Antioxidant Properties of Organic and Non-organic Tea Brews
Department of Studies in Food Science and Nutrition, University of Mysore, Mysuru, India
- *Corresponding Author:
- Prof. Jamuna Prakash
Department of Studies in Food Science and Nutrition
University of Mysore, Mysuru 570 006, India
Tel: 91-821-2510054
E-mail: jampr55@hotmail.com
Received Date: January 19, 2018; Accepted Date: February 09, 2018; Published Date: February 15, 2018
Citation: Prakash J, Nagar MN, Devisetti R, Prabhavathi SN (2018) Antioxidant Properties of Organic and Non-organic Tea Brews. J food Biotechnol Res. Vol.2 No.1:5
Abstract
The objective was to determine the antioxidant properties of brews obtained from different types of organic and non-organic tea. Twelve different varieties of commercial tea from organic and non-organic source (four each from black, green and flavoured green tea) were procured. Methanolic and aqueous extracts (obtained from brewing tea in hot water for different time periods) of tea were analyzed for antioxidant components and activities. A wide variation was seen in antioxidant components among tea varieties with higher levels in green tea. The total phenols content was highest followed by flavonoids and condensed tannins. Free radical scavenging activity and ferric reducing antioxidant power revealed high antioxidant activity in tea brews, while metal chelating activity was lesser with significant differences between type of tea and between organic and nonorganic samples. All tea samples had high antioxidant potential, though a specific trend between organic and non-organic could not be observed.
Keywords
Green tea; Black tea; Phenolics; Condensed tannins; Aqueous extracts
Introduction
Consumption of tea is generally considered a social and a habitual concept by most consumers, though awareness regarding health benefits of tea in on the rise. The present day consumers are also conscious about the origin of food and prefer organically grown foods. This has served as a boom for newer products to enter the market such as organic tea and green tea comprising more of natural flavor, fruits and herbal infusions. In addition, consumers opt for ‘green buying behavior’ through the purchase of organic foods grown and packed in environmental friendly conditions [1]. Among the organic foods, organic tea is one of the major products that is being produced and marketed in India. Annually India produces about 6.5 million kg of organic tea, out of which 25% is utilized for domestic consumption. Organic foods are comparatively costlier due to the high logistic cost involved and low volume operation [2]. Organic foods are associated with health promoting property which has been identified as the major motivational factor for their purchase [3,4].
It is generally perceived that organic foods are free from harmful chemical residues, additives and preservatives. They are also considered superior on account of their antioxidant properties in comparison to conventionally grown foods [5,6]. A consumer survey indicated that 70.3% of consumers bought organic foods to avoid pesticides, 68.3% for freshness, 67.1% for healthy and nutritious alternatives, and 55% to avoid genetically modified foods [7]. Use of fertilizers and pesticides is linked with limited synthesis of bioactive components such as phenolic metabolites in the plants [8].
Tea is generally consumed in various forms as green, oolong, pu-erh, and black tea, all of which originate from the leaves of the plant Camellia sinensis L. The techniques of brewing tea also differ among population groups for the proportion of tea leaf used and the brewing time. Hence the strength of the tea brew also varies considerably ranging from 1-2% [9]. The objective of the study was to determine the antioxidant properties of brews obtained from different types of organic and non-organic tea brewed for different time periods.
Materials and Methods
Selection of sample
Twelve different varieties of tea, i.e., six organic and six nonorganic were purchased from super market. The samples were collected in such a way that each conventional/non-organic sample had one organic counterpart sample. The organic tea was purchased from source with authentic organic certification. A total of four black tea samples, four green tea samples and four flavoured green tea samples from different brands were selected as given in Table 1.
Table 1 List of selected tea samples.
| Type of Tea | Organic Tea | Abbreviation used | Non-organic Tea | Abbreviation uses |
|---|---|---|---|---|
| Group A: Black Tea | 1 | OBT1 | 1 | NBT1 |
| 2 | OBT2 | 2 | NBT2 | |
| Group B: Green Tea | 1 | OGT1 | 1 | NGT1 |
| 2 | OGT2 | 2 | NGT2 | |
| Group C: Green Tea Flavoured | 1 | OGTF1 | 1 | NGTF1 |
| 2 | OGTF2 | 2 | NGTF2 |
All samples were analyzed for their antioxidant properties by extraction in methanolic and aqueous media. In aqueous media, tea samples were extracted in hot water for different time periods to mimic the process of brewing tea. The chemicals used for the study were procured from Sigma-Aldrich (St. Louis, USA); and the reagents used were of analytical grade. All analysis was carried out in triplicate and mean values reported. Glass double distilled water was used for all analysis.
Methods
Sample preparation
Extraction with water (brewing): A 2.5 g of tea sample was weighed and added to 50 mL boiling water, stirred thoroughly and brewed for different time intervals of 2, 5, 7 and 10 min. After brewing, extracts were cooled in ice water bath and collected by straining. These extracts were used for analysis of phenolic constituents and antioxidant activities.
Extraction with methanol: Total phenolics from tea samples were extracted with methanol containing 1% HCl (1:10 w/v) by refluxing in water bath. Briefly, 1.0 g sample was accurately weighed in conical flask and 10 mL of 1% HCl in methanol was added. The conical flask was placed in water bath at 50°C for 3 hrs, taken out, cooled, centrifuged and supernatant was collected. Extraction was repeated three times, extracts pooled and used for analysis.
Estimation of antioxidant components
The total phenolic content (TPC) of tea extracts was determined using the method described by Chandrasekara and Shahidi [10]. Briefly, 0.5 mL of the diluted sample was mixed with 0.5 mL of Folin- Ciocalteu’s reagent in centrifuge tubes, followed by addition of 1.0 mL of saturated sodium carbonate and distilled water (8.0 mL). Tubes were allowed to rest in dark for 35 min followed by centrifugation. The absorbance of the resulting blue colour supernatant was measured at 760 nm and TPC was determined using a standard curve prepared using gallic acid and expressed as gallic acid equivalent per gram (mg of GAEq./g). Total flavonoid content (TFC) of the diluted tea extracts (1/20 dilution) were determined by mixing 0.25 mL of sample 1.25 mL of distilled water and 75 μL of 5% NaNO2 solution. After 6 min, 150 μL of 10% AlCl3_6H2O was added rested for 5 min and then 0.5 mL of 1M NaOH was added. Volume of reaction mixture was made up to 2.5 mL with distilled water and absorbance measured against the blank at 510 nm. The TFC was determined using catechin as the standard and expressed as catechin equivalent (mg CATEq./g) [11]. For estimation of condensed tannins (CT) to 1.0 mL of diluted tea extracts, 5.0 mL of 0.5% vanillin-HCl reagent (0.5% vanillin (w/v) in 4% concentrated HCl in methanol] was added and the mixture incubated for 20 min at room temperature. A separate blank for each sample was prepared with 4% HCl in methanol. The absorbance was read at 500 nm, and the CT content was determined using a standard curve with catechin and expressed as mg of catechin equivalents per gram (mg of CATEq./g) [10].
Antioxidant properties
For measuring free radical scavenging activity (FRSA) by α, α-diphenyl-β-picrylhydrazyl (DPPH) method, a 0.2 mL diluted tea extract was added to 3.8 mL methanol solution of DPPH radical (final concentration, 0.1 mM). The mixture was shaken vigorously for 1 min by vortexing and allowed to stand at room temperature in dark for 30 min. A separate blank for each sample was prepared in methanol and the absorbance measured at 517 nm. FRSA was determined using Trolox standard and expressed as mg TROLOX Eq./g [12].
For ferric reducing antioxidant power (FRAP) assay, 100 μL of tea extract and 300 μL of distilled water was mixed with 3.0 mL of FRAP reagent and allowed to stand at room temperature for 4 min. The absorbance was measured at 593 nm against reagent blank using Trolox as standard and expressed as mg Trolox Eq./g [13].
Metal chelating activity was measured as follows - a 0.4 mL of tea extracts in distilled water was added to a solution of 50 μL of 2 mM FeCl2. The reaction was initiated by adding 200 μL of 5 mM Ferrozine and the total volume made up to 4 mL with distilled water. The mixture was vigorously shaken and left at room temperature for 10 min. The absorbance of the reaction mixture was measured at 562 nm. For the control, distilled water was used instead of the extract. Appropriate blanks were prepared with 0.4 mL of the sample and 3.6 mL of distilled water for background subtraction. Metal chelating activity was determined by using EDTA as standard and is expressed as mg EDTA Eq./g [14]. Total antioxidant activity was determined by mixing 0.1 mL of diluted sample solution and 1.0 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) in test tubes. The test tubes were wrapped and incubated in a water bath at 95°C for 90 min. Then the samples were allowed to cool and absorbance measured at 695 nm against blank. Total antioxidant capacity was determined using TROLOX as standard and expressed as mg TROLOX Eq./g [15].
Statistical analysis
The data were analyzed to obtain mean and standard deviation. ANOVA was applied employing Duncan’s multiple range test (DMRT) at 5% level of significance to know the significant differences between the samples. Three different types of tea samples viz. organic and non-organic black tea, green tea, and green tea flavoured samples were compared between each group and within the entire set to know the level of significant differences.
Results and Discussion
The results of the study are compiled in Tables 2-5 and Figure 1. The statistical analysis data is compiled in supplementary files.
Table 2 Total phenolic, flavonoids and condensed tannins in methanolic extracts of tea.
| Group | Organic Te1 | Total phenolics (mg GA Eq./g) | Total flavonoid (mg CAT Eq./g) | Condensed tannins (mg CAT Eq./g) | Non-organic Te1 | Total phenolics (mg GA Eq./g) | Total flavonoid (mg CAT Eq./g) | Condensed tannins (mg CAT Eq./g) |
|---|---|---|---|---|---|---|---|---|
| Groupa | OBT 1 | 91.75 ± 2.17a | 53.72 ± 7.81a | 146.14 ± 9.72a | NBT 1 | 41.13 ± 6.23d | 29.27 ± 2.37d | 52.85 ± 6.11c |
| OBT 2 | 68.2 ± 2.43b | 42.75 ± 2.06b | 66.49 ± 3.64b | NBT 2 | 58.49 ± 3.45c | 36.2 ± 3.14c | 60.95 ± 2.8b | |
| Groupb | OGT 1 | 108.42 ± 5.12a | 64.76 ± 2.6a | 87.94 ± 2.62c | NGT 1 | 109.58 ± 7.99a | 49.2 ± 1.22c | 102.71 ± 8.19b |
| OGT 2 | 88.66 ± 3.5b | 52.53 ± 8.56b | 75.95 ± 8.03d | NGT 2 | 110.73 ± 0.34a | 59.9 ± 4.37b | 114.09 ± 8.93a | |
| Groupc | OGTF 1 | 96.81 ± 1.87b | 20.12 ± 0.38c | 75.9 ± 4.64b | NGTF 1 | 89.98 ± 0.17c | 30.57 ± 6.26a | 52.41 ± 11.09d |
| OGTF 2 | 108.99 ± 3.88a | 31.41 ± 0.72a | 114.15 ± 1.98a | NGTF 2 | 105.75 ± 4.64a | 28.67 ± 0.31b | 68.18 ± 8.18c |
Note: Means with different superscript for organic and non-organic tea in a group are significantly different at p<0.05
Table 3 Total phenolic, flavonoids and condensed tannins in aqueous brew extracts of tea.
| Group | Organic Tea | Brewing time (min) | Non-organic tea | Brewing time (min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 7 | 10 | 2 | 5 | 7 | 10 | |||
| Total phenolics (mg GA Eq./g) | ||||||||||
| Group a | OBT 1 | 25.69 ± 1.79a | 25.95 ± 2.2a | 26.27 ± 1.9a | 31.69 ± 1.04a | NBT 1 | 19.88 ± 1.54b | 24.17 ± 1.79a | 25.76 ± 1.07a | 28.33 ± 2.72b |
| OBT 2 | 24.57 ± 1.49a | 26.09 ± 1.31a | 30.06 ± 1.9b | 31.02 ± 1.99a | NBT 2 | 18.42 ± 0.74b | 18.62 ± 0.59b | 25.14 ± 0.91a | 25.2 ± 1.47b | |
| Group b | OGT 1 | 26.74 ± 0.57b | 41.75 ± 10.28a | 41.93 ± 1.14b | 43.02 ± 0.72c | NGT 1 | 26.19 ± 3.11b | 26.61 ± 1.84b | 45.98 ± 4.89b | 46.04 ± 5.86c |
| OGT 2 | 41.29 ± 8.08a | 48.29 ± 1.43a | 54.53 ± 1.3a | 66.4 ± 2.2a | NGT 2 | 40.06 ± 0.88a | 45.3 ± 1.94a | 47.76 ± 3.33b | 55.53 ± 21b | |
| Group c | OGTF 1 | 18.43 ± 1.27b | 17.49 ± 0.13c | 23.85 ± 0.25b | 26.64 ± 1.09b | NGTF 1 | 33.32 ± 0.97a | 37.46 ± 1.97a | 38.25 ± 1.49a | 43.1 ± 2.34a |
| OGTF 2 | 10.73 ± 0.59c | 10.55 ± 0.38d | 14.53 ± 0.49c | 15.04 ± 0.64c | NGTF 2 | 18.33 ± 0.6b | 25.05 ± 0.76b | 28.86 ± 1.54b | 28.61 ± 0.6b | |
| Total flavonoids (mg CAT Eq./g) | ||||||||||
| Group a | OBT 1 | 13.91 ± 0.88a | 13.37 ± 1.48a | 13.47 ± 0.46a | 16.56 ± 2.23a | NBT 1 | 8.6 ± 0.46c | 11.6 ± 0.64b | 11.26 ± 1.08b | 12.66 ± 1.06b |
| OBT 2 | 10.54 ± 0.84b | 10.48 ± 0.66b | 11.09 ± 1.07b | 12.35 ± 0.81b | NBT 2 | 8.55 ± 1.5c | 9.35 ± 0.88b | 11.06 ± 0.37b | 11.45 ± 0.21c | |
| Group b | OGT 1 | 15.94 ± 3.06b | 24.88 ± 1.06a | 27.37 ± 2.88a | 25.98 ± 2.23a | NGT 1 | 15.96 ± 0.85b | 17 ± 5.13b | 21.58 ± 1.52a | 22.5 ± 0.83a |
| OGT 2 | 21.73 ± 3.31a | 23.35 ± 0.75a | 27.07 ± 4.01a | 29.11 ± 2.36a | NGT 2 | 21.63 ± 3.75a | 23.56 ± 7.37a | 24.26 ± 0.49a | 26.36 ± 1.86a | |
| Group c | OGTF 1 | 11.94 ± 0.13b | 14.01 ± 0.37b | 16.02 ± 0.27b | 18.82 ± 0.49b | NGTF 1 | 16.8 ± 0.4a | 17.43 ± 1.23a | 20.79 ± 0.35a | 21 ± 0.59a |
| OGTF 2 | 9.26 ± 0.17c | 9.59 ± 0.23c | 13.36 ± 0.37c | 14.59 ± 2.44c | NGTF 2 | 9.82 ± 0.47c | 13.07 ± 0.17b | 14.79 ± 1.41c | 15.37 ± 0.29c | |
| Condensed tannins (mg CAT Eq./g) | ||||||||||
| Group a | OBT 1 | 6.95 ± 0.16a | 8.11 ± 0.35a | 8.26 ± 0.14a | 10.12 ± 0.21a | NBT 1 | 2.57 ± 0.24b | 3.47 ± 0.18c | 3.9 ± 0.24c | 3.95 ± 0.14d |
| OBT 2 | 5.81 ± 0.32a | 5.75 ± 0.1b | 6.15 ± 0.23b | 8.93 ± 0.15b | NBT 2 | 2.48 ± 0.00c | 3.62 ± 0.12c | 4.58 ± 0.21c | 6.08 ± 0.1c | |
| Group b | OGT 1 | 4.99 ± 0.1b | 5.21 ± 0.39b | 5.88 ± 0.15b | 6.05 ± 0.14b | NGT 1 | 3.27 ± 0.2c | 4.7 ± 0.16c | 5.98 ± 0.09b | 5.98 ± 0.08c |
| OGT 2 | 4.65 ± 0.26b | 5.53 ± 0.12b | 5.69 ± 0.16b | 6.17 ± 0.09b | NGT 2 | 5.76 ± 0.1a | 6.35 ± 0.11a | 7.21 ± 0.9a | 9.43 ± 0.14a | |
| Group c | OGTF 1 | 0.75 ± 0.08d | 1.22 ± 0.2c | 1.72 ± 0.41c | 3.78 ± 0.58b | NGTF 1 | 5.42 ± 0.09a | 5.95 ± 0.18a | 6.33 ± 0.15a | 6.9 ± 0.12a |
| OGTF 2 | 1.08 ± 0.14c | 1.94 ± 0.22c | 2.34 ± 0.14c | 2.66 ± 0.46b | NGTF 2 | 4.27 ± 0.8b | 4.83 ± 0.1b | 5.31 ± 0.14b | 6.17 ± 0.62a | |
Note: Means with different superscript for organic and non-organic tea in a group are significantly different at p<0.05
Table 4 Antioxidant activity of methanolic extracts of tea as measured by different assays.
| Tea | Free radical scavenging activity (mg TROLOX Eq./g) | Ferric reducing antioxidant power (mg TROLOX Eq./g) | Metal chelating activity (mg EDTA Eq./g) | Total antioxidant activity (mg TROLOX Eq./g) |
|---|---|---|---|---|
| OBT 1 | 47.15 ± 3.39e | 151.58 ± 8.78c | 1.07 ± 0.02c | 515.97 ± 10.09c |
| OBT 2 | 83.58 ± 2.41c | 98.59 ± 17.99d | 2.67 ± 0.1a | 354.82 ± 4.16d |
| OGT 1 | 353.52 ± 9.1a | 151.25 ± 8.61c | 1.08 ± 0.07c | 668.42 ± 17.82a |
| OGT 2 | 150.78 ± 9.27b | 159.09 ± 3.88c | 0.67 ± 0.0bc | 582.09 ± 27.97b |
| OGTF 1 | 62.9 ± 12.89d | 225.28 ± 2.5a | 0.99 ± 0.07c | 418.55 ± 17.11d |
| OGTF 2 | 68.74 ± 14.86d | 164.5 ± 7.84b | 1.17 ± 0.05b | 321.05 ± 11.83e |
| NBT 1 | 143.37 ± 4.68c | 85.09 ± 7.82e | 1.67 ± 0.08b | 248.06 ± 7.23f |
| NBT 2 | 135.49 ± 3.17c | 88.4 ± 3.03e | 1.73 ± 0.09b | 307.02 ± 3.61e |
| NGT 1 | 141.88 ± 4.58c | 164.12 ± 2.66c | 1.01 ± 0.08c | 600.00 ± 13.81b |
| NGT 2 | 180.84 ± 3.91a | 215.08 ± 8.91b | 0.66 ± 0.08c | 781.08 ± 37.15a |
| NGTF 1 | 65.94 ± 2.47d | 124.97 ± 6.62d | 1.05 ± 0.09c | 512.88 ± 11.51c |
| NGTF 2 | 159.7 ± 2.16b | 363.38 ± 3.54a | 2.31 ± 0.05a | 538.41 ± 16.45c |
Note: Means with different superscript for organic and non-organic tea in a column are significantly different at p<0.05
Table 5 Antioxidant activity of aqueous brew extracts of tea as measured by different assays.
| Organic Tea | Brewing time (min) | Non-organic Tea | Brewing time (min) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 7 | 10 | 2 | 5 | 7 | 10 | ||
| Free radical scavenging activity (mg TROLOX Eq./g) | |||||||||
| OBT 1 | 5.2 ± 0.94a | 8.81 ± 2.71c | 14.17 ± 5.37c | 23.21 ± 6.38c | NBT 1 | 2.94 ± 1.1a | 3.4 ± 0.85d | 6.93 ± 2.71d | 10.21 ± 0.82d |
| OBT 2 | 15.04 ± 1.15b | 18.49 ± 7.51b | 27.48 ± 0.95b | 33.16 ± 0.49b | NBT 2 | 13.14 ± 2.68b | 39.93 ± 1.64a | 87.32 ± 3.9a | 108.97 ± 7.13a |
| OGT 1 | 17.87 ± 0.64c | 29.02 ± 4.41c | 48.83 ± 2.26c | 69.5 ± 1.37c | NGT 1 | 11.54 ± 1.74c | 20.14 ± 5.81d | 40.54 ± 3.47d | 40.7 ± 1.2d |
| OGT 2 | 43.91 ± 8.66a | 66.03 ± 34.2b | 81.21 ± 2.09b | 113.57 ± 4.22b | NGT 2 | 30.13 ± 6.51b | 81.86 ± 1.64a | 98.62 ± 4.88a | 136.4 ± 2.08a |
| OGTF 1 | 25.34 ± 4.65b | 65.97 ± 6.44b | 90.54 ± 6.22b | 111.81 ± 6.59b | NGTF 1 | 126.44 ± 2.51a | 139.5 ± 3.31a | 144.31 ± 9.65a | 238.1 ± 5.49a |
| OGTF 2 | 5.78 ± 1.08d | 28.5 ± 2.46c | 37.81 ± 1.44d | 50.09 ± 2.28d | NGTF 2 | 34.13 ± 3.91c | 61.94 ± 4.47b | 76.87 ± 3.04c | 85.23 ± 5.58c |
| Ferric reducing antioxidant power (mg TROLOX Eq./g) | |||||||||
| OBT 1 | 49.55 ± 1.3a | 59.16 ± 19.28a | 65.58 ± 15.21a | 68.96 ± 3.58a | NBT 1 | 37.21 ± 1.07d | 39.85 ± 1.47c | 42.05 ± 6.27c | 47.4 ± 1.18d |
| OBT 2 | 42.81 ± 0.4b | 43.99 ± 0.93b | 47.07 ± 1.96b | 58.89 ± 0.91b | NBT 2 | 38.68 ± 1.13c | 38.46 ± 2.75c | 43.2 ± 1.32c | 51.65 ± 0.76c |
| OGT 1 | 50.11 ± 1.18a | 52.1 ± 1.44b | 59.52 ± 4.06b | 65.01 ± 0.66c | NGT 1 | 43.31 ± 1.67b | 46.93 ± 0.2c | 50.22 ± 0.97b | 52.08 ± 1.38d |
| OGT 2 | 53.98 ± 2.71a | 58.96 ± 2.22b | 69.73 ± 1.04a | 71.97 ± 3.27b | NGT 2 | 54.1 ± 0.75a | 66.15 ± 4.88a | 70.65 ± 1.71a | 86.51 ± 5.07a |
| OGTF 1 | 40.64 ± 0.37b | 52.56 ± 1.66b | 54.38 ± 2.14b | 55.17 ± 2.19c | NGTF 1 | 89.7 ± 3.55a | 90.62 ± 7.01a | 93.66 ± 3.59a | 95.2 ± 8.09a |
| OGTF 2 | 23.87 ± 0.48d | 30.74 ± 1.11d | 40.36 ± 1.6c | 43.31 ± 1.92dJ | NGTF 2 | 34.63 ± 1.48c | 43.65 ± 0.75c | 51.32 ± 1.43b | 66.17 ± 4.83b |
| Metal Chelating Activity (mg EDTA Eq./g) | |||||||||
| OBT 1 | 0.43 ± 0.03c | 0.83 ± 0.1c | 0.9 ± 0.05c | 0.92 ± 0.11c | NBT 1 | 0.77 ± 0.05b | 1.06 ± 0.02b | 1.2 ± 0.02b | 1.21 ± 0.14a |
| OBT 2 | 0.99 ± 0.05a | 1.07 ± 0.07b | 1.13 ± 0.06b | 1.13 ± 0.09b | NBT 2 | 1.21 ± 0.13a | 1.23 ± 0.03a | 1.36 ± 0.08a | 1.37 ± 0.05a |
| OGT 1 | 0.52 ± 0.13a | 0.55 ± 0.06a | 0.56 ± 0.13a | 0.59 ± 0.07a | NGT 1 | 0.33 ± 0.03b | 0.37 ± 0.04b | 0.44 ± 0.11b | 0.77 ± 0.18a |
| OGT 2 | 0.36 ± 0.11b | 0.36 ± 0.01b | 0.51 ± 0.01a | 0.6 ± 0.12a | NGT 2 | 0.15 ± 0.05c | 0.18 ± 0.05c | 0.31 ± 0.08c | 0.57 ± 0.08a |
| OGTF 1 | 0.51 ± 0.06b | 0.79 ± 0.03b | 0.85 ± 0.03b | 0.94 ± 0.16a | NGTF 1 | 0.58 ± 0.06b | 0.68 ± 0.14c | 0.7 ± 0.13b | 0.93 ± 0.05a |
| OGTF 2 | 0.86 ± 0.1a | 0.99 ± 0.02a | 1.03 ± 0.01a | 1.06 ± 0.04a | NGTF 2 | 0.39 ± 0.02c | 0.42 ± 0.14d | 0.55 ± 0.09c | 0.56 ± 0.12b |
| Total Antioxidant Activity (mg TROLOX Eq./g) | |||||||||
| OBT 1 | 38.06 ± 7.94c | 54.9 ± 27.29c | 75.15 ± 32.51b | 80.9 ± 15.02b | NBT 1 | 57.86 ± 1.72b | 69.45 ± 3.22b | 71.55 ± 4.15b | 84.72 ± 2.25b |
| OBT 2 | 74.58 ± 6.09a | 83.65 ± 8.02a | 89.18 ± 2.36a | 97.73 ± 26.13a | NBT 2 | 59.62 ± 1.5b | 63.68 ± 4.9b | 69.45 ± 3.22c | 71.35 ± 4.14c |
| OGT 1 | 61.86 ± 7.06c | 63.15 ± 6.92c | 78.42 ± 5.61d | 135.19 ± 5.74b | NGT 1 | 51.92 ± 1.18d | 65.78 ± 7.88c | 96.04 ± 9.04c | 96.85 ± 7.58d |
| OGT 2 | 76.37 ± 4.5b | 88.04 ± 3.45b | 103.51 ± 12.47b | 120.79 ± 17.41c | NGT 2 | 87.76 ± 4.32a | 151.02 ± 11.32a | 177.73 ± 2.77a | 179.12 ± 8.01a |
| OGTF 1 | 53.55 ± 2.97b | 66.26 ± 14.65c | 106.73 ± 4.04b | 116.38 ± 19.35b | NGTF 1 | 112.99 ± 15.75a | 181.74 ± 22.11a | 183.94 ± 16.45a | 185.25 ± 5.12a |
| OGTF 2 | 16.48 ± 5.39d | 33.92 ± 13.79d | 50.76 ± 4.26d | 57.41 ± 4.53d | NGTF 2 | 47.65 ± 7.8c | 76.62 ± 1.11b | 77.51 ± 2.19c | 82.77 ± 6.04c |
Note: Means with different superscript for organic and non-organic tea in a column are significantly different at p<0.05
Antioxidants components in organic and nonorganic tea
The antioxidants components of tea were measured and results are compiled in Table 2 (for methanolic extracts), and Table 3 (for aqueous extracts). The total amount of polyphenols in a tea sample is regarded as a major quality determinant of tea. The catechins present in green tea are normally referred as polyphenols. Catechins and other polyphenols have been known to have antioxidant activities. They possess antioxidant property via sequestering metal ion and by scavenging reactive O2 and nitrogen species [16]. Among the organic tea samples, the highest TPC was found in methanolic extracts of OGT1 and OGTF2 with similar values. Other samples had significantly lower amounts with least value in OBT2. For the non-organic tea samples, higher range of TPC was seen in NGT1, NGT2 and NGTF2 (range, 105.75- 110.73 mg GAEq./g). Black tea samples had the least TPC among all in non-organic category. When compared between the group, OGT1 and NGT1 had almost similar TPC. Comparison within the group indicated organic tea samples to have higher phenolics, though major differences were not observed among green tea varieties. Statistical analysis indicated significant differences within the group and for the entire set of samples.
The TFC of organic black tea and green tea was higher than non-organic counterpart, though one variety of non-organic flavoured green tea had high TFC. Between organic and nonorganic, former had higher TFC with differences being significant. The total amount of condensed tannins in methanolic extracts of tea samples showed higher concentration in organic black and flavoured green tea, whereas green tea among non-organic category had higher content. OBT1 was found to have the highest content (146.14 mg CATEq./g) followed by OGTF2 (114.158 mg CATEq./g). Among the non-organic tea, the higher content was observed in NGT2 (114.09 mg CATEq./g). The differences between the samples were found to be statistically significant.
Data pertaining to TPC in aqueous brew extracts of both organic and non-organic tea samples are compiled in Table 3. As evident, for all tea samples the TPC increased with increase in brewing time. The highest TPC observed in OGT2 was in the range of 41.29 – 66.40 mg GAEq./g and for non-organic counterpart, it ranged from 40.06 to 55.53 mg GAEq./g. The pattern of increase in total phenolic content was observed to be similar for both the varieties with maximum phenolics leaching at 10 min of brewing. Comparison between organic and non-organic tea revealed significant groupwise differences. Among black tea samples, organic varieties were better; green tea did not differ significantly, whereas among flavoured green tea, non-organic had higher phenolics. Overall analysis indicated wide variations among all samples with significant differences.
Anesini et al. determined the TPC and antioxidant capacity of twelve commercially available tea samples, of which three were green and the rest were black tea samples [17]. All the samples were subjected to extraction and determination of TPC, antioxidant activity and FRSA. The range of TPC as reported in green tea ranged from 21.02-14.32 GAE%, whereas for black tea, the range was 8.42 to 17.62 GAE%. This difference in various brands was attributed to post maturation process where black tea subjected for an extended period of fermentation would normally result in the oxidation of phenolic compounds. Tea from India or Sri Lanka have been shown to have higher polyphenol content (up to 30%) than those from Chinese variety (upto 20%) [18].
The TFC in aqueous brew extracts of tea samples showed similar trend of increase with brewing time as that of total phenols for both organic and non-organic tea samples. The most significant factors which are found to influence flavonoid content per serving of tea are the weight and tea variety. Studies have also been performed using different infusion rates and are known to yield varied results depending upon the strenght of infusion. The flavonol profile in teas, the flavour and color of tea are known to be greatly affected by the type of tea such as black, green, oolong, pu-erh and herbal tea. Among the organic variety a considerably higher value was noted in OGT2. Brewing for 2 min yielded a total flavonoid content of 21.73 mg CATEq./g, which increased to 29.11 mg CATEq./g after brewing for 10 min. Black tea and flavoured green tea had comparatively lesser flavonoids. Compared to total phenols, flavonoid content in a group were in a nearer range. Statistically significant differences were noted with respect to both the varieties.
Black teas have been estimated to have a characteristic profile of flavonoids. Their presence in tea is influenced by their content and brewing conditions. Theaflavins and polymeric thearubigins are known to be present in large quantities. Brewing techniques are said to vary considerably across the world. Peterson et al. examined the influence of variety and brewing technique on the characteristic of flavonols of ten samples of black tea [19]. Total catechins, theaflavins and thearubigins were analyzed and exhibited cosiderable varietal differences. The results revealed that the blended teas had lowest catechin content. Among the unblended varieties, Darjeeling had highest and Ceylon variety the least catechin content. Theaflavin content was high in both Assam and Kenya varieties with significant differences among samples. China and Darjeeling teas had lowest theaflavin content, whereas Ceylon and blended teas exhibited average values. Analysis of thearubigin showed highest content in Assam and Kenya variety, followed by Ceylon variety. Darjeeling variety had least content and was significantly different from all the other varieties.
Proanthocyanidins or condensed tannins are one of the important groups of di-oligomeric flavonoids present in plants, foods and in pharmaceutical compounds [20]. They are recognized as potential antioxidant compounds. Proanthocyanidin tend to yield anthocyanidins upon heating with aqueous acids. Proanthocyanidins isolated from tea comprises about 2% of the total weight of the leaf on dry weight basis. In the present study, condensed tannin content in aqueous brew extracts of tea were very low compared to total phenols and flavonoids in all tea samples (Table 3). Among samples, organic green flavoured tea had least content followed by green tea and black tea varieties. The non-organic varieties had flavonoid content in the range of 2.4 - 5.76 mg CATEq./g initially and increased to 3.95 – 9.43 mg CATEq./g after 10 min of brewing with no specific trend among green tea or black tea. The mean difference in the increase of the flavonoid content was not very large irrespective of the tea being from organic or non-organic source on brewing. The differences within each group and the variety were statistically significant.
Luximon-ramma et al. characterized the antioxidant functions of flavonoids and proanthocyanidins in freshly plucked nine commercially available tea and extracts of Mauritian black tea [21]. The flavonid content of the infused tea samples was found to be in the range of 15 ± 2 to 26 ± 3 mg/g whereas the proanthocyanidin content varied from 25 ± 2 to 74 ± 10 mg/g on dry weight basis. The infused samples of fresh tea leaves were found to contribute 184 ± 36 mg/g of total phenols, 34 ± 5 mg/g of total flavonoids and 64 ± 11 mg/g of proanthocyanidins on dry weight basis. Results clearly indicated that fresh tea leaves had much higher antioxidant components in comparison to black tea leaves.
Antioxidant activity of organic and non-organic tea
The FRSA in methanolic extracts of tea sample presented in Table 4 showed a wide variation among organic tea samples. The highest scavenging activity was observed in OGT1 (353.52 mg Trolox Eq./g) and the least was seen in OBT1 (47.15 mg Trolox Eq./g). In non-organic tea samples, NBT1, NBT2 and NGT1 had nearing range of scavenging activity (135.49 to 143.37 mg Trolox Eq./g), while others had ether higher or lower levels, the difference being significant.
The results of FRAP assay in methanolic extracts of tea samples showed varied results with some varieties under organic and some under non-organic showing higher FRAP (Table 4). Overall green tea samples had higher FRAP value than black tea in both categories. FRAP values were also much higher that FRSA indicating that this assay could estimate the antioxidant capacity of tea samples efficiently. Statistical analysis revealed that both the varieties were observed to have significant differences.
Metal chelating activity in methanolic extract of tea samples showed that all samples from both organic and non-organic tea variety had extremely low activity. The observed activity was found to be unique for each variety of tea samples. Non-organic tea samples also exhibited relatively low metal chelating activity, though differences were significant for all samples.
The total antioxidant activity of the methanolic extracts of tea samples presented in Table 4 shows that the samples from both the varieties i.e., organic and non-organic had considerably higher total antioxidant activity. Among the organic variety OGT1 had the highest activity (668.42 ± 17.82 mg TROLOX Eq./g). Comparison between OGT2 and IGT2 indicated IGT2 to be superior with higher activity as indicated by the observed values (781.08 ± 37.15 mg TROLOX Eq./g). Between OGTF1 and IGTF1 the methanolic extract provided widely varying levels of activity. On the whole, it can be concluded that all the selected samples showed considerable higher activity level and these tea samples could be utilized as a potential source of antioxidant activity for long term health benefits.
The FRSA in aqueous brew extracts of organic tea and non-organic tea samples are given in Table 5. In comparison to methanolic extracts most of the aqueous extracts showed low levels of FRSA with the exception of IGTF1. The scavenging activity of IGTF1 was found to be in the range of 126.44-238.1 mg TROLOX Eq./g.
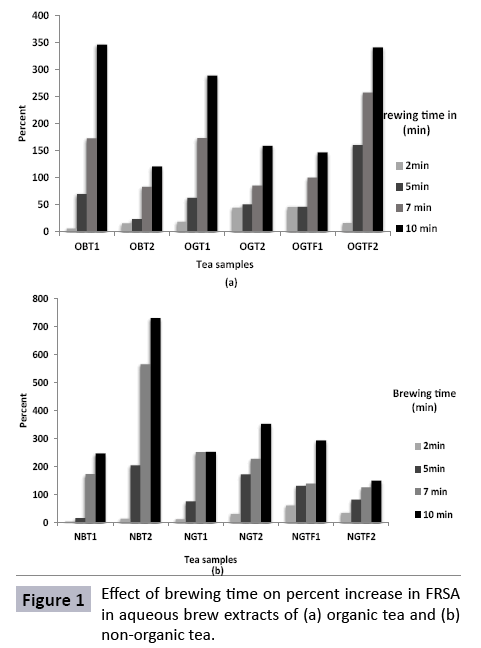
Figure 1 shows percent increase in FRSA in aqueous brew extracts of tea samples as a function of brewing time. All the samples from both the groups indicated a gradual increase in scavenging activity on brewing. The highest amount of percent increase was noted for OBT1 and OGTF2 with 346% and 341% respectively. A proportionately lower percent increase was observed for OBT2 which was in the range of 15 - 120% over the brewing time. Among the non-organic tea samples, a considerably highest level of percent increase was seen in NBT2 (maximum of 729% at 10 min) and lowest was seen for NGTF2 (150%) at highest brewing time.
Zhang et al. evaluated the antioxidant activity of 10 compounds in different tea samples and reported the range of TROLOX equivalent in the range of 1321 to 2550 mM TROLOX [22]. Green teas had high mean TROLOX equivalent of 2143 mM and was significantly different. Green teas also exhibited higher antioxidant activity than white tea with an IC50 value ranging between 33.95-35.80 μg/mL. Similar results have been reported by other workers also. Unachukwu et al. reported a mean DPPH IC50 value of 23.26 μg/mL for green tea samples. Furthermore, these values indicate that the tea samples possess good FRSA [23]. The different antioxidant capacity showed by polyphenolic compound was found to be consistent with their chemical structure with respect to the number and position of phenolic hydroxyl groups. The investigators concluded that catechin component, particularly EGCG (Epigallocatechin-3-gallate) greatly contribute to the antioxidant activity of tea. The catechins were found to decrease during tea fermentation and could result in the reduced antioxidant capacity. This may be the reason for the lower antioxidant capacity of the black tea when compared with the green tea.
The estimated FRAP in aqueous brew extracts of tea samples presented in Table 5 were much lower than methanolic extracts. Overall range of values for organic and non- organic samples were 43.31 – 71.97 and 47.4 – 95.2 mg TROLOX Eq./g respectively. The trend in increase in the reducing power with each brewing time did not show a wide variation. The amount of increase was found to be in very narrow range. Therefore, it is difficult to arrive at any conclusion to declare whether organic or non-organic samples are better as per this assay. Statistically significant differences were noticed with respect to all the selected samples.
It is a well-established fact that, tea antioxidants affords protection against strong mutagens [24]. The antioxidant composition varies with each type of tea. Some tea antioxidants may be more active and are more easily absorbed than the others [25,26]. Benzie et al. analyzed 25 tea varieties for total antioxidant capacity by the FRAP assay [27]. Freshly prepared infusion of different types of tea was indicated to have widely varied antioxidant activities, which ranged from 132 μmol/g for one variety of black tea to 1144 μmol/g for green tea. A stability study was also performed, by keeping the tea infusions at 4°C for 48 hr which indicated no significant change over the designated period of storage, which implies that antioxidants in teas are stable. A linear relationship was noted between the strength of tea infusion and the FRAP value. Also a strong correlation was observed between FRAP value and the estimated total phenolic content. This indicates that the number of phenolic hydroxyl groups in a tea could be considered as the major determinants of antioxidant power in a tea sample. It is estimated that a cup of green tea would contribute an amount of antioxidant power similar to that encountered in 100-200 mg of pure ascorbic acid. Hence, green tea has enormous potential to be a significant dietary source of antioxidants.
Table 5 also shows the metal chelating activity in aqueous brew extracts of tea samples. Similar to methanolic extracts, even aqueous extracts showed very low metal chelating activity with very small insignificant increase with brewing time. Group wise differences were not observed though there were differences between organic and non-organic varieties.
The flavonoid compounds particularly the catechins have the ability to chelate metal ions such as iron and copper. These in turn may contribute to their antioxidant activity through the inhibition of transitions metal catalyzed free radical formation. The most likely metal binding site for catechins is the orthodihydroxy group in the B ring [28]. One of the study, attempted to examine the effects of pH and metal ions as the antioxidative activites of catechins. The observations suggested that, the gallate moiety of the gallocatechins were also evaluated to bind metals [29]. In the same study it was also found that binding of Cu2+ was known to increase the antioxidant activity of EGCG and binding of Fe2+ was observed to have inhibitory effects on its antioxidant activity. Catechins are also known to exert inhibitory effect on copper mediated low density lipoprotein oxidation and other metal catalyzed oxidation in vitro could be probably due to their ability to chelate metals as well as their radical scavenging activities [30].
The results of the analysis of total antioxidant activity in aqueous brew extracts of tea samples are presented in Table 5. Between organic and non-organic tea samples a wide variation in the analysed values were recorded. Few samples from non-organic variety exhibited relatively higher level of activity (IGT2 with 179.12 ± 8.01 mg TROLOX Eq./g vs OGT2 with 120.79 ± 17.41 mg TROLOX Eq./g). Similar pattern of observation was recorded even for IGTF1 (185.25 ± 5.12 mg TROLOX Eq./g) and OGTF1 (116.38 ± 19.35 mg TROLOX Eq./g). For all the samples it was observed that with brewing time, the obtained values were found to have been increased reaching the highest level at the last variation i.e., brewing for 10 min. For OBT1 and IBT1 the activity level was in the range of 38.06 ± 7.94 - 80.9 ± 15.02 and 57.86 ± 1.72 - 84.72 ± 2.25) respectively. Comparative analysis of the data with each group and for the entire set of sample was found to have statistically significant differences.
Conclusion
The present study gave some importance inferences that the total content of bioactive components could be affected by extracting solvent particularly the polyphenolic content and antioxidant activity. Samples extracted with methanol were shown to have higher amounts of phenolic and flavonoids. Significant variations in the content and composition of polyphenols also were noted. Organic green teas were shown to have better antioxidant potentialities in comparison to inorganic tea varieties. It was also found that the amount of brewing time was found to have greater impact on the content of bioactive components. With maximum brewing time all the bioactive components were observed to follow an increasing trend. All the assayed components differed with each variety of tea samples and they were statistically significant. Various factors such as tea leaf variety, growth condition, climate, processing and analytical methods used would tend to affect the overall composition. However, further long term, well controlled human trials are required before any firm conclusions can be made regarding the proposed health benefits of tea consumption.
Acknowledgement
Financial support from Department of Science and Technology, Govt. of India, under DST-PURSE project for this research is gratefully acknowledged.
References
- Willer H, Kilcher L (2010) The world of organic agriculture - Statistics and emerging trends.
- Chakrabarti S (2010) Factors influencing organic food purchase in India–expert survey insights. Brit Food J 112: 902-915.
- Zanoli R, Naspetti S (2002) Consumer motivations in the purchase of organic food: A means-end approach. Brit Food J 104: 643-653.
- Sun YHC (2008) Health concern, food choice motives, and attitudes toward healthy eating: The mediating role of food choice motives. Appetite 51: 42-49.
- Padel S, Foste C (2005) Exploring the gap between attitudes and behaviour: Understanding why consumers buy or do not buy organic food. Brit Food J 107: 606-625.
- Williams CM (2002) Nutritional quality of organic food: shades of grey or shades of green. ProceedNutriSoc61: 19-24.
- Siderer Y, Maquet A, Anklam E (2005) Need for research to support consumer confidence in the growing organic food market. Trends Food Sci Technol 16: 332-343.
- Winter CK, Davis SF (2006) Organic foods.J Food Sci 71: 117-214.
- Simrany J (2003) A tea tour of Sri Lanka. Tea Coffee Trade J 175: 15-19.
- Chandrasekara A, Shahidi F (2010) Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agri Food Chem 58: 6706-6714.
- Heimler D, Vignolini P, Dini MG, Romani A (2005) Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J Agri Food Chem 53: 3053-3056.
- Chen CW, Ho CT (1995) Antioxidant properties of polyphenols extracted from green and black teas. J Food Lipids 2: 35-46.
- Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299: 15-27.
- Dinis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch BiochemBiophy 315: 161-169.
- Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Annals Biochem 269: 337-341.
- Frei B, Higdon JV (2003) Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutri1 33: 3275-3284.
- Anesini C, Ferraro GE, Filip R (2008) Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agri Food Chem 56: 9225-9229.
- HarbowyME, Balentine DA, Davies AP, Cai Y (1997) Tea chemistry. CRC Crit Rev Plant Sci16: 415-480.
- Peterson J, Dwyer J, Jacques P, Rand W, Prior R, et al. (2004) Tea variety and brewing techniques influence flavonoid content of black tea. J Food Comp Anal 17: 397-405.
- Galensa R, Engelhardt UH (1997) Analytik und Bedeutung von Polyphenolen in Lebensmitteln. In: Gunzler H (ed.). Analytiker- Taschenbuch.Springer, Berlin Heidelberg, New York.pp:147-178.
- Luximon-Ramma A, Bahorun T, Crozier A, Zbarsky V, Datla KP, et al. (2005) Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas. Food Res Intern 38: 357-367.
- Zhang Y, Li Q, Xing H, Lu X, Zhao L, et al. (2013) Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC–DPPH assay. Food Res Intern 53: 847-856.
- Unachukwu UJ, Ahmed S, Kavalier A, Lyles JT, Kennelly EJ (2010) White and green teas (Camellia sinensis var. sinensis): variation in phenolic, methylxanthine, and antioxidant profiles. J Food Sci 75: 541-548.
- Leanderson P, Faresjö ÅO, Tagesson C (1997) Green tea polyphenols inhibit oxidant-induced DNA strand breakage in cultured lung cells. Free Rad Biol Medi 23: 235-242.
- Van Acker SA, Tromp MN, Griffioen DH, Van Bennekom WP, Van Der Vijgh WJ,et al. (1996) Structural aspects of antioxidant activity of flavonoids. Free Rad Biol Medi 20: 331-342.
- Hollman PCH (1997) Bioavailability of flavonoids. Euro J ClinNutri 51: 66-69.
- Benzie IF, Szeto YT (1999) Total antioxidant capacity of teas by the ferric reducing/ antioxidant power assay. J Agri Food Chem 47: 633-636.
- Hider RC, Liu ZD, Khodr HH (2001) Metal chelation of polyphenols. Methods Enzymol 335: 190-203.
- Kumamoto M, Sonda T, Nagayama K, Tabata M (2001) Effects of pH and metal ions on antioxidative activities of catechins. Biosci Biotechnol Biochem 65: 126-132.
- Brown EJ, Khodr H, Hider CR, Rice-Evans CA (1998) Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem J 330: 1173-1178.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences