ISSN : 2348-9502
American Journal of Ethnomedicine
Two Flavonoid Compounds Isolated From Nepeta septemcrenata Growing in South Sinai, Egypt
1Botany Department, Faculty of Science, Suez Canal University, Egypt
2Chemistry Department, Faculty of Science, Suez Canal University, Egypt
3Zoology Department faculty of Science, Suez Canal University, Egypt
4Biochemistry and Molecular Biology National Organization for Drug Control and Research
Abstract
Nepeta septemcrenata is a perennial herb grows in Sinai Mountains and used in folk medicine as a laxative antispasmodic, carminative, diuretic and to treat liver diseases. The present work aimed to study phytochemical, biological and toxicological effects of Nepeta septemcrenata. The study includes phytochemical screening of N. septemcrenata and investigation of volatile oil and flavonoids of the plant. The results of preliminary studies showed that the aerial parts of N. septemcrenata contain volatile oil, carbohydrates, flavonoid and unsaturated sterols or triterpenoids while the stem contains carenolides, tannins, saponins, alkaloids and coumarins. The analysis of volatile oil of the plant (petroleum ether fraction) using GC/MS revealed that it contains 15 compounds, the main components of it was ester which represent 18.15% from the volatile oil contents. Methylene chloride fraction subjected to preparative TLC that led to the isolation of two flavonoid compounds Ne2, Ne4. Ethyle acetate fraction was subjected to a chromatographic polyamide column. The residue obtains from fractions showed by TLC and PC contained a mixture of two flavonoid compounds (Ne2 & Ne4). It was further purified by preparative paper chromatography. The two isolated compounds (Ne2 and Ne4) were identified using different techniques as preliminary chemical test, thin chromatographic examination with or without treatment with ammonia and aluminum chloride, UV spectral analysis and mass spectroscopy. The isolated compounds were identified as apigenin -7- methyl ether and flavones aglycone.
Keywords
Labiatae, South sinai, Medicinal species, Folk medicine, Flavonoids.
Introduction
Medicinal plants have a very important role from the medical and economical point of view all over the world, where the folk medicine made a wide base used for searching new drug. The Egyptian desert has many wild medicinal plants which have a great economic and therapeutic value. Family Lamiaceae comprises many species of special economic and medicinal importance due to their different constituents [1]. It is represented in Egypt by 21 genera: Thymus, Marrbium, Nepeta, Otostegia, Mentha, Salvia, Ocimum, Teucrium, Orthosiphon, Lavandula, Origanum, Satureja, Micromeria, Ziziphora, Stachys, Lamium, Ballota, Leuces, Phlomis, Prasium and Ajuga [2]. Several species from lamiaceae are used in folk medicine. The pharmacological activities of these plants are anti-inflammatory, antiallergic, antitumor, antimicrobial and specific therapeutic activity on gastrointestinal and respiratory tracts [3]. Plants belonging to the family lamiaceae are characterized by their volatile oil content. On this basis, the family is divided into two subfamilies; one of them is rich in its volatile oil content (Nepetoideae) such as Nepeta, and Rosmary, While the other is poor in its volatile oil content (Lamioideae) such as Otostegia and Teucrium [4].
Nepeta is one of the most important genera of lamiaceae family with regard to the number of its species. Some species of this genus are important medicinal plants and their extracts have been used for medicinal purposes [5]. Some species of Nepeta germs are utilized in folk medicine for treatment of contusions, rheumatic pains, fever, cutaneous eruption and some species are used for their anti-inflammatory properties [6]. Nepeta plants were prepared as tea and used in traditional medicine as anthelmintics, febrifuges, expectorants and to treat bronchitis, bites as well as stings of scorpions [2]. They have antispasmodic, astringent properties [7], cardiotonic (N. ciliaris) diaphoretic, and anti-catarrhal effects (N. hederacea) [8]. The genus Nepeta is characterized by the presence of volatile oils, terpenes, flavonoids and phenolic acid. There is only one species found in Egypt (Nepeta septemcrenata) [2].
Genus Nepeta, family lamiaceae, includes 250 species of perennial plants. They are aromatic plants widely grown in Mediterranean countries [9]. The genus Nepeta (lamiaceae) is a wide spread weed in Egypt [10]. Nepeta septemcrenata Eherenb is an erect slender plant with branches at base leaves are oppositely alternated, ovate with crenate or slightly dentate margins. This plant is found in Saint Catherine, South Sinai, Egypt [9]. Nepeta septemcrenata known to be used by the native Bedouins in folk medicine as antipyretic, sedative, cardiotonic, eye wash and as a gargle in sore throat [3]. The present study was undertaken to investigate the following: A phytochemical analysis of N. septemcrenata plant includes; phytochemical screening, investigation of some chemical constituents that could be present and evaluation of the biological and therapeutic activities of the ethanolic extract of the plant including determination of its LD50, anti-Inflammatory, antipyretic and the antimicrobial effect.
MATERIALS AND METHODS
Plant collection and Preparation of plant extract
The fresh Nepeta septemcrenata was collected from Saint Catherine mountains in South Sinai (Egypt) during the flowering season. Following [11] method the extract was prepared, where 440 gm of the air dried powder of the aerial parts (leaves and flowers) of Nepeta septemcrenata were defatted with petroleum ether (40-600 C) in a soxhlet apparatus. The defatted powder was then extracted with 95% alcohol; the alcohol extract was evaporated to dryness under reduced pressure.
Phytochemical analysis
The powdered samples of the aerial parts were subjected to chemical screening according to the standard procedures for the following constituents; volatile oil [12], carbohydrates and/or Glycosides [13], alkaloids [14] and/or nitrogenous bases, cardinolides [15], anthraquinon [16], tannins [17-19], flavonoids [20], saponins [21], sterols and/or triterpenes [17], lactones and/or esters and cumarines [22].
The isolation of volatile oil and extraction of flavonoid from leaves were performed according to the method of [23] and [11]. The survey of flavonoid was employed using paper chromatography (Whatman1), tertiary butanol: 15% acetic acid: Water (3:1:1) were used as solvents. The different fractions were also subjected to paper chromatography (PC) using 30% acetic acid as a solvent. Flavonoid profiles were viewed under UV- light and colors were recorded before and after fuming with ammonia vapour. Compounds were indentified using standard UV- visible spectroscopy (Beckmam-26 spectrophotometer), Mass Spectrophtometer (finnigan SSQ7000 model) and Gas Chromatography (Thermo- Trace 2000 model), and thin layer chromatography (TLC) recoated silica gel 60F254 plates (Merck) and polyamide plates (Merck).
Identification of the volatile constituents
The volatile constituents were prepared from the fresh aerial parts by hydro-distillations. The volatile constituents were extracted with petroleum ether (40- 60 °C) and dried over anhydrous sodium sulphate12. The prepared volatile constituents were analyzed by gas chromatography (GC/MS).
Identification of the volatile constituents was achieved by library searched data base Willey 229 LIB and by comparing their mass fragmentation patterns with those of the available published data [24].
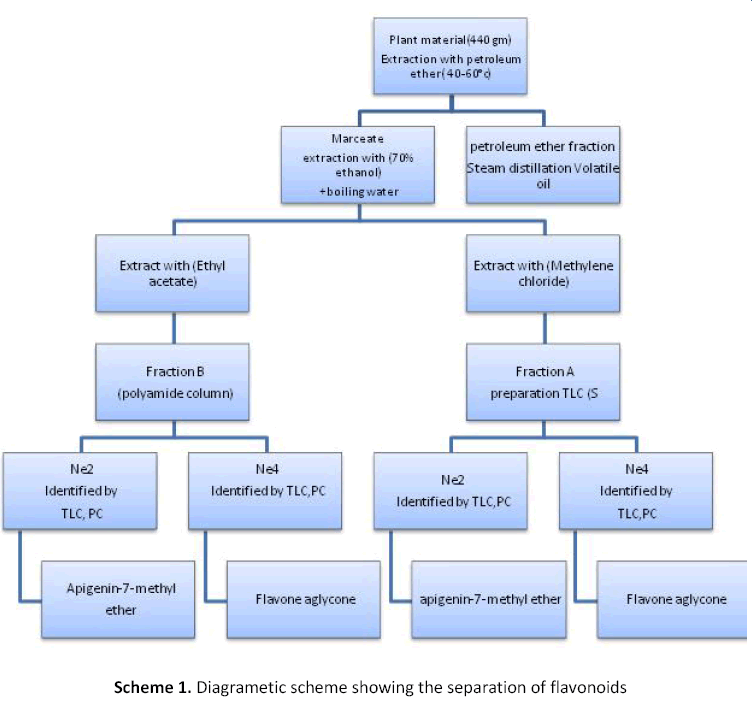
Separation of the flavonoid
After extraction with petroleum ether (40-60°c), about 440 gm of dried powder of the plant material (leaves and flowers) was extracted with ethanol till exhaustion. The ethanolic extract was concentrated and the residue was dissolved in hot distilled water. The aqueous solution was extracted by shaking with successive portion of methylene chloride (4×100 ml). The methylene chloride extract (fraction A) was dried over anhydrous sodium sulphate then concentrated to small volume. The aqueous mother liquor was extracted by shaking with successive portions of ethyl acetata (4× 100) extract (fraction B), then it was dried over anhydrous sodium sulphate and concentrated to a small volume [11].
Thin layer chromatography of the contained fractions was carried out using silica gel G, and polyamide pates 0.25 mm thick coated plates. Detection of the flavonoide constituents was done by examining the chromatoplates under the U.V light at 366 nm, as by spraying with aluminum chloride before and after exposure to ammonia vapour. Whereas fluorescence spots of the flavonoid component appeared with varying colors and fluorescence [11]. Plates coated with silica gel G and developed with chloroform- Methanol (98:2) gave the best resolution (Scheme 1).
Investigation of methylene chloride fraction
The methylene chloride fraction subjected to preparative thick layer chromatography on silica gel (SG) (0.5 mm thick) coated plates. The methanolic solution of methylene chloride fraction was applied to the plates as homogenous bands. The chromatoplates were developed with chloroform: methanol (98:2) as a solvent then the plates were dried by air. The separation bands were localized in the UVlight, scrapped off, and transferred into flasks then eluted with methanol, then filtered. The solution of the eluted bands was evaporated until dryness and the residue was dissolved in methanol [11].
Investigation of ethyl acetate fraction
Ethyl acetate fraction was subjected to a chromatographic polyamide column. About 400 mg of ethyl acetate fraction was applied on the top of the column. Elution was made with 70% methanol and 100 ml fraction was collected. The chromatographic separation was followed by the thin layer chromatography on SG plates. The residue obtained from fraction number (20-34) showed by TLC and PC contained a mixture of two flavonoid compounds. It was further purified by preparation paper chromatography on 3 MM paper which developed in 30% acetic acid as a solvent, then dried by air. The separation bands were localized in the UV-light [11].
RESULTS
Phytochemical screening of Nepeta septemcrenata
Table (1) showed the preliminary screening of the aerial parts "flowers, leaves and stem" of Nepeta septemcrenata. It is clear from the table that, aerial parts of Nepeta septemcrenata containing a highly concentration of volatile oils, carbohydrate, flavonoides and unsaturated sterols. In the mean time the other constituents "cardenolides, tannins, saponins, alkaloids and coumarins" were found in trace amount.
Table 1. Preliminary screening of the aerial parts (flower, Leaves and stem) of Nepeta septemcrenata
| Constituents | Leaves and flowers | Stem |
|---|---|---|
| Volatile oil | +++ | +++ |
| carbohydrate and /or glycosides: (Molisch`s test) |
+++ | +++ |
| Test for cyanogentic glycosides (Guignard reaction) |
- | - |
| Cardenolides | - | + |
| Anthraquinone Free anthraquinone aglycones Combined aglycnes |
- - - |
- - - |
| Tannins | - | + |
| Flavonoids: | +++ | +++ |
| Saponins (Forth test) | - | + |
| Unsaturated sterols and/or triterpenoids |
+++ | +++ |
| Alkaloids or nitrogenous base | - | + |
| Lacto and / or ester | - | - |
| Coumarins | - | + |
+++High concentration, ++Moderate concentration, + Trace concentration, -Absent
Volatile oils
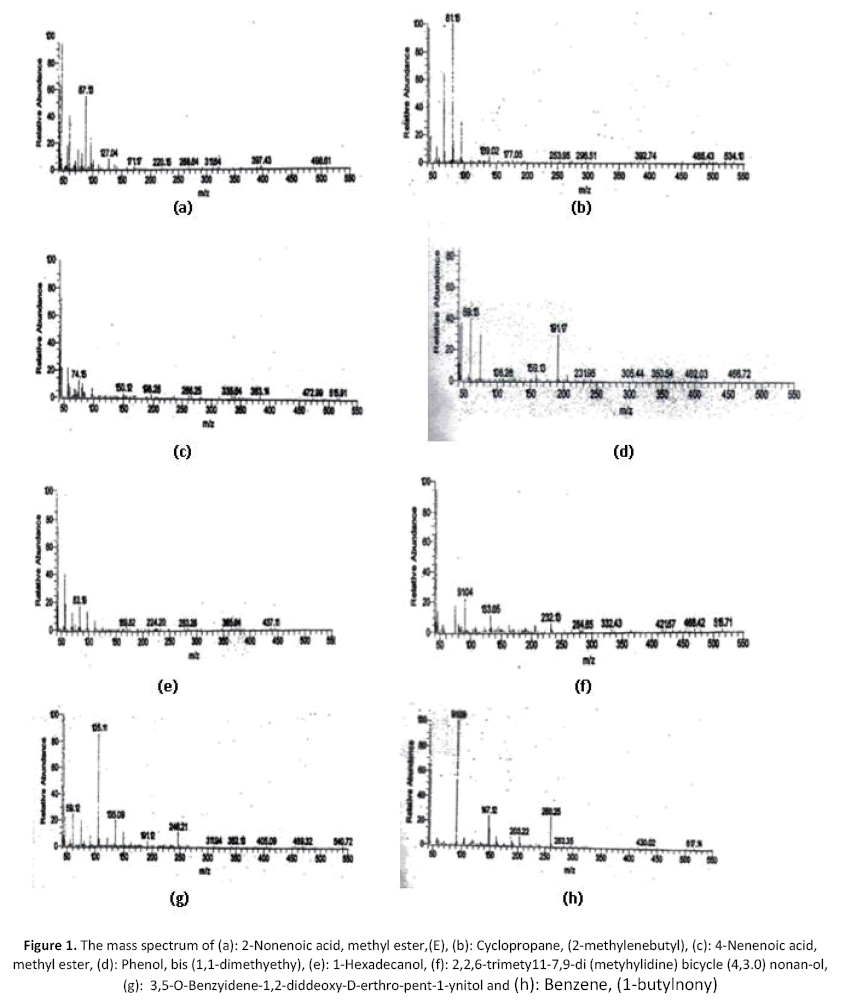
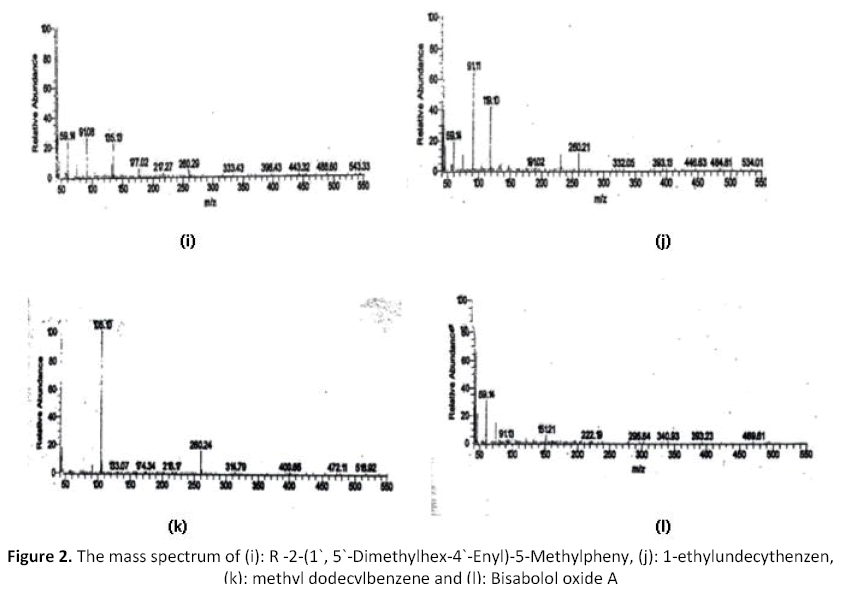
The yielded oil of Nepeta septemcrenata was yellow color and has a characteristic odor. Table (2) revealed the analysis of the volatile oil of Nepeta septemcrenata by GC/MC. These results indicated the presence of fifteen compounds which represent 60.82% of the total oil. The identified compounds represent several chemical classes; ester 21.5% which represent the main constituent (no. 1, 3, 5, 12 & 15 in the table and their mass illustrated in figure1 (a),(c) and figure 2 (i) respectively), alcohol 10.23 % ( no. 6, 7, & and 10 in the table and their mass illustrated in figure 1 (e), (f) and (g)), keton 6.31% (no. 11 in the table and its mass illustrated in figure 1 (h)) hydrocarbons 5.79 % (no. 2, 13, & 14 in the table and illustrated their mass in figures1 (b) and figure 2 (k), (l) ) and phenol 0.95 % (no. 4 in the table and its mass illustrated in Figure 1 (d)).
Table 2. GC/MS analysis of the volatile oil of Nepeta septemcrenata
| Compound | R.T. | % | M.F. | B.P. | M + |
|---|---|---|---|---|---|
| 2-Nonenoic acid, melthyl ester, (E) | 24.07 | 6.10 | C10 H16 O2 | 87 | 170 |
| Cyclopropapane, (2- methyylenebutyl) | 24.78 | 2.21 | C8 H14 | 81.16 | 110 |
| 4-Nenenoic acid, methyl ester | 27.04 | 1.32 | C10 H18 O2 | 81.16 | 110 |
| Phenol, bis (1,1- dimethylethyl) | 30.73 | 0.95 | C14 H22 O | 59.13 | 206 |
| Butanoic acid, 3-methyl ester | 30.96 | 0.58 | C6 H12 O2 | 59.16 | 117 |
| 1-Hexadecanol | 32.25 | 5.88 | C16 H34O | 83.16 | 242 |
| 2,2,6-trimethyl-7,9-di(methylidine) bicycle(4,3.0) nonan-1- ol | 33.49 | 0.95 | C14 H22 O | 91.O4 | 206 |
| Bisabolol oxide A | 35.82 | 7.95 | C15 H 26O2 | 59.14 | 238 |
| Germacrane-D | 35.82 | 8.10 | C15 H30 | 97.11 | 210 |
| 3,5 –O- Benzylidene-1,2-dideoxy-D-erythro-pent-1-ynitol | 37.37 | 3.36 | C14 H14 O4 | 105.11 | 246 |
| Benzene, (1- butynony1) | 37.75 | 6.31 | C19 H32 | 91.09 | 260 |
| ®-2-(1`,5`- Dimethylhex-4`-Enyl)-5-Methylphenylacetate | 38.19 | 3.43 | C17 H24 O2 | 91.08 | 260 |
| 1-ethylundecylbenzene | 38.66 | 1.13 | C19 H32 | 91.11 | 260 |
| 1-Methyldodecylbenzene | 39.59 | 2.45 | C19 H32 | 105.10 | 260 |
| 2-pyridinepropanoic,a`-methyl-a`-oxo-ethyl ester | 61.89 | 10.15 | C11 H13 NO3 | 73.12 | 207 |
R.T.: Retention time, M.F.: Molecular formula, M+: Molecular weight, B.P.: Base peak.
Figure 1: The mass spectrum of (a): 2-Nonenoic acid, methyl ester,(E), (b): Cyclopropane, (2-methylenebutyl), (c): 4-Nenenoic acid, methyl ester, (d): Phenol, bis (1,1-dimethyethy), (e): 1-Hexadecanol, (f): 2,2,6-trimety11-7,9-di (metyhylidine) bicycle (4,3.0) nonan-ol, (g): 3,5-O-Benzyidene-1,2-diddeoxy-D-erthro-pent-1-ynitol and (h): Benzene, (1-butylnony)
The following figures from (a-h) represent the mass spectrum of the 8 compounds of the volatile oils.
The following figures from (i-l) represent the mass spectrum of the compounds 4 of the volatile oils.
Isolation and Identification of Flavonoid Compounds
As we describe before in the material and methods section, two flavonoides components, Ne2 and Ne4, were isolated from methylene chloride. TLC and PC of the isolated flavonoids showed to be single compound, identified by UV and MS spectral analysis as apigenin 7-methyl ether and flavones glycoside. Also the residue which obtained from the fraction B of the ethyl acetate extract showed by TLC and PC to be a mixture of two flavonoides compounds and the methanolic solution of the eluted bands gave upon concentration of yellow crystalline compounds Ne2 and Ne4 as the same in methylene chloride fraction.
The flavonoid substance Ne2 showed a single spot when purified by sephadex LH- 20 column (pure substance), thin layer and paper chromatogram. The ultra-violet absorption spectra of the flavonoid substance Ne2 in methanol as well as in the presence of shift reagent were measured, and the results obtained are shown in table (3). The results obtained as shown in table (3) are in agreement with these reported for apigenin -7- methyl ether. The ultra-violet spectrum in methanol showed that the flavonoid substance belong to the flavones type free hydroxyl group at C-5 was proved by the bathochromic shift in B-I-(54 nm) with ALCL3 no free hydroxyl group at C-7 was confirmed by sodium Acetate (No shift of B-ΙΙ was observed). The large bathchromic shift of b-i with sodium methoxide, is indicating of a free hydroxyl group at C-4. The mass spectrum showed a molecular ion peak (M+) at m/z 280 (100%) which corresponds to the molecular formula (C16H14O5). The fragmentation pathway of the molecular ion (scheme 2) Undergoes the retro- Diets- Alder reaction, giving ring- A fragment at m/z 166 (6%) and ring – C fragment at m/z 118 (4%), fragment ion peak at obtained data the identity of the flavonoid substance Ne2 was proved to be apigenin-7- methyl ether.
Table 3. Ultra- violet absorption spectra of Ne2 in methanol
| Band | Max (n.m.) | |||||
|---|---|---|---|---|---|---|
| MeOH | NaOMe | AlCl3 | AlCl3/ HCl | NaOAc | NaOAc/H3BO3 | |
| ΙΙ | 267 | 266 | 270 | 243 | 266 | 267 |
| Ι | 329 | 380 | 383,346 | 379,339 | 382 | 332 |
The flavonoid substance Ne4 showed a single spot when purified by Sephadex LH20 column (pure substance), on thin layer and paper chromatogram. The ultra-vilot absorption spectra of shift reagent were measured, and the results obtained are shown in table (4). The UV- spectrum showed that the flavonoid substance belong to the flavone type. Mass spectrum showed that the compound belong to the flavone glycoside.
Table 4. Ultra- violet absorption spectra of shift reagent of Ne4
| Band | Max (n.m.) | |||||
|---|---|---|---|---|---|---|
| MeOH | NaOMe | AlCl3 | AlCl3/ HCl | NaOAc | NaOAc/H3BO3 | |
| ΙΙ | 267 | 258,247 | 272 | 275 | 269 | 242 |
| Ι | 398 | 398 | 390 | 355 | 438 | 348 |
DISCUSSION
Family lamiaceae have many medical plants, which characterized by the presence of volatile oils, flavonoids, phenolic acids, terpens and coumarins. The studied plant Nepeta septemcrenata is belonging to the family lamiaceaa. N. septemcrenata is common plant in Sinai and Saint Cathrine, Egypt [9]. An isopimarance type diterpence and 7-omethyl apigenin were isolated from the ethanolic extract of Nepeta septemcrenata Ehrenb herb [25]. Investigated the chemical constituents of Nepeta septemcrenata [26] analyzed the volatile oil of the same plant species and found that it is used in folk medicine by the native Bedouins as sedative, antiseptic, antiplasmodic, antipyretic cardiotonic, eye wash and as a gargle in sore throat. The present study was aimed to investigate the phytochemical analysis of the plant, evaluate the biological and therapeutic activities of the ethanolic extract of the plant and to study the biochemical effect of the plant extract.
In the present study petroleum ether extract of Nepeta septemcrenata yielded volatile oil with yellow color and characteristic odor. The results of GC/MS analysis of this oil showed that it is contain a mixture of 15 compounds represent several chemical classes. The obtained results are in agreement with [26] who detected sixteen major volatile compounds of Nepeta septemcrenata and with3 who mention that N. septemcrenata is rich by volatile oil. In this respect [4] divided family lamiaceae into two subfamilies on the basis of their volatile oil content. The lamiodeae is poor in its volatile oil content while the subfamily neptoideae is rich in its volatile oils content N. septemcrenata belongs to subfamily Neptoideae.
In the present study, two flavonoids have been isolated from N. septemcrenata, the first one was apigenin-7-methyl ether. This result is in agreement with that of [25] who isolated and indentified 7-O-methy apigenin from the ethanol extract of the aerial parts of N. septemcrenata. The second flavonoids were a flavones glycoside. Identification of the isolated flvonoids was achieved through chromatographic studies and spectroscopic measurements (UV and MS/GC).
Administration of the ethanolic extract of Nepeta septemcrenata to the hyperthermeric rats decreased the body temperature significantly. This result is in agreement with that of [27] who reported that methanol extract of Leucas lavandulaefolia (lamiaceae) produced significant dosedependent lowering of body temperature in yeast-provoked elevation of body temperature in rats.
The interaperiotenal injection of ethanolic extract of Nepeta septemcrenata into rats with inflamed paw significantly decreased the thickness of the inflamed rats' paws. This anti-inflammatory effect was reported by [6] who studied the antiinflammatory activity of Nepeta sibthorpii methanol extract and they observed a significant inhibition of paw oedema. They suppose that the ant-inflammatory effect of methanol extract could be related to free radical scavenging activity and it depends on a synergic effect of component of the alcoholic extract. So, it appears that the antinflammatory action of the alcoholic extract of N. septemcrenata could be due to its antioxidant properties.
It was found that apigenin as a flavonoid of Nepeta setemcrenata extract inhibit the induction of β-galactosidase activity in Sw480 cells stably transfected with β-galactosidase. Apigenin demonstrated potent anti-inflammatory activity in carrageenan induced rat paw edema and it was concluded that flavonoids offer important therapeutic potential for the treatment of a variety of inflammatory diseases [28]. It was investigated that the bioflavonoids on lysosomal acid hydrolylases, viz., β-glucuronidase, β-Nacetyl glucosaminidase and cathepsinn D in serum, liver, kidney and spleen and the stability of liver lysosomes was studied by [29]. The activity of these enzymes in arthritic tissue and serum increased significantly. The total liver was appreciably decreased, while its release was significantly. The total activity of β-glucurandase in the lysosomerich fraction from arthritic liver was appreciably decreased, while its release was significantly increased.
It was found that one of the most potent flavones, apigenin as a flavonoid exhibited a dose and time dependent, reversible effect on adhesion protein expression as well as inhibitory adhesion protein up regulation at transcriptional level28. Also, apigenin inhibited IL-1α- induced prostaglandin synthesis and TNF- α-INDUCED 1L-6 and 1L-8 productions, suggesting that the hydroxyl flavones may acts as general inhibitors of cytokineinduced gene expression. As well as, this flavonoid did inhibit TNF- α-induced β- glactosidase activity in SW 480 cells stably tranfected will a β- glactosidase.
It was found that the natural extract of some medical plants protector free radicals damage [30]. In addition, it has been shown that the content of the mucosal non protein. Sulfhydrxyl group may increase by the effect of the active ingredinents of the medicinal plants [31].
It was found that the protective effects of four flavonoids "quercetin, rutin, luteolin and apigenin" against induced DNA damage in human leukemia cells (K562). These flavonoids are characterized by the number of hydroxyl groups on the B-ring, the presence of an O-dihydroxy structure on the B-ring confers a higher degree of stability to the flavonoid phenoxyl radicals by participating in electron delocalization and is, therefore, an important determinant for antioxidant potential32. The strongest inhibition against DNA damage (44%, 42%) was found in a range of luteolin and quercetin concentrations of 20-100 u mol/L.
It has been investigated that DNA protective capacity of three flavonoids, apigenin, luteolin and quercetin against the free radical generated by H2O2. The quantitive analysis has shown that luteolin possesses the highest DNA protective effect of flavonoids [33]. Also, it has been found that thirteen isoflavonoids, flavonoids, and lignans were evaluated for their effects on DNA synthesis in MCF-7 and human breast cancer cells by [34]. At 0.1-10 uM of coumestrol, genistein, biochanin A, luteolin, apigenin kaempferol and enterolactone induced DNA synthesis 150-235% and at 20-90 uM, inhibited DNA synthesis by 50%. The biphasic effects showing inhibition of DNA synthesis at high concentrations but induction at concentration close to probable levels in humans [35]. Investigated that the flavonoides "qurestin, rhamnetin, isorhamnetin, apigenin, and luteolin" did not introduce any damage into the DNA.
In conclusion, N. septemcrenata has many promising active constituents that exert significant pharmacological and therapeutic effects with high margine of safety. We recommend further phytochemical and pharmacological studies on each purified active constituent of this plant on different body systems and on fatal diseases such as cancer and AIDS.
REFERENCES
- Authman, A. F. (2001). Phenolics, terpenoids and bioactivity of sideritia taurica family lamiaceae. M.Sc. thesis, Faculty of Pharm., Cairo University (Egy.), Center library.
- Abdel-Shafiq, K. A. (1997). Chemical studies on certain plants of family Labiate. M.Sc. thesis, Chemistry Dep. Faculty of Science, Cairo University. PP.174.
- Tachholm, V. (1974). Student`s Flora of Egypt. Cairo University, 2 nd Edition. Coop. Beirut.
- Barberan, F. A. T., and Wollenweber, E. (1990). Pl. Syst. Evol., 173:109-118.
- Sajjadi S (2005). Analysis of essential oil of Nepeta sintenisii Bornm from Iran. Journal of Faculty of Pharmacy-Teheran- University-of-Medical-Sciences, 13 (2): 61- 64.
- Miceli, N., Taviano, M., Giuffreda, D., Trovate, A., and Galati, E. (2005). Anti- inflammatory activity of extract fractions from Nepeta sibthorpii Bentham. J- Ethnopharmacol., 97(2): 261-266.
- Arnold, A., Valentini, G., Bellomaria, B., and Arnold, H. J., (1993). Contribution a l’e´tude chimique de l’huile es de Nepeta parnassica Heldr. & Sart. Ex Boiss. biflora. Turk. J. Chem. 23, 99D104. Plant. Med. Phytother., Tome XXVI, n. 2, 149D157.
- Treas, G. E., and Evans, W. C. (2001). Pharmacognosy, 14 th Ed. Wb, Saundres Company Ltd, London, 48.
- Pagni, A. M., Catalano, P. I., and Morelli, I., (1990). Plants Medicinal- et- Phytotherpie, (24): 3, 203-213.
- Saker, M. M., and Kawashity, S. A. (1998). Tissue Culture and Flavonoid Content of Nepeta and Plantago Species Endemic in Egypt. Fitoterapia., LXIX (4): 358-364.
- Harborne, J. B., Mabry, T. J., and Mabry, H. (1975). The flavonoides "Chapman and Hall, London, 84.
- Clauss, E., and Tyler, V. (1968). Pharmacognosy 4th edtion. Lea and febiger. Philadelphia, pp. 321.
- Molish, H. (1972). Projective methodologies. Annu Rev Psychol., 23: 577- 614.
- Tewari, S. (1968). Separation and identification of alkaloids by paper electrophoresis and its application in medico- legal cases. Pharmazie., 23 (2): 58- 60.
- Casmada, S. M. (1954). Photocolorimetry of cardiotonic glucosides: application of Baljet reaction to digitalin for photocolorimetry. Farmacogosia., 14(31): 15-26.
- Wallis, T. E. (1967). "Text book of Pharmacognosy" 5 th edition. J. and A. Churchill Itd, London, PP. 949.
- Wall, M. E., Krieder, M. M., Krewson, C. F., Eddy, C. R., William, J. J., Corell, D. S., and Gentry, H. S. (1954). Steroidal sapogenins. VII. Survey of plants for steroidal sapogenins ans other constituents. J. Amer. Pharm. Assoc., 43(1): 1-7.
- Gonzalez, E. E., and Delgado, J. N. (1962). Phytochemical investigation of amphipterygium adstringes J. Pharm. Sci., 51: 786-790.
- Treas, G., And Evans, W. C. (1989). "Text Book of pharmacognosy" 13 th edition. London, PP. 113.
- Peach, K., And Tracy, M. V. (1955). "Modern Methods of Plant Analysis" Springer- Velarg, Berlin, Vol.3. PP. 478.
- Oleszek, W. (2002). Chromatographic determination of plant saponins. J. Chromatorgr A., 967 (1): 147-162.
- Murray, R. D., Mendez, J., and Brouns, S. (1982). "The Natural Coumarins occurrence Chemistry and Biochemistry". John Wiley and sons ltd, Chichester, New York., PP. 355-356.
- Mabry, T. J., Markham, K. And Thomas, M. (1970). "The Systematic Identification of Flavonoids". Springer-Verlag, New York, PP. 46-62.
- Adams, R. P. (1989). Identification of essential oils by ion trap mass spectrometry, Academic press, New York.
- Khalil, A. T., Gedarasr, S. R., Lahoub, M. F., Halim, A. F., and Voehler M (1997). Aditrepene from Nepeta septemcrenata. Phytochemistry, 44(3): 475-478.
- El-Hamouly, A. M., and El-Hela, A. A. (2004). Phytochemical and biological investigation of the volatile constituents of Nepeta septemcernata Ehrenb., growing in Egypt. Bull-Pharm-Sci-Assiut-Univ., 27(6): 95-98.
- Mukherjee, K., Saha, B., and Mukherjee, p. (2002). Ealuation of antipyretic potential of Leucas laandulaefolia (labiatae) aerial parts extract. Phytother Res., 16 (7): 686-688.
- Gerritsen, M. E., Carley, W. W., Range, G. F., Shen, C. P., Phan, S. A., Ligon, G. F., And Perry, C. A. (1995). Flavonoids inhibit cytokine induced endothelial cell adhesion protein gene expression. Amj. Pathol., 147 (2): 235-237.
- Rao, C. N., Rao , V. H., Verbuggen, L., and Orloff, S. (1980). Effect of bio flavonoids on lysosomal acid hydrolyases and lysosomal stability in adjuvant-induced arthritis. Scand J. Rheumatol., 9(4):280-284.
- Calabrese, V., Scapagnini, G., Catalano, C., Dinotta, F., Geraci, D., and Morganti, P. (2000). Biochemical studies of a natural antioxidant isolated from rosemary and its application in cosmetic dermatology. Int. J. Tissue React. 22(1):5-13.
- Dias, P., Foglio, M. A., Possenti, A., and De Carvalho, J. E. (2000). Anti-ulcerogenic activity of crude hydroalcoholic extracts of Rosmarinus officnalis. L. J. Ethno pharmacol., 69: 57-57.
- Horvathova, K., Novotny, L., Tothova, D., and Vachalkova, A. (2004). Determination of free radical scavenging activity of quercetin, rutin, luteolin and apignin in H2O2-Treated human MLulls K562. Neoplasma, 51(5): 395-399.
- Ramanova, D., Vachalkova, A., Cipak, L., Ovesna, Z., and Rauko, P. (2001). Study of antioxidant of apigenin, luteolin and quercetin by DNA protective method. Neoplasma, 48(2): 104-107.
- Wang, C., And Kurzer, M. S. (1997). Phytoestrogen concentration determines effect of DNA synthesis in human breast cancer cells. Nutr Cancer, 28(3): 236-247.
- Czeczot, H., and Kusztelak, J. (1993). A study of the genotoxic potential of flavonoids using short-term bacterial assays. Acta. Biochem. Pol., 40(4): 549-554.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences