesis of silver nanoparticles using leaves extract of Centella asiatica L. For studies against human pathogens. Int J Pharm Biol Sci 4: 661-674.
- Thombre R, Parekh F, Patil N (2014) Green synthesis of silver nanoparticles using seed extract of Argyreia nervosa. Int J Pharm Biol Sci 5: 114-119.
- Sunita D, Tambhale D, Parag V, Adhyapak A (2014) Facile green synthesis of silver nanoparticles using Psoralea corylifolia: Seed extract and their in-vitro antimicrobial activities. Int J Pharm Biol Sci 5: 457-467.
- Narayanan K, Park H (2014) Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur J Plant Pathol 140: 185-192.
- Kathiravan V, Ravi S, Ashokkumar S (2014) Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 130: 116-121.
- Rasheed T, Bilal M, Iqbal HMN, Li C (2017) Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf B Biointerfaces 158: 408-415.
- Kirubaharan CJ, Kalpana D, Lee YS, Kim AR, Yoo DJ, et al. (2012) Biomediated silver nanoparticles for the highly selective copper (II) ion sensor applications. Ind Eng Chem Res 51: 7441-7446.
- Reddy KR (2017) Green synthesis, morphological and optical studies of CuO nanoparticles. J Mol Struct 1150: 553-557.
- Ijaz F, Shahid S, Khan SA, Ahmad W, Zaman S (2017) Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop J Pharm Res 16: 743-753.
- Gopinath M, Subbaiya R, Selvam MM, Suresh D (2014) Synthesis of copper nanoparticles from Nerium oleander leaf aqueous extract and its antibacterial activity. Int J Curr Microbiol App Sci 3: 814-818.
- Kaur P, Thakur R, Chaudhury A (2016) Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem Lett Rev 9: 33-38.
- Hemmati S, Mehrazin L, Hekmati M, Izadi M, Veisi H (2018) Biosynthesis of CuO nanoparticles using Rosa canina fruit extract as a recyclable and heterogeneous nanocatalyst for CN Ullmann coupling reactions. Mater Chem Phys 214: 527-532.
- Yugandhar P, Vasavi T, Rao YJ, Devi PUM, Narasimha G, et al. (2018) Cost effective, green synthesis of copper oxide nanoparticles using fruit extract of Syzygium alternifolium (Wt.) Walp., characterization and evaluation of antiviral activity. J Clust Sci 29: 743-755.
- Thakur S, Sharma S, Thakur S, Rai R (2018) Green synthesis of copper nano-particles using Asparagus adscendens roxb. Root and leaf extract and their antimicrobial activities. Int J Curr Microbiol Appl Sci 7: 683-694.
- Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, et al. (2012) Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B Biointerfaces 95: 284-288.
- Rafique M, Shaikh AJ, Rasheed R, Tahir MB, Bakhat HF, et al. (2017) A review on synthesis, characterization and applications of copper nanoparticles using green method. Nano 12: 1750043.
- Karimi J, Mohsenzadeh S (2015) Rapid, green, and eco-friendly biosynthesis of copper nanoparticles using flower extract of Aloe vera. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 45: 895-898.
- Saranyaadevi K, Subha V, Ravindran RSE, Renganathan S (2014) Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int J Chem Tech Res 6: 4533-4541.
- Kulkarni VD, Kulkarni PS (2013) Green synthesis of copper nanoparticles using Ocimum sanctum leaf extract. Int J Chem Stud 1: 1-4.
- Parikh P, Zala D, Makwana BA (2014) Biosynthesis of copper nanoparticles and their Antimicrobial activity. OALib Journal 1200067: 1-15.
- Trang N, Dung P, Ngoc L, Van P (2018) Green synthesis of copper nanoparticles using mandarin (citrus reticulata) peel extract and antifungal study. Asian J Biotechnol Bioresour Technol 3: 1-9.
- Shende S, Ingle AP, Gade A, Rai M (2015) Green synthesis of copper nanoparticles by Citrus medica Linn.(Idilimbu) juice and its antimicrobial activity. World J Microbiol Biotechnol 31: 865-873.
- Chung IM, Rahuman AA, Marimuthu S, Kirthi AV, Anbarasan K, et al. (2017) Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med 14: 18-24.
- Padma PN, Banu ST, Kumari SC (2018) Studies on green synthesis of copper nanoparticles using Punica granatum. Annu Res Rev Biol 23: 1-10.
- Hassanien R, Husein DZ, Al-Hakkani MF (2018) Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon 4: e01077.
- Tu HL (2019) Biosynthesis, characterization and photocatalytic activity of copper/copper oxide nanoparticles produced using aqueous extract of lemongrass leaf. Composite Materials 3: 30-35.
- Shah R, Pathan A, Vaghela H, Ameta SC, Parmar K (2019) Green synthesis and characterization of copper nanoparticles using mixture (Zingiber officinale, Piper nigrum and Piper longum) extract and its antimicrobial activity. Chem Sci Trans 8: 63-69.
- Batoool M, Masood B (2017) Green synthesis of copper nanoparticles using Solanum lycopersicum (tomato aqueous extract) and study characterization. J Nanosci Nanotechnol 1: 1-5.
- Ismail MIM (2020) Green synthesis and characterizations of copper nanoparticles. Mater Chem Phys 240: 122283.
- Amatya SP, Joshi LP (2020) Bio-Synthesis of copper nanoparticles (CuNPs) using garlic extract to investigate antibacterial activity. BIBECHANA 17: 13-19.
- Baskar G, Sakthivel D, George GB (2016) Synthesis, characterization and anticancer activity of copper nanobiocomposite synthesized by leaf extract of Catharanthus roseus. Int J Mod Sci Eng Technol 1: 92-96.
- Ibrahim S, Jakaria NZ, Rozali S, Ghazali NNN, Ab-Karim MS, et al. (2020) Biosynthesis of copper oxide nanoparticles using camellia sinensis plant powder. Adv Mater Sci Eng.
- Hasheminya SM, Dehghannya J (2019) Green synthesis and characterization of copper nanoparticles using Eryngium caucasicum Trautv aqueous extracts and its antioxidant and antimicrobial properties. Part Sci Technol 38: 1019-1026.
- Naika HR, Lingaraju K, Manjunath K, Kumar D, Nagaraju G, et al. (2015) Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J Taibah Univ Sci 9: 7-12.
- Hariprasad S, Bai GS, Santhoshkumar J, Madhu CH, Sravani D (2016) Green synthesis of copper nanoparticles by Arevalanata leaves extract and their anti-microbial activites. Int J Chemtech Res 9: 98-105.
- Prasad S, Patra A, Govindaraju S, Shivamallu C (2017) Aqueous extract of Saraca indica leaves in the synthesis of copper oxide nanoparticles: Finding a way towards going green. J Nanotechnol 2017: 1-6.
- Sankar R, Manikandan P, Malarvizhi V, Fathima T, Shivashangari KS, et al. (2014) Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim Acta A Mol Biomol Spectrosc 121: 746-750.
- Altikatoglu M, Attar A, Erci F, Cristache C, Isildak I (2017) Green synthesis of copper oxide nanoparticles using Ocimum basilicum extract and their antibacterial activity. Fresenius Environ Bull 26: 7832-7837.
- Nasrollahzadeh M, Sajadi SM, Khalaj M (2014) Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Advances 4: 47313-47318.
- Mukherjee R, Kumar R, Sinha A, Lama Y, Saha AK (2016) A review on synthesis, characterization, and applications of nano zero valent iron (nZVI) for environmental remediation. Crit Rev Environ Sci Technol 46: 443-466.
- Prasad PR, Kanchi S, Naidoo EB (2016) In-vitro evaluation of copper nanoparticles cytotoxicity on prostate cancer cell lines and their antioxidant, sensing and catalytic activity: One-pot green approach. J Photochem Photobiol B 161: 375-382.
- Nazar N, Bibi I, Kamal S, Iqbal M, Nouren S, et al. (2018) Cu nanoparticles synthesis using biological molecule of P. granatum seeds extract as reducing and capping agent: Growth mechanism and photo-catalytic activity. Int J Biol Macromol 106: 1203-1210.
- Nasrollahzadeh M, Momeni SS, Sajadi SM (2017) Green synthesis of copper nanoparticles using Plantago asiatica leaf extract and their application for the cyanation of aldehydes using K4Fe (CN)6. J Colloid Interface Sci 506: 471-477.
- Yedurkar SM, Maurya CB, Mahanwar PA (2017) A biological approach for the synthesis of copper oxide nanoparticles by Ixora coccinea leaf extract. J Mater Environ Sci 8: 1173-1178.
- Kalpana VN, Chakraborthy P, Palanichamy V, Rajeswari VD (2016) Synthesis and characterization of copper nanoparticles using Tridax procumbens and its application in degradation of bismarck brown. Int J ChemTech Res 9: 498-507.
- Ranjitham AM, Ranjani GS, Caroling G (2015) Biosynthesis, characterization, antimicrobial activity of copper nanoparticles using fresh aqueous Ananas comosus L.(Pineapple) extract. Int J Pharmtech Res 8: 750-769.
- Al-Hakkani MF (2020) Biogenic copper nanoparticles and their applications: A review. Appl Sci 2: 1-20.
- Rajesh KM, Ajitha B, Reddy YAK, Suneetha Y, Reddy PS (2018) Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 154: 593-600.
- Lotfabad TB, Madadi Z (2016) Aqueous extract of acanthophyllum laxiusculum roots as a renewable resource for green synthesis of nano-sized titanium dioxide using Sol-gel Method. Adv Ceram Prog 2: 26-31.
- Ganesan S, Babu IG, Mahendran D, Arulselvi PI, Elangovan N, et al. (2016) Green engineering of titanium dioxide nanoparticles using Ageratina altissima (L.) King & HE Robines. medicinal plant aqueous leaf extracts for enhanced photocatalytic activity. Ann Phytomed 5: 69-75.
- Roopan SM , Bharathi A, Prabhakarn A, Rahuman AA, Velayutham K, et al. (2012) Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 98: 86-90.
- Marimuthu S, Rahuman AA, Jayaseelan C, Kirthi AV, Santhoshkumar T, et al. (2013) Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac J Trop Med 6: 682-688.
- Velayutham K, Rahuman AA, Rajakumar G, Santhoshkumar T, Marimuthu S, et al. (2012) Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol Res 111: 2329-2337.
- Valli G, Geetha S (2015) A green method for the synthesis of titanium dioxide nanoparticles using cassia auriculata leaves extract. J Biomed Pharm Sci 2: 490-497.
- Kashale AA, Gattu KP, Ghule K, Ingole VH, Dhanayat S, et al. (2016) Biomediated green synthesis of TiO2 nanoparticles for lithium ion battery application. Composites Part B: Engineering 99: 297-304.
- Naik GK, Mishra PM, Parida K (2013) Green synthesis of Au/TiO2 for effective dye degradation in aqueous system. Chem Eng Technol 229: 492-497.
- Jalill A, Raghad DH, Nuaman RS, Abd AN (2016) Biological synthesis of titanium dioxide nanoparticles by Curcuma longa plant extract and study its biological properties. World Scientific News 49: 204-222.
- Sahu N, Soni D, Chandrashekhar B, Sarangi BK, Satpute D, et al. (2013) Synthesis and characterization of silver nanoparticles using Cynodon dactylon leaves and assessment of their antibacterial activity. Bioprocess Biosyst Eng 36: 999-1004.
- Rajakumar G, Rahuman AA, Priyamvada B, Khanna VG, Kumar DK, et al. (2012) Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater Lett 68: 115-117.
- Zahir AA, Chauhan IS, Bagavan A, Kamaraj C, Elango G, et al. (2015) Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob Agents Chemother 59: 4782-4799.
- Hudlikar M, Joglekar S, Dhaygude M, Kodam K (2012) Green synthesis of TiO2 nanoparticles by using aqueous extract of Jatropha curcas L. latex. Mater Lett 75: 196-199.
- Sundrarajan M, Bama K, Bhavani M, Jegatheeswaran S, Ambika S, et al. (2017) Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J Photochem Photobiol A Chem 171: 117-124.
- Sivaranjani V, Philominathan P (2016) Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Medicine 12: 1-5.
- Sundrarajan M, Gowri S (2011) Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett 8: 447-451.
- Jayasinghe C, Gotoh N, Aoki T, Wada S (2003) Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). Agric Food Chem 51: 4442-4449.
- Hunagund SM, Desai VR, Kadadevarmath JS, Barretto DA, Vootla S, et al. (2016) Biogenic and chemogenic synthesis of TiO2 NPs via hydrothermal route and their antibacterial activities. RSC advances 6: 97438-97444.
- Santhoshkumar T, Rahuman AA, Jayaseelan C, Rajakumar G, Marimuthu S, et al. (2014) Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac J Trop Med 7: 968-976.
- Bao SJ, Lei C, Xu MW, Cai CJ, Jia DZ (2012) Environment-friendly biomimetic synthesis of TiO2 nanomaterials for photocatalytic application. Nanotechnology 23: 205601.
- Subhapriya S, Gomathipriya P (2018) Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb Pathog 116: 215-220.
- Boca SC, Potara M, Gabudean AM, Juhem A, Baldeck PL, et al. (2011) Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer lett 311: 131-140.
- Baharara J, Namvar F, Ramezani T, Mousavi M, Mohamad R (2015) Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: Apoptosis induction in MCF-7 cells via caspase activation and regulation of Bax and Bcl-2 gene expression. Molecules 20: 2693-2706.
- Sathishkumar P, Preethi J, Vijayan R, Yusoff ARM, Ameen F, et al. (2016) Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J Photochem Photobiol B 163: 69-76.
- Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, et al. (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochemistry 47: 651-658.
- Raman S, Priyanka KM, Jacob JA, Kamalakkannan S, Thangam R, et al. (2012) Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochemistry 47: 273-279.
- Vasanth K, Ilango K, MohanKumar R, Agrawal A, Dubey GP (2014) Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces 117: 354-359.
- Mittal AK, Thanki K, Jain S, Banerjee UC (2016) Comparative studies of anticancer and antimicrobial potential of bioinspired silver and silver-selenium nanoparticles. J Mat NanoSci 3: 22-27.
- Govindaraju K, Krishnamoorthy K, Alsagaby SA, Singaravelu G, Premanathan M (2015) Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnology 9: 325-330.
- Nayak D, Pradhan S, Ashe S, Rauta PR, Nayak B (2015) Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci 457: 329-338.
- Salehi S, Shandiz SAS, Ghanbar F, Darvish MR, Ardestani MS, et al. (2016) Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int J Nanomedicine 11: 1835-1846
- Govindaraju K, Krishnamoorthy K, Alsagaby SA, Singaravelu G, Premanathan M (2015) Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnology 9: 325-330.
- He Y, Du Z, Ma S, Liu Y, Li D, et al. (2016) Effects of green-synthesized silver nanoparticles++ on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int J Nanomedicine 11: 1879-1887.
- Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, et al. (2013) Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces 108: 80-84.
- Sre PRR, Reka M, Poovazhagi R, Kumar MA, Murugesan K (2015) Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 135: 1137-1144.
- Castro-Aceituno V, Ahn S, Simu SY, Singh P, Mathiyalagan R, et al. (2016) Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother 84: 158-165.
- Venugopal K, Rather HA, Rajagopal K, Shanthi MP, Sheriff K, et al. (2017) Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J Photoch Phoyobio B 167: 282-289.
- Gurunathan S, Jeong JK, Han JW, Zhang XF, Park JH, et al. (2015) Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res Lett 10: 35.
- Venkatesan B, Subramanian V, Tumala A, Vellaichamy E (2014) Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac J Trop Med 7: S294-S300.
- Banerjee K, Das S, Choudhury P, Ghosh S, Baral R, et al. (2017) A novel approach of synthesizing and evaluating the anticancer potenlntial of silver oxide nanoparticles in vitro. Chemotherapy 62: 279-289.
- Inbakandan D, Kumar C, Bavanilatha M, Ravindra DN, Kirubagaran R, et al. (2016) Ultrasonic-assisted green synthesis of flower like silver nanocolloids using marine sponge extract and its effect on oral biofilm bacteria and oral cancer cell lines. Microb Pathog 99: 135-141.
- Khanra K, Panja S, Choudhuri I, Bhattacharyya N (2015). Evaluation of antibacterial activity and cytotoxicity of green synthesized silver nanoparticles using Scoparia dulcis. Nano Biomed Eng 7: 128-133.
- Singh A, Kaur K (2019) Biological and physical applications of silver nanoparticles with emerging trends of green synthesis. Engineered Nanomaterials-Health and Safety.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences