The Acute Vestibular Syndrome
Hearing and Speech Center, Egypt
- *Corresponding Author:
- Mahmoud Farouk, MBBCH, M.sc ENT, M.sc
Audiovestibular Medicine, Hearing and Speech Center, Minia City, Egypt
Tel: 00201014727869s
E-mail: Dr.mahmoudfarouk31@gmail.com
Received date: December 21, 2018; Accepted date: January 17, 2019; Published date: January 24, 2019
Citation: Farouk M (2019) The Acute Vestibular Syndrome. Research J Ear Nose Throat. Vol.3 No.1:1.
Copyright: © 2019 Farouk M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Acute vestibular syndrome (AVS) is defined as the sudden onset of acute, ‘continuous’ vertigo (lasting longer than 24 hours), associated with nausea, vomiting and head motion intolerance, the term was initially introduced by Hotson and Baloh in 1998. Vestibular neuritis (VN), the most common cause of an AVS, It is also estimated that 10%-20% of dizzy patients visiting emergency departments (ED) have an AVS. The second most common cause of an AVS is an ischaemic stroke involving the cerebellum or the brainstem (probably 5%-10% of all patients with AVS). Vertigo and nystagmus ranked as the most common symptom/sign in patients with posterior circulation stroke.
Misdiagnosis of posterior fossa infarcts in emergency-care settings is frequent. A three-step bedside oculomotor exam (H.I.N.T.S.: Head-Impulse-Nystagmus-Test-of-Skew) appears more sensitive for stroke than early MRI in AVS.
This review illustrates the clinical hints of these tests and applying the diagnostic predictors in clinical practice.
Keywords
Acute vestibular syndrome; Ischaemic stroke; Hearing loss
Introduction
Acute vestibular syndrome (AVS) is defined as the sudden onset of acute, ‘continuous’ vertigo (lasting longer than 24 hours), associated with nausea, vomiting and head motion intolerance, the term was initially introduced by Hotson and Baloh in 1998. Vestibular neuritis (VN), the most common cause of an AVS, It is also estimated that 10%-20% of dizzy patients visiting emergency departments (ED) have an AVS. The second most common cause of an AVS is an ischaemic stroke involving the cerebellum or the brainstem (probably 5%-10% of all patients with AVS) [1,2]. Vertigo and nystagmus ranked as the most common symptom/sign in patients with posterior circulation stroke.
‘HINTS’, widely used acronym refers to HI: Head Impulse, N: Nystagmus direction and TS: Testing Skew. In addition, a second acronym and mnemonic: INFARCT summaries the application of HINTS: IN: Impulse normal, FA: Fast Alternates (referring to the nystagmus fast phase) and RCT: Refixation on cover test (skew deviation).
HINTS examination is a 3-step ocular motor examination that can differentiate central (e.g., posterior fossa stroke and demyelinating lesion) from peripheral (e.g., vestibular neuritis) etiologies of the AVS, with a higher sensitivity and specificity than DW-MRI when performed within the first 72 hours.
When combined with an assessment of unilateral hearing loss in HINTS-plus examination, the sensitivity of the examination further increases with minimal tradeoff in specificity. In a large validation study performed by subspecialists, the 3-step HINTS examination had 96.8% sensitivity and 98.5% specificity for detection of a central etiology for AVS, whereas HINTS-plus examination had 99.2% sensitivity and 97.0% specificity for detection of a central etiology for AVS, most commonly due to stroke.
DWMRI had only 85.7% sensitivity in the first 24-72 hours, and the ABCD2 (age, blood pressure, clinical features, duration of symptoms, and diabetes) score had only 61.1% sensitivity and 62.3% specificity [3]. The physiologic basis of the basic 3-step examination rests primarily on semicircular canal and utricular pathway imbalance. Acute horizontal canal (HC) hypofunction causes dynamic semicircular canal imbalance, demonstrated by a positive or abnormal ipsilateral HIT. Static HC imbalance causes a contralesional unidirectional horizontal-torsional nystagmus, which is contralateral to the positive HIT.
Skew deviation is the result of utricleocular motor pathway imbalance and should be absent with an acute peripheral vestibulopathy with the following exceptions: cases of chemical or surgical labyrinthectomy or neurectomy, or a particularly destructive peripheral etiology such as bacterial labyrinthitis. The first step is to access the nystagmus. Spontaneous nystagmus should be present for the 3-step HINTS test to be valid, and the nystagmus should be evaluated in primary and eccentric gaze [4]. The nystagmus associated with vestibular neuritis is unidirectional, and should follow Alexander law in which the nystagmus increases in the direction of the fast phase and decreases without reversal in the direction of the slow phase (e.g., RBN is maximal in right gaze and less pronounced in left gaze), However, small unilateral posterior fossa lesions commonly produce unidirectional nystagmus as well. AICA infarctions usually produce contralesional nystagmus, which may be in accordance with Alexander law. However, in a large series of patients with AICA infarctions, 6 of 55 patients had ipsilesional nystagmus, which may have been due to selective lesions of the superior and medial vestibular nuclei, damage to inhibitory or balancing pathways between the vestibular nuclei and/or vestibulocerebellum, or potentially local irritation due to hemorrhage [5].
In addition, when assessing nystagmus, if gaze-evoked nystagmus is present, or if spontaneous nystagmus is vertical, vertical-torsional, or pure torsional, then, this should be considered central. Although it has been traditionally taught that peripheral vestibular nystagmus is suppressed with fixation and accentuated with removal of fixation (e.g., with occlusive ophthalmoscopy, Frenzel, or infrared video-oculography goggles), central vestibular nystagmus due to a posterior fossa lesion can behave in the same way. Although an increase in peripheral vestibular nystagmus with removal of fixation is almost always evident weeks or months after the symptom onset, a significant difference between fixation and fixation removal may not be appreciated in the acute setting without eye movement recordings [6]. The second step is to assess for a skew deviation, which is a nonparalytic (usually), comitant vertical misalignment of the eyes due to interruption of the pathways between the utricle of the inner ear and the ocular motor nuclei. Vertical alignment is best assessed by cross-cover testing, whereby each eye is alternately covered while the patient fixates on a distant target.
Although skew deviation can rarely be due to a peripheral etiology, demonstration of a vertical refixation saccade or newonset vertical diplopia should be considered central until proven otherwise. Cross-cover testing in the primary position is generally sufficient for the non-neuro-ophthalmologist, but because it is possible that a false-positive result could occur from longstanding vertical strabismus or an unrecognized congenital fourth nerve palsy, quantification of the hyperdeviation in all directions of gaze and with head tilt is preferred. The last step is to perform the HIT. Although generally well tolerated, the HIT is usually the last of the 3 maneuvers to be assessed, given the fact that patients’ symptoms are usually aggravated by head movements. The patient should be sitting upright and is (usually) asked to fixate on the examiner’s nose. The examiner grasps the patient’s head and quickly thrusts/ rotates the head from the center to the left or right or starts 10-20° eccentric and thrusts back to the center (the preferred method in older patients with cervical spine disease or poor range of motion [7]; when robust nystagmus is present in eccentric gaze; or in the novice examiner to minimize the risk of cervical discomfort or injury), each time observing the eyes to make sure that they stay on the examiner’s nose.
In addition, the rotational speed of the head impulse must be high (but with low amplitude), and the HIT should be unpredictable in timing and amplitude, thereby uncovering covert saccades when present. Covert saccades occur during head movements and commonly develop as a compensatory response after unilateral vestibular loss. Covert saccades can make the HIT appear normal (which would suggest a central etiology), although overt saccades can usually be uncovered by an experienced examiner (by varying the amplitude of the impulses) [8].
Blinking during the HIT may also make the test appear falsely normal (or central), as the patient’s corrective saccade may occur during the patient or examiner’s blink. However, if a patient is inattentive and cannot maintain fixation on the examiner’s nose, the test may appear falsely abnormal (or peripheral) because the patient will saccade away from the target during or before the head impulse and then return with a refixation saccade once the head rotation is complete. In the AVS, a negative or normal HIT is highly suggestive of a central etiology, whereas a positive or abnormal result is usually reassuring for a benign peripheral etiology. However, a positive HIT can be seen with central or dangerous processes involving the vestibular nucleus, root entry zone (or other intraparenchymal) lesions of the eighth cranial nerve, and with AICA territory infarcts involving the labyrinth In these cases, however, other clues to a central etiology are almost always found with a comprehensive ocular motor and vestibular examination including an assessment of auditory function.
A negative HIT can rarely be seen with a benign peripheral etiology, especially with an inferior division vestibular neuritis in which the HC function is spared. However, this is another case in which the etiology should be presumed to be central until proven otherwise.
other potentially misleading HINTS results can be seen in the case of a skew deviation with severe peripheral injury with hearing loss due to bacterial infection of the labyrinth (or with a positive HIT that is central in origin. In addition, studies have shown that nearly half of patients with confirmed central vestibulopathy due to stroke may have unidirectional nystagmus [9]. Therefore, observation of unidirectional nystagmus that follows Alexander law can be either peripheral or central in origin. The specificity of the HINTS examination in daily practice remains high, with very few false-positive or false negative results. However, findings of gaze evoked nystagmus, vertical, or pure torsional nystagmus are considered highly suggestive of a central etiology. Head-shaking nystagmus, although not part of the HINTS examination, can also be useful for differentiating central and peripheral etiologies of AVS. The examiner passively shakes the patients’ head at 2 Hz for 15-20 seconds and then looks for Nystagmus; this transiently accentuates vestibular asymmetry and may reveal distinctive patterns of central and peripheral Nystagmus [10]. Ideally, this test is performed with fixation removed, which is easily accomplished with bedside techniques (following the head-shaking procedure) and include occlusive ophthalmoscopy (viewing one optic nerve while occluding the other eye), placing a 20-diopter ophthalmic lens over one eye while occluding the fellow eye, or with Frenzel goggles). These can help unmask peripheral nystagmus and magnify the movements under observation [11].
Down beat or upbeat HSN (also known as “perverted” nystagmus) implies a central etiology. Horizontal nystagmus in the opposite direction of the spontaneous baseline nystagmus and a vigorous HSN in the absence of unilateral vestibular loss are also patterns suggesting a central etiology following the headshake maneuver [12,13]. Mono-symptomatic attacks of vertigo and nystagmus without any other brainstem symptoms and signs would be unusual in brainstem ischemia. Selective damage to the vestibular nuclei and root entry zone of the eighth nerve in the pontomedullary junction can cause isolated vertigo. Because the root entry zone of the eighth cranial nerve has a rich network of anastomotic vessels arising from the neighboring arteries, the possibility of focal infarction in that area is extremely low. Although some case reports showed central isolated vertigo due to a demyelinating lesion localized to the root entry zone of the eighth nerve, isolated vertigo due to focal infarction in the root entry zone of the vestibular nerve has not been reported in the literature. Focal ischemia involving the vestibular nuclei can cause isolated vertigo and nystagmus mimicking acute vestibular neuritis [14]. Several studies have described patients with an isolated vestibular nucleus infarction who presented with isolated prolonged vertigo, spontaneous horizontal nystagmus, a positive HIT, and unilateral canal paresis [15]. All of these findings are consistent with acute peripheral vestibulopathy. These reports emphasize that isolated vestibular nucleus infarction should be considered in the differential diagnosis of central vascular vertigo syndrome, even though when the patients have unilateral canal paresis and positive HIT on the side of the canal paresis, and other neurologic symptoms or signs are absent. Vertigo in the lateral medullary infarction is usually associated with other neurological symptoms or signs, but tiny infarct in the lateral medulla can present with vertigo without other localizing symptoms. In this case, the HIT might be positive, if the medial vestibular nucleus is involved.
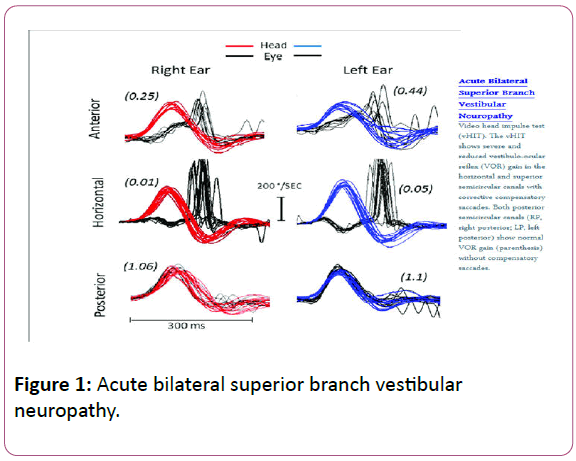
Importantly, graphic recording of h-HIT has been possible for the last few years, using Video head impulse goggles (vHIT) which has been described as an ECG of the eyes. There are several advantages of these recordings which provide VOR gain values for all six semicircular canals, vHIT records both overt and covert saccades, in addition, it records the number of corrective saccades, their latency and the detection of artefacts that may lead to equivocal clinical responses Figure 1.
In conclusion, although HINTS testing demonstrates outstanding sensitivity and specificity in the evaluation of the AVS, the results of testing must still be considered in the setting of the overall clinical context and in the setting of a more detailed ocular motor, otologic, and neurologic examination, particularly if a false-positive or false-negative HINTS result is suspected.
In an era of increasing costs from neuroimaging, however, this valuable tool should allow for more focused diagnostic testing and decision making, helping to avoid near-misses of MRInegative strokes, while also helping to avoid unnecessary testing in patients with benign peripheral pathology.
Case Study
1-A 42 year-old man presents to the emergency department because of continuous dizziness, nausea, vomiting and unsteady gait that began 16 hours earlier. He denies auditory or neurologic symptoms, headache, neck pain or recent trauma. He has no relevant medical or exposure history, including no cerebrovascular or vestibular disorders, no recent or remote ear surgery. The emergency physician notes his eyes jerking horizontally. Neither a neurology consultation nor neuroimaging is readily available. Although only symptomatic for 16 hours so far, the patient will soon probably fit the full clinical picture of acute vestibular syndrome (continuous vertigo lasting more than a day, accompanied by nausea or vomiting, intolerance to head motion, nystagmus and unstable gait). The most likely cause is vestibular neuritis, but cerebellar stroke must be excluded [16]. The absence of vascular risk factors and age less than 50 years reduce the patient’s risk of vertebrobasilar atherosclerosis, but dissection of the vertebral artery remains a concern even though he has no head or neck pain. There are no auditory symptoms to raise concerns about ischemic disturbance of the inner ear.
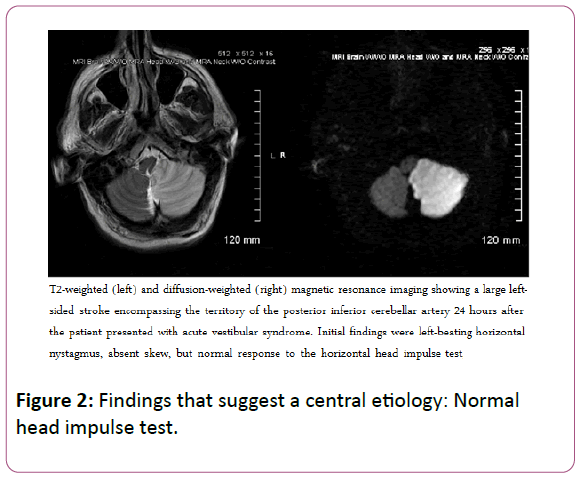
General neurologic findings are normal, which is compatible with a peripheral cause. The patient feels unsteady when standing but is able to sit with arms crossed unaided, which is also compatible with a peripheral lesion. Eye examination reveals direction-fixed, left-beating horizontal nystagmus worse in left gaze, and no skew deviation on alternate cover testing, all compatible with the leading potential diagnosis of vestibular neuritis. The head impulse test was normal responses during rapid head rotation in either direction [17,18]. The normal response in the setting of acute vestibular syndrome strongly suggests a stroke, despite the lack of other symptoms, signs or risk factors. MRI obtained the next morning reveals a large infarction of the left posterior inferior cerebellar artery (an example of such an MRI is shown in Figure 2).
Case 2
A 55-year-old woman was evaluated in the emergency department for severe vertigo and dizziness. She denied hearing loss but reported postural instability while walking. She had a history of hypertension but had no other significant medical history. She had horizontal left-beating nystagmus (LBN) in primary gaze. Her LBN increased in left gaze and diminished but remained left beating in right gaze. The nystagmus was more evident with (fixation-blocking) Frenzel goggles. Crosscover testing demonstrated normal vertical alignment. Her HIT showed a catch-up saccade with head impulses to the right, a positive or “abnormal” HIT result. Hearing was intact. The remainder of the neurologic examination was normal aside from gait imbalance, although she could walk independently. She was diagnosed with right sided vestibular neuritis, and her vertigo, nystagmus, and gait imbalance improved significantly over the next few weeks [19].
Findings that Suggest a Benign Etiology
• Unidirectional (contralesional) nystagmus in accordance with Alexander law
• Negative test of skew • Abnormal (positive) ipsilesional HIT
• Absence of acute hearing loss (e.g., labyrinthine ischemia)
References
- Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE (2009) HINTS to diagnose stroke in the acute vestibular syndrome. Stroke 40: 3504-3510.
- Kerber KA, Meurer WJ, Brown DL, Burke JF, Hofer TP, et al. (2015) Stroke risk stratification in acute dizziness presentations. Neurology 85: 1869-1878.
- Newman-Toker DE, Kerber KA, Hsieh YH, Pula JH, Omron R, et al. (2013) HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med 20: 987-996.
- Kruschinski C, Hummers-Pradier E, Newman-Toker D (2008) Diagnosing dizziness in the emergency and primary care settings. Mayo Clinc Proc 83: 1297-1298
- Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, et al. (2006) Cerebellar infarction presenting isolated vertigo: Frequency and vascular topographical patterns. Neurology 67: 1178-1183.
- Ahn BY, Bae JW, Kim DH, Choi KD, Kim HJ, et al. (2010) Pseudo-vestibular neuritis associated with isolated insular stroke. J Neurol 257: 1570-1572.
- Leigh RJ, Zee DS (2006) The neurology of eye movements. IV ed. New York: Oxford University Press.
- Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ (2008) Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology 70: 2378-2385.
- Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE (2011) Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. Canadian Medical Asso J 183: E571–E592.
- Chen L, Lee W, Chambers BR, Dewey HM (2011) Diagnostic accuracy of acute vestibular syndrome at the bedside in a stroke unit. J Neurol 258: 855-861.
- Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M (2008). Bedside differentiation of vestibular neuritis from central "vestibular pseudoneuritis". J Neurol Neurosurg Psychiatry 79: 458-460.
- Kirchner H, Kremmyda O, Hufner K, Stephan T, Zingler V, et al. (2011) Clinical, electrophysiological, and MRI findings in patients with cerebellar ataxia and a bilaterally pathological head-impulse test. Ann N Y Acad Sci 1233: 127-138.
- Park HK, Kim JS, Strupp M, Zee DS (2013) Isolated floccular infarction: Impaired vestibular responses to horizontal head impulse. J Neurol 260: 1576-1582.
- Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, et al. (2006) Cerebellar infarction presenting isolated vertigo: Frequency and vascular topographical patterns. Neurology 67: 1178-1183.
- Huh YE, Kim JS (2011) Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: Imaging correlations. Brain 134: 3662-3671.
- Baier B, Dieterich M (2011) Incidence and anatomy of gaze-evoked nystagmus in patients with cerebellar lesions. Neurology 76: 361-365.
- MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP (2013) The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS One 8: e61488.
- MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS (2009) The video head impulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology 73: 1134-1141.
- Kim HJ, Lee SH, Park JH, Choi JY, Kim JS (2014) Isolated vestibular nuclear infarction: Report of two cases and review of the literature. J Neurol 261: 121-129.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences