Effect of Selective Laser Trabeculoplasty in Glaucoma Patients with High or Low Central Corneal Thickness
1University of Antwerp, Department of Medicine, Universiteitsplein 1, B-2610 Antwerp, Belgium
2University of Antwerp, Department of Medicine, Universiteitsplein 1, B-2610 Antwerp, Belgium
3University Hospital Antwerp, Department of Ophthalmology, Wilrijkstraat 10, B-2650 Edegem, Belgium
- *Corresponding Author:
- Myrjam De Keyser
University of Antwerp, Department of Medicine
Universiteitsplein 1, Den Brem 93
B-2610 Antwerp, Belgium.
Tel: 0032/473893001
E-mail: Myrjam@bijzonder.be
Received date: December 11, 2016; Accepted date: January 22, 2017; Published date: January 28, 2017
Citation: De Keyser M, De Belder M, De Belder J, et al. Effect of Selective Laser Trabeculoplasty in Glaucoma Patients with High or Low Central Corneal Thickness. Ins Ophthal. 2017, 1:1.
Abstract
Purpose: Comparison of the effect of selective laser trabeculoplasty (SLT) in glaucoma patients with high and low central corneal thickness (CCT).
Materials and methods: Clinical trial on 72 glaucoma patients, controlled on medication that received SLT as replacement therapy. We compared the effect of SLT on patients with CCT ≥ 550 μm with patients having CCT <550 μm. Primary end point was intraocular pressure and number of medications taken. Measures were made at 1 h, 1 week, 1 month, 3, 6, 12 and 18 months after SLT.
Results: Mean IOP reduction after SLT was comparable between the low and the high CCT group at most time points (p>0.05); only at 18 months after SLT, the low CCT group showed less IOP reduction compared to the high CCT group (p=0.04). The mean number of medications showed no significant differences between the high and the low CCT group at any point in time. In both groups, the mean number of medications lowered significantly; from 1.43 to 0.17 medications in the high CCT group and from 1.55 to 0.33 in the low CCT group.
Conclusion: CCT does not influence the outcome of SLT in terms of mean IOP reduction and number of medications needed.
Keywords
Selective laser trabeculoplasty; Central corneal thickness; Glaucoma; Ocular hypertension; SLT; Intraocular pressure reduction.
Introduction
In 1997, Latina et al. launched selective laser trabeculoplasty (SLT) as a new treatment to lower intraocular pressure (IOP) [1]. Since then, SLT has proven to be efficient [2,3] and its use has become widespread. Several studies have been performed to investigate which factors influence the outcome of SLT. Up to now, only higher pre-laser IOP seems to be correlated to higher IOP lowering effect of the SLT [4-6]; other factors like race 6, trabecular meshwork pigmentation [7,8], number and type of previously used anti-glaucoma medications [9,10], previous laser [11,12] and phakic or pseudophakic eyes [13] showed no impact on SLT efficiency. Shazly et al. examined whether central corneal thickness had influence on SLT outcome and suggested that thinner corneas gave better IOP reduction after SLT [14].
CCT is known to have an effect on IOP measurement [15] but some studies suggest that CCT may also give information about tissue properties of the eye that are otherwise difficult to measure [16,17].
The Ocular Hypertension Treatment Study [18] showed that CCT was a strong predictor for the development of open angle glaucoma in patients with ocular hypertension. This was confirmed by the European Glaucoma Prevention Study [19] and the Barbados Eye Studies [20]. Several studies have found that CCT is an important variable in patients with existing glaucoma [17,21-24].
The Early Manifest Glaucoma Trial however, reported that CCT was not a significant predictor of glaucoma progression [25]. Medeiros et al. did identify CCT as a risk factor for development of visual field loss among patients diagnosed with optic neuropathy [21].
The aim of this study is to examine the possible effect of CCT on SLT outcome.
Materials and Methods
Study design and subjects
This is a post hoc analysis of data collected in a randomized interventional clinical trial conducted to evaluate use of antiinflammatory drops after SLT. The design of that study has been reported previously [21].
Inclusion criteria concerned primary open angle glaucoma (POAG) or ocular hypertension (OHT) controlled with medical therapy.
Exclusion criteria were other types of glaucoma than open angle glaucoma, previous glaucoma surgery or previous laser trabeculoplasty. Patients with corneal disease that inhibited good visualization of the trabecular meshwork and those taking systemic steroids were also excluded from the study.
POAG was defined as IOP above 21 mmHg on two separate occasions, open angle on gonioscopy and either glaucomatous visual field defects on Humphrey visual field analyzer, optic disc changes on funduscopy and/or loss of retinal nerve fiber layer on Optical Coherence Tomography (OCT). The diagnosis of OHT was made when an IOP higher than 21 mmHg was measured on two separate occasions in the absence of field or disc changes or defects in retinal nerve fiber layer.
The study was not designed to create additional IOP lowering effect, because IOP was already controlled with medication before treatment with SLT. The main goal of this study was lowering the number of medications needed to maintain adequate IOP control and examine effect of CCT on SLT outcome.
Baseline examinations
At baseline a full ophthalmological examination of each study participant was conducted, including a medical history review, best corrected visual acuity (BCVA) measurement, IOP measurement using Goldmann applanation tonometry (mean of two measurements was taken), slit lamp examination of the anterior segment, central corneal thickness (CCT) measurement, dilated fundus examination, visual field examination by computerized perimetry (program 24-2, Humphrey Field Analyzer 745i, Zeiss, Jena, Germany), optical coherence tomography (OCT) of the optic nerve head and recording of glaucoma medications.
All OCT scans were performed with the spectral-domain OCT RTVue (Optovue, Fremont, USA). We used focal loss of volume (FLV) as determinant for the OCT [26]. CCT was measured at baseline using a handheld pachymeter (iPac Pachymeter, Reichert, Buffalo, NY, USA); the same pachymeter was used for every patient.
IOP before treatment was calculated as the mean of three measurements taken before starting glaucoma medication at three different days, at least 3 to 6 months apart. IOP at baseline was calculated as the mean of the Goldman measurements made on different time points on the three last examinations before laser treatment.
The same examiner performed all examinations.
Patients were divided into a group with high CCT (≥ 550 μm) or low CCT (<550 μm). Doughty et al. described a normal CCT in white adults could be expected to be 535 μm ± 2SD, i.e., 473- 597 μm [15]. Shih et al. found a mean CCT of 544 ± 34 μm based on a large review [23]. The Ocular Hypertension Treatment Study Group (1636 subjects) arrived at a mean CCT of 573.0 ± 39.0 μm [24]. We took the mean of these three results to come to a cut-off value of 550 μm for the CCT in our study.
Laser technique
A frequency doubled, Q-switched Nd:YAG laser was used, emitting a wavelength of 532 nm, coupled to a slit lamp delivery system (Selecta Duet laser, Lumenis, Dreieich, Germany). We used single pulses with pulse duration of 3 ns and spot size of 400 μm. The laser energy was initially set at 0.9 mJ and a single laser pulse was delivered at the 12 O’clock position. If a cavitation bubble appeared, the laser energy was reduced by 0.1 mJ increments until minimal bubble formation was observed. Treatment was then continued at this energy level. If no cavitation bubble was observed, the pulse energy was increased by steps of 0.1 mJ until bubble formation [1].
Immediately before the laser procedure a drop of pilocarpine 1% and apraclonidine 0.5% were instilled into the treated eye. After the laser treatment, indomethacin 3 times daily, dexamethasone 3 times daily for one week or no anti-inflammatory drops were administered, following study protocol. The observation that the use of anti-inflammatory drops did not influence SLT outcome was previously published [27].
Postoperative management
Patients were examined 1 h, 1 week, 1, 3, 6, 12 and 18 months after SLT. A full clinical examination, comparable to the examinations at baseline, was performed at 6 and at 12 months. After SLT, glaucoma drops were continued until IOP was more than 2 mm Hg below target pressure, at which point they were stopped one by one. A fixed combination of drugs was considered as a combination of two medications; the first step entailed a switch to a single medication. The second drug was stopped if possible, after respecting a wash out period of three months. The number of applications daily was not changed during the study; medication was given at the normal frequency or stopped.
Target pressure was calculated using the formula proposed by Jampel (Target IOP=maximum IOP=maximum IOP% - z, where z is an optic nerve damage severity factor) [28].
Statistical methods
A paired samples t-test was performed to compare baseline differences between the low and the high CCT group for continuous variables (i.e., age, IOP at baseline with medication, BCVA, cup-disc ratio, CCT, visual field mean deficit, OCT FLV, IOPmax before treatment, number of medications at baseline). A χ2-test was used to compare baseline differences in sex and type of glaucoma.
A second paired samples t-test was executed to investigate the difference in mean IOP reduction for both groups. A χ2-test was performed to examine the difference in mean number of medications needed. Results of statistical analysis with p-values <0.05 were considered to be significant.
Results
Population
Demographic and baseline characteristics are shown in Table 1.
135 eyes of 72 patients underwent SLT. We met no significant complications or side effects after SLT; data collection was stopped for practical reasons.
No significant differences were present between the high and the low CCT groups in terms of mean age, sex, BCVA, visual field deficits, OCT and medication taken at baseline. This excluded all these factors as possible confounders.
A significant difference was found in IOP before start of medication and IOP at baseline; patients with low CCT had significantly lower IOP at these two time points. Since a thin cornea leads to measuring a lower IOP than the ‘true’ IOP [15], this is not unexpected. There were significantly less patients in the low CCT group diagnosed with ocular hypertension.
A significant baseline difference was also present in the cup disc ratio of the two groups; optic discs were more excavated in the low CCT group. This concurs with other studies that showed glaucoma patients with thinner cornea have higher cups disc ratio [17,22,29]. The visual field and OCT examinations however, showed no significant difference between the two groups [17,30].
Laser technique
All patients received a 360° treatment of the trabecular meshwork. We used a mean number of 102.37 ± 9.34 non-overlapping spots with a mean energy of 1.09 ± 0.31 mJ. The same experienced practitioner (MDK) applied all treatments.
Evolution of IOP and medication
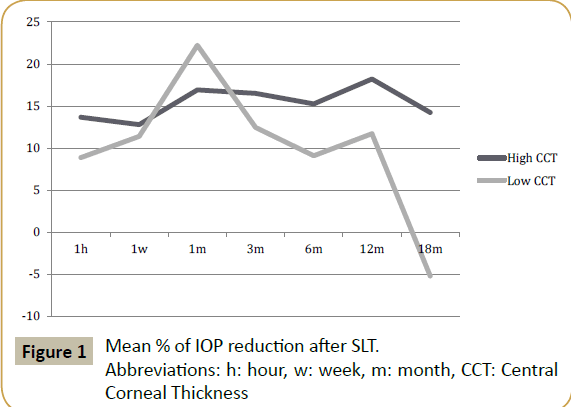
The mean percentage of IOP reduction in eyes with thinner corneas (CCT<550 μm) showed no significant difference compared to the percentage of IOP reduction in thicker corneas at 1 h, 1 week, 3, 6 and 12 months. At 18 months, there was a small rise in mean IOP in the low CCT group, resulting in a negative mean reduction percentage and a significant difference to the high CCT group (Table 2 and Figure 1). The results at 18 months may be somewhat biased by the smaller group of patients at that time.
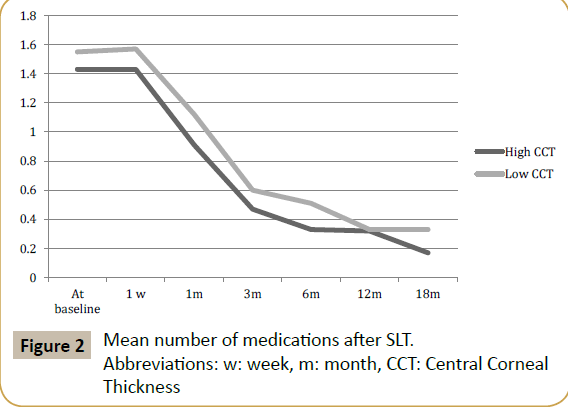
The mean number of medications showed no significant differences between the high and the low CCT group at alltime points (Table 3). In both groups, the mean number of medications lowered significantly; from 1.43 to 0.17 medications in the high CCT group and from 1.55 to 0.33 medications in the low CCT group. Both groups started with comparable amount of medications at baseline (Table 3 and Figure 2).
Discussion
It is well known that measuring IOP with the Goldmann applanation tonometer (GAT) is influenced by the thickness of the cornea, since the design of the GAT was based on a CCT of 500 μm. Consequently, thicker corneas generally yield higher IOP values and thinner corneas lower IOP readings than the ‘true’ IOP [15,19,22,23,31]. There is evidence to suggest however, that the association between CCT and POAG severity is more than just a tonometry artifact. Several studies have proposed CCT as an independent risk factor for POAG and an important predictor of the evolution of OHT to POAG [18,19,25]. CCT could also be an indicator for an independent mechanism of glaucoma based on the biological properties of ocular tissues like the posterior sclera and the lamina cribrosa [16,29,31,32].
| High CCT groupN=58 | Low CCT groupN=77 | t-test/?2 testp-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 68.10 ± 13.10 | 68.53 ± 12.28 | 0.85 |
| Sex (F/M) | 27 (46.55%)/31 (53.45%) | 40 (51.95%)/37 (48.05%) | 0.54 |
| Glaucoma parameters | |||
| IOP baseline with medication (mmHg) | 15.10 ± 3.89 | 13.07 ± 2.94 | 0.001* |
| POAG/OHT | 43 (74.14%)/15 (25.86%) | 72 (93.51%)/5 (6.49%) | 0.002* |
| BCVA | 0.83 ± 0.23 | 0.78 ± 0.28 | 0.24 |
| CCT (µm) | 584.05 ± 26.47 | 516.78 ± 28.02 | <0.001* |
| Cup disc ratio | 0.66 ± 0.25 | 0.80 ± 0.18 | 0.001* |
| Visual field MD | 5.09 ± 7.38 | 5.73 ± 6.22 | 0.59 |
| Visual field PSD | 3.96 ± 3.48 | 4.95 ± 3.89 | 0.13 |
| OCT FLV | 4.31 ± 4.74 | 4.70 ± 4.16 | 0.61 |
| Mean follow up (months) | 13.45 ± 6.08 | 13.79 ± 5.31 | 0.72 |
| IOPmax before medication (mm Hg) | 24.53 ± 5.91 | 22.30 ± 5.31 | 0.02* |
| Medication at start | |||
| Total number (mean) | 1.43 ± 0.70 | 1.55 ± 0.94 | 0.44 |
| Prostaglandin analogs | 49 (84.48%) | 71 (92.21%) | 0.09 |
| Betablocker | 23 (39.66%) | 29 (37.66%) | 0.54 |
| Carbo anhydrase inhibitor | 6 (10.34%) | 14 (18.18%) | 0.06 |
| Alphamimetics | 5 (8.62%) | 5 (6.49%) | 0.67 |
Abbreviations: F: Female; M: Male; IOP: Intraocular Pressure; POAG: Primary Open Angle Glaucoma; OHT: Ocular Hypertension; BCVA: Best Corrected Visual Acuity; CCT: Central Corneal Thickness; MD: Mean Deviation; PSD: Pattern Standard Deviation; OCT: Optical Coherence Tomography; FLV: Focal Loss Of Volume
*Statistically significant difference (p<0.05).
Table 1 Baseline characteristics.
| High CCT group | Low CCT group | t-test p-value |
|
|---|---|---|---|
| 1 hour | 13.67 (n=58) | 8.88 (n=77) | 0.40 |
| 1 week | 12.80 (n=58) | 11.40 (n=77) | 0.80 |
| 1 month | 16.92 (n=58) | 22.23 (n=77) | 0.32 |
| 3 months | 16.51 (n=58) | 12.46 (n=77) | 0.49 |
| 6 months | 15.26 (n=58) | 9.10 (n=77) | 0.21 |
| 12 months | 18.22 (n=41) | 11.72 (n=60) | 0.21 |
| 18 months | 14.24 (n=24) | -5.19 (n=33) | 0.04* |
Table 2: Mean % of IOP reduction after SLT.
Abbreviations: n: number of patients
* Statistically significant difference (p<0.05).
| High CCT group | Low CCT group | χ2-test p-value |
|
|---|---|---|---|
| At baseline | 1.43 ± 0.70 (n=58) | 1.55 ± 0.94 (n=77) | 0.44 |
| 1 week | 1.43 ± 0.70 (n=58) | 1.57 ± 0.94 (n=77) | 0.34 |
| 1 month | 0.91 ± 0.71 (n=58) | 1.12 ± 1.04 (n=77) | 0.20 |
| 3 months | 0.47 ± 0.63 (n=58) | 0.60 ± 0.85 (n=77) | 0.32 |
| 6 months | 0.33 ± 0.54 (n=58) | 0.51 ± 0.75 (n=77) | 0.29 |
| 12 months | 0.32 ± 0.79 (n=41) | 0.33 ± 0.63 (n=60) | 0.91 |
| 18 months | 0.17 ± 0.38 (n=24) | 0.33 ± 0.60 (n=33) | 0.23 |
Table 3 Mean number of medications after SLT.
Abbreviations: n: number of patients
No statistically significant differences (p<0.05).
Since SLT relies on biochemical properties of the tissue of the eye [33-35] and CCT may be an index for tissue qualities, it is not unthinkable that CCT should influence the outcome of SLT.
In our study, we recorded a significantly larger cup disc ratio in patients with thinner cornea compared to those with thicker cornea. This concurs with the study of Pakravan et al. who suggested that thinner corneas may be a marker for more deformable discs, prone to the effects of increased IOP [29]. Jonas et al. also described the high association between thin corneas and more pronounced glaucomatous optic nerve, but noticed that thin corneas were not associated with a higher risk for glaucomatous visual field progression [22]. We can agree with this; although the patients with low CCT and those with high CCT showed a difference in cup to disc ratio, we did not record a significant difference in visual field parameters or OCT of the optic nerve head between the two groups.
Shazly et al. and Kooner et al. recorded a greater reduction of IOP in patients with low CCT following SLT [12,36].
In our trial, we recorded a comparable percentage of IOP reduction between the high and the low CCT groups at all-time point, except at 18 months. At that point, the number of patients was reduced to about half the number at baseline; this makes the results less reliable.
At baseline, our groups showed a significantly different IOP. This may have been a tonometry artifact; mean CCT was 584 μm in the high CCT group compared to 517 μm in the low CCT group. This significant difference can be due to overestimation of the IOP in the high CCT group and an underestimation in the low CCT group, while the ‘true’ IOP is less different between the two groups [29].
As can be expected after SLT [37-39], SLT lowered the number of glaucoma medications; from 1.43 to 0.17 in the high CCT group and from 1.55 to 0.33 in the low CCT group.
CCT was obtained using a hand held pachymeter, which is only one of many methods of measuring CCT. Studies have shown that, depending on the method used, different CCTs may be obtained [23]. Also, a larger number of patients would add significance to the results.
Conclusion
At no point in time was there a significant difference between the IOP of the group with high CCT compared to the group with low CCT. SLT can be considered an efficient therapy in glaucoma in patients with both thinner and thicker corneas.
References
- Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G (1998) Q-switched 532 nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty) - A multicenter, pilot, clinical study. Ophthalmology 105: 2082-2088.
- Wong MO, Lee JW, Choy BN (2015) Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol 60: 36-50.
- Li X, Wang W, Zhang X (2015) Meta-analysis of selective laser trabeculoplasty versus topical medication in the treatment of open-angle glaucoma. BMC Ophthalmol, pp: 1-9.
- Hodge WG (2005) Baseline IOP predicts selective laser trabeculoplasty success at 1 year post-treatment: results from a randomised clinical trial. Br J Ophthalmol 89: 1157-1160.
- Rhodes KM, Weinstein R, Saltzmann RM (2009) Intraocular pressure reduction in the untreated fellow eye after selective laser trabeculoplasty. Curr Med Res Opin 25: 787-796.
- Lee JY, Lee YK, Moon JI (2014) Long-term outcomes and predictive factors for success of selective laser trabeculoplasty. J Kor Ophth Soc 55: 1347-1354.
- McIlraith I, Strasfeld M, Colev G, Hutnik CML (2006) Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma 15: 124-130.
- Weinand FS, Althen F (2006) Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol 16: 100-104.
- Singh K, Shrivastava A (2009) Intraocular pressure fluctuations: How much do they matter? Curr Opin Ophthalmol 20: 84-87.
- Lai JSM, Chua JKH, Tham CCY, Lam DSC (2004) Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Experiment Ophthalmol 32: 368-372.
- Bovell AM, Damji KF, Hodge WG, Rock WJ, Buhrmann RR, et al. (2011) Long term effects on the lowering of intraocular pressure: Selective laser or argon laser trabeculoplasty? Can J Ophthalmol 46: 408-413.
- Hong BK, Winer JC, Martone JF, Wand M, Altman B, et al. (2009) Repeat selective laser trabeculoplasty. J Glaucoma 18: 180-183.
- Shazly T, Latina M, Dagianis JJ, Chitturi S (2011) Effect of prior cataract surgery on the long-term outcome of selective laser trabeculoplasty. OPTH 377-380.
- Shazly TA, Latina MA, Dagianis JJ, Chitturi S (2012) Effect of central corneal thickness on the long-term outcome of selective laser trabeculoplasty as primary treatment for ocular hypertension and primary open-angle glaucoma. Cornea 31: 883-886.
- Doughty MJ, Zaman ML (2000) Human corneal thickness and its impact on intraocular pressure measures. Surv Ophthalmol 44: 367-408.
- Lesk MR (2006) Relationship between central corneal thickness and changes of optic nerve head topography and blood flow after intraocular pressure reduction in open-angle glaucoma and ocular hypertension. Arch Ophthalmol 124: 1568-1572.
- Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA (2006) Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 141: 868-875.
- Gordon MO (2002) The ocular hypertension treatment study. Arch Ophthalmol 120: 714-720.
- Group EGPSE (2007) Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology 114: 3-9.
- Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B (2008) Risk factors for incident open-angle glaucoma. Ophthalmology 115: 85-93.
- Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, et al. (2003) Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol 136: 805-813.
- Jonas JB, Stroux A, Velten I, Juenemann A, Martus P, et al. (2005) Central corneal thickness correlated with glaucoma damage and rate of progression. Invest Ophthalmol Vis Sci 46: 1269-1274.
- Shih CY (2004) Clinical significance of central corneal thickness in the management of glaucoma. Arch Ophthalmol 122: 1270.
- Brandt JD, Beiser JA, Kass MA (2001) Central corneal thickness in the ocular hypertension treatment study. Ophthalmol 108: 1179-1788.
- Leske MC, Heijl A, Hussein M (2003) Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch Ophthalmol 121: 48-56.
- Zhang X, Loewen N, Tan O (2016) Predicting development of glaucomatous visual field conversion using baseline fourier-domain optical coherence tomography. Am J Ophthalmol 163: 29-37.
- Nagar M, Ogunyomade A, O'Bart DP (2005) A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol 89: 1413-1417.
- Jampel HD (1997) Target pressure in glaucoma therapy. J Glaucoma 6: 133-138.
- Pakravan M, Parsa A, Sanagou M, Parsa CF (2006) Central corneal thickness and correlation to optic disc size: A potential link for susceptibility to glaucoma. Br J Ophthalmol 91: 26-28.
- Chauhan BC, Mikelberg FS, Artes PH (2010) Canadian Glaucoma Study: Impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Ophthalmol 128: 1249-1255.
- Dimasi DP, Burdon KP, Craig JE (2010) The genetics of central corneal thickness. Br J Ophthalmol 94: 971-976.
- Chauhan BC (2005) Central corneal thickness and progression of the visual field and optic disc in glaucoma. Br J Ophthalmol 89: 1008-1012.
- Damji KF, Shah KC, Rock WJ, Bains HS, Hodge WG (1999) Selective laser trabeculoplasty vs. argon laser trabeculoplasty: A prospective randomised clinical trial. Br J Ophthalmol 83: 718-722.
- Bylsma SS, Samples JR, Acott TS, Van Buskirk EM (1988) Trabecular cell division after argon laser trabeculoplasty. Arch Ophthalmol 106: 544-547.
- Bradley JM, Anderssohn AM, Colvis CM (2000) Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest Ophthalmol Vis Sci 41: 422-430.
- Kooner KS, Tokunaga J (2005) Central corneal thickness and selective laser trabeculoplasty outcomes. Invest Ophthalmol Vis Sci 46: 107.
- Francis BA, Ianchulev T, Schofield JK, Minckler DS (2005) Selective laser trabeculoplasty as a replacement for medical therapy in open-angle glaucoma. Am J Ophthalmol 140: 524-525.
- Eltanamly R, Abdelrahman A (2014) Selective laser trabeculoplasty in pseudophakic patients with open angle glaucoma. J Egypt Ophthalmol Soc 107: 268-271.
- Werner M, Smith MF, Doyle JW (2007) Selective laser trabeculoplasty in phakic and pseudophakic eyes. Ophthalmic Surg Lasers Imaging 38: 182-188.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences