ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Spectral and Thermal Characterization of Cu(II) Complexes with two New Schiff Base Ligand towards Potential Biological Application
Md. Saddam Hossain, C M Zakaria, Md. Kudrat-E-Zahan* and B Zaman
Department of Chemistry, University of Rajshahi, Rajshai, Bangladesh and Barind medical college, Rajshahi
Abstract
The Cu(II) complexes [C15H11N3O3Cu] and [C15H11N3O2SCu] with two new Schiff base ligands HL1(C15H11N3O3) and HL2 (C15H11N3O2S) prepared derived from the condensation reaction of salicylaldehyde with semicarbazide and thiosemicarbazide respectively. where, HL1=bis(2-hydroxybenzylidene)hydrazinecarboxamide and HL2=bis(2-hydroxybenzylidene) hydrazinecarbothioamide. The ligands and complexes were isolated from the reaction in the solid form and characterized by IR ,UV-visible,1H NMR, thermal analysis and some physical measurements. Spectroscopic evidence indicated that the Schiff bases were behaved as N,O coordinating chelating agents. Magnetic susceptibility data coupled with electronic spectra suggested a distorted square planar structure of the complexes. The overall reaction is monitored by TLC and UV-Visible spectral analysis. The Schiff bases and their metal complexes have been shown moderate to strong antimicrobial activity.
Keywords
Schiff base, Semicarbazone, Thiosemicarbazones, Transition metal complexes, UV-visible, IR, 1H NMR TGA, DTG, Antibacterial activity
Introduction
Schiff bases are condensation products of primary amines with carbonyl compounds and they were first reported by German chemist noble prize winner Hugo Schiff. The common structural feature of these compounds is the azomethine group with a general formula RHC=N-R/ where R and R/ are alkyl, aryl, cyclo alkyl or heterocyclic groups which may be variously substituted. These compounds are also known as anils, imines or azomethines [1]. Metals have played a significant role in biological systems over the years. Many are important to our diets in varying quantities, although people have only recently realized their significance. Introducing metal ions into a biological system may be carried out for therapeutic or diagnostic purposes, although these purposes overlap in many cases. Metals not only provide path for synthesis, but they also introduce functionalities that enhance drugs action. The chemistry of the transition metal complexes of thiosemicarbazones became largely appealing because of their broad profile of pharmacological activity that provides a diverse variety of compounds with different activities [2-5]. Bis- Schiff base ligands and their coordination compounds having multifunctional groups play an important role in the areas of stereochemistry, structure of science, spectroscopy, and magnetic fields [6]. In recent years, sulfur containing ligands such as thiosemicarbazones and their transition metal complexes have received more attention in the area of medicinal chemistry, due to their pharmacological properties, such as antiviral, antibacterial, antifungal, antiphrastic, antitumor, anticancer and anti HIV activities [7-19]. Mei-Hsiu Shih et al [20] were synthesized Nickel (II) complexes from novel semicarbazone ligands with chloroformyl arylhydrazine, benzimidazole and salicylaldehyde moieties. Sulekh Chandra, et al. [21] synthesized copper (II) complexes with semicarbazones and thiosemicarbazones also determined their biological activity. Raman, et al. [22] synthesized Cu(II), Co(II), Ni(II) and Zn(II) complexes with Schiff base ligand derived from semicarbazide hydrochloride and shown their microbial activity. Recently, we have synthesized Mn(II), Fe(II), Co(II) and Sn(II) complexes of Schiff base ligand [23] and Cr(III), Sn(II) complexes of N,O coordinating novel Schiff base ligand [24] also investigated their thermal and antibacterial activity. Keeping these facts in view the significance of transition metal complexes in biological system, we here in report the synthesis and characterization with antibacterial activity of two novel Schiff base ligands and their Cu(II) complexes.
Materials and Methods
Reagents and chemicals
All the reagents used were of AnalaR grade or chemically pure grade. All metal (II) salts were used as chloride and sulphate. The solvents such as ethanol, methanol, chloroform, diethyl ether, petroleum ether, DMSO (dimethyl sulfoxide), dichloromethane and acetonitrile were purified by distillation procedure.
Physical measurement
The melting point or the decomposition temperature of all the prepared ligand and metal complexes were observed in an electro thermal melting point apparatus model No.AZ6512. Vibrational spectra (IR) were recorded with SHIMADZU-8400, FTIR spectrophotometer, (Japan), in the range 4000-400 cm-1 with a KBr disc as reference. UV-Visible spectra of the complexes in DMF (0.0005 molar) were recorded in the region 200-800 nm on a THERMOELECTRON NICOLET evolution 300 UV-Visible spectrophotometer. The SHERWOOD SCIENTIFIC Magnetic Susceptibility Balance that following the Gouy Method were used to measure the magnetic moment of the solid complexes. The electrical conductance measurements were made at room temperature in freshly prepared solution (10-3 M) in DMF using a WPACM35 conductivity meter and a dip-cell with a platinum electrode. The thermogravimetric analysis (TGA) was performed on Perkin Elmer Simultaneous Thermal Analyzer, STA-8000. The purity of the ligand and metal complexes was tested by Thin Layer Chromatography (TLC).
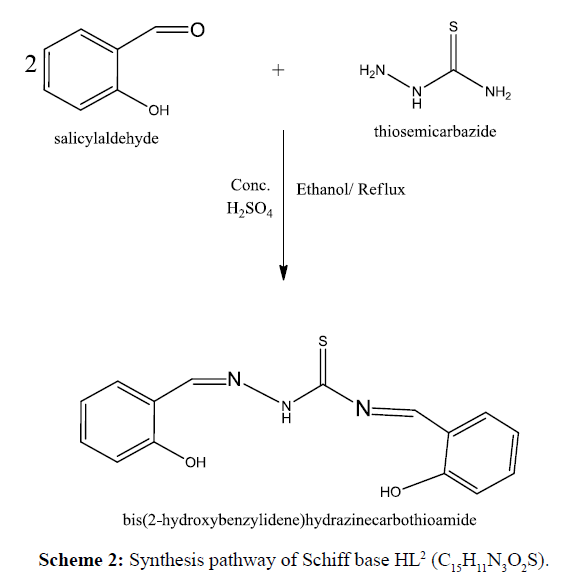
Preparation of Schiff base ligand HL1 and HL2
Salicyaldehyde (20 mmol, 2.4 mL) dissolved in absolute ethanol/(20 mL) was added slowly to a constant stirring solution of semicarbazide (0.75 g,10 mmol)/thiosemicarbazide (0.91 g, 10 mmol) in 20 mL hot ethanol in a two necked round bottom flask with 5 ml of conc. H2SO4 and the mixture was refluxed for 6 h. On cooling, a solid orange product was formed which was filtered, washed with ethanol and diethyl ether and dried in vacuum over anhydrous CaCl2. The synthesized reaction of ligand and complexes were monitored by TLC using petroleum ether, ethyl acetate, toluene and methanol solvents. The product was found to be soluble in methanol, DMF and DMSO and insoluble in ethanol, acetone, diethyl ether, petroleum ether and isopropanol. The synthesis route of Schiff base is shown in Schemes 1 and 2 respectively.
Preparation procedure of Schiff base metal complexes
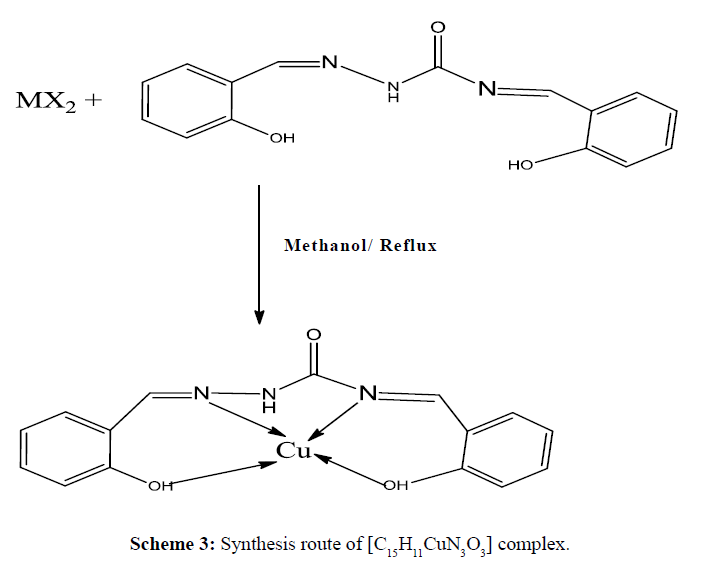
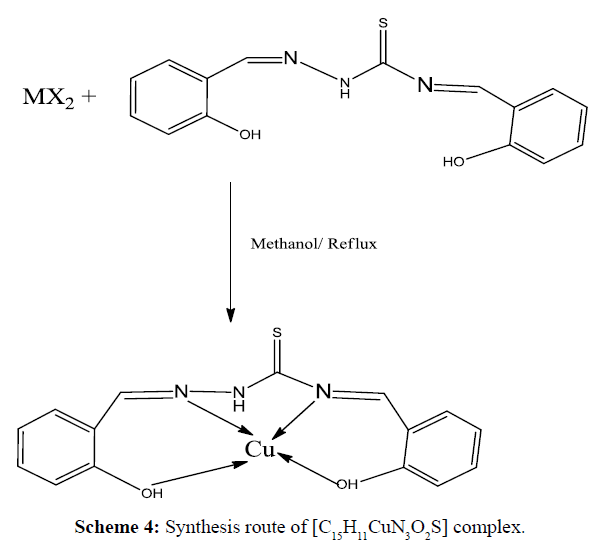
The complexes have the general formula [Cu(HL1)/HL2]; where HL1 =Synthesized Schiff base ligand C15H11N3O3 and HL2 = Synthesized Schiff base ligand C15H11N3O2S. Methanolic solution 20 mL of (HL1, 0.562 g, 2 mmol/ HL2,0.594g, 2 mmol )was taken in a two necked round bottom flask and kept on magnetic stirring. A warm methanolic solution (10 mL) of Cu(NO3)2.6H2O (0.482 g, 2 mmole) was added drop wise and stirred with heating for 4 h. On cooling, precipitates were formed which were filtered, washed with ethanol, acetone, and diethyl ether and dried in vacuum desiccators over anhydrous CaCl2. The purity of each complexes were tested by TLC using petroleum ether, ethyl acetate, toluene and methanol solvents. The complexes were soluble in DMSO and DMF. The synthesis pathway of complexes is shown in Schemes 3 and 4.
Physical properties
Some physical properties of the Schiff base ligand and its metal complexes such as melting point, color, magnetic moment etc. are shown in Table 1. The complexes are intensely colored, powdered solids, which decomposes above 300°C. Molar conductance values in DMSO (10-3 M) showed low values (25-41 μS/cm) indicating [25] them to be non-electrolyte.
| Compound/Mol. formula | Color | Yield(%) | Melting point /0C |
Conductivity /(µS/cm) |
|---|---|---|---|---|
| HL1 C15H11N3O3 |
Orange | 59 | 195 | 25 |
| HL2 C15H11N3O2S |
Gold | 62 | 205 | 35 |
| Cu- HL1 [C15H11 CuN3O3] |
Saddle brown | 65 | ˃300 | 38 |
| Cu- HL2 [C15H11CuN3O2S] |
Coffee | 60 | ˃300 | 41 |
Table 1: Physical and analytical data of the Schiff bases and metal complexes.
Selected 1H NMR spectral component of (C15H11N3O2S)
1H-NMR spectra of Schiff base ligand (C15H11N3O2S) was recorded in DMSO-d6 on a Bruker 300 MHz spectrophotometer using TMS as internal standard δ 6.786(s OH), δ 6.803(s CH), δ 8.303(s NH), δ 7.173-7.221(t, ph-H), 7.777-7.846 (d, ph-H),
Infrared spectral analysis
IR-Spectral analysis of Schiff base C15H11N3O3 and [C15H11CuN3O3] complex:
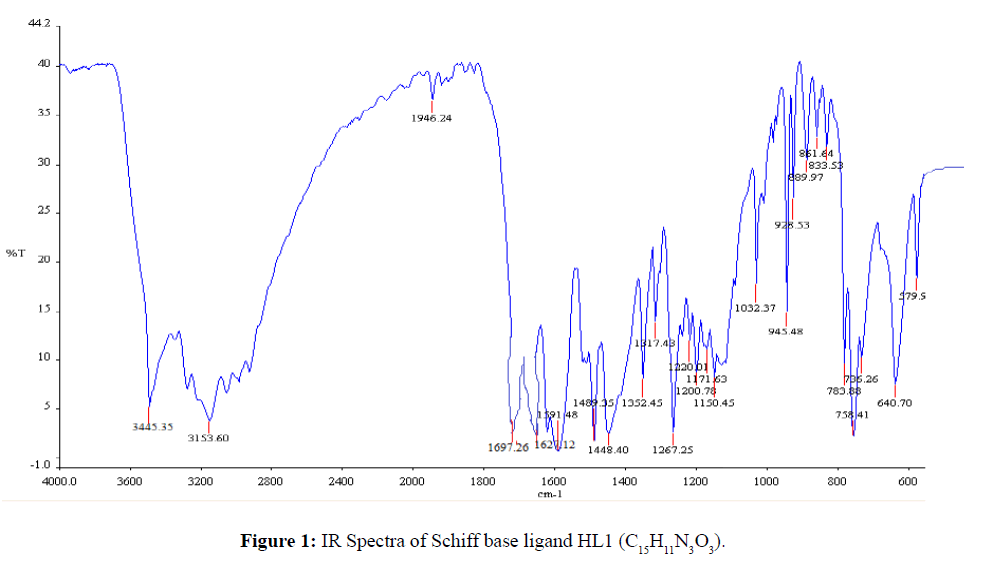
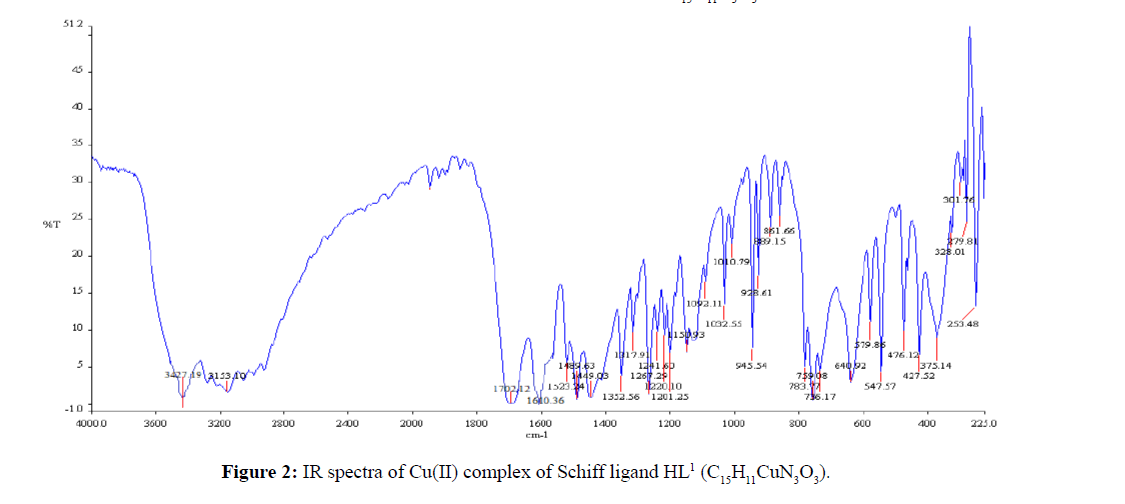
The strong band 1627 cm-1 that was assigned to ν(CH=N) in the Schiff base ligand (Figure 1) indicated that the free- NH2 group of semicarbazide was converted to azomethine group. The appeared of band 3445 cm-1 that was assigned to ν(O-H) in free Schiff base ligand confirmed that hydroxyl group of salicylaldehyde was present in Schiff base. The strong band 1697 cm-1 for ν(C=O) indicated that C=O bond for amide group was present in the Schiff base ligand. In C15H9CuN3O3 complex the band 3445 cm-1 for ν(O-H) in free Schiff base ligand was shifted to lower absorption frequency revealed that it was coordinated to metal atom (Figure 2). The shifting of bands from 1627 cm-1 to lower absorption frequency suggested that CH=N group was also coordinated to metal atom.
IR-Spectral analysis of Schiff base HL2(C15H11N3O2S) and[C15H11CuN3O2S] complex
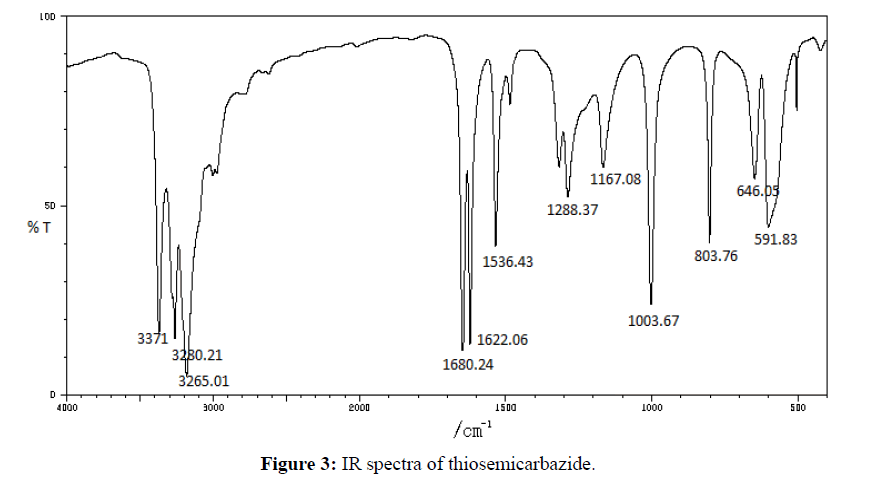
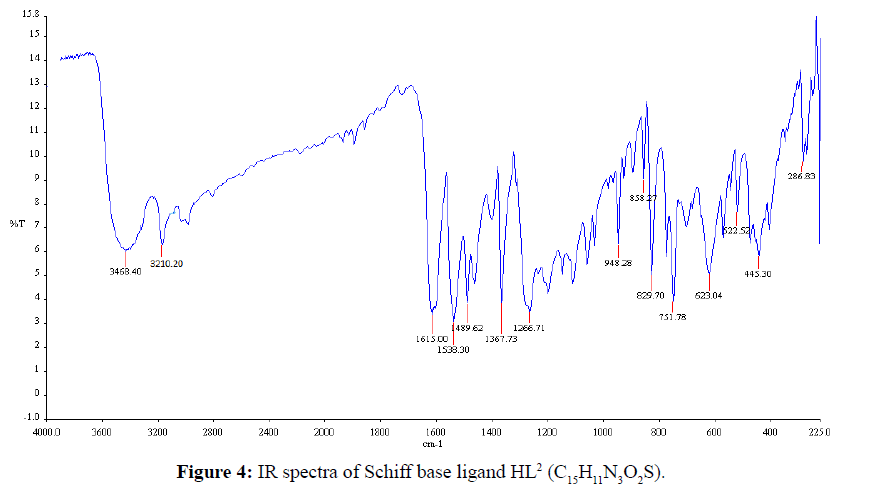
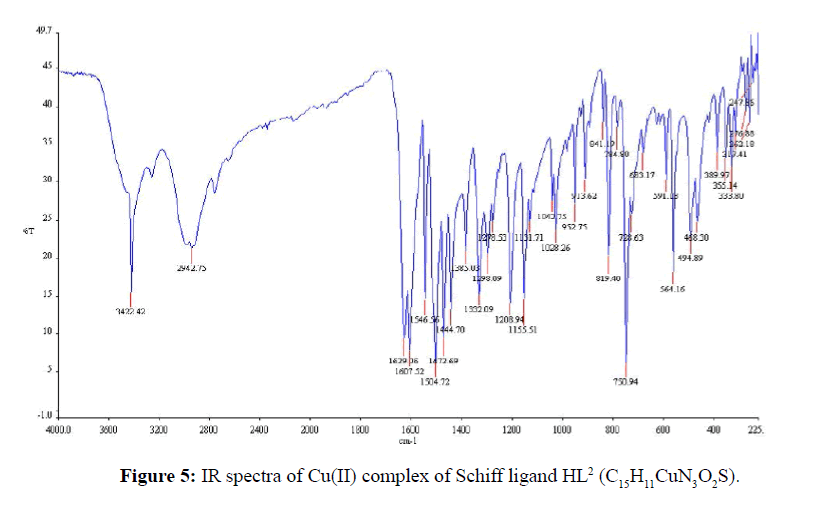
The IR-absorption bands 3371 and 3280 cm-1 was due to free -NH2 group of thiosemicarbazide (Figure 3) was absent in the free Schiff base ligand (Figure 4). The strong band 1615 cm-1 that was assigned to ν(CH=N) in the Schiff base ligand represented that free -NH2 group of thiosemicarbazide was converted to azomethine group. The appeared of band 3468 cm-1 that was assigned to ν(O-H) in Schiff base confirmed that hydroxyl group of salicylaldehyde was present in ligand. The strong band 1266 cm-1 for ν(C=S) indicated that C=S bond was present in the Schiff base ligand [26]. During complexation the band 3468 cm-1 for ν(O-H) shifted to lower absorption frequency evident that it was coordinated to metal atoms. The shifting of bands from 1615 cm-1 to lower absorption frequency suggested that CH=N group was coordinated to central metal atom. Also the appearance of new low absorption bands at (468-494), (317- 389) that was assigned to ν(M-N), ν(M-O) respectively [27] confirmed that O and N atoms are coordinated to central metal atoms. IR spectra of C15H11CuN3O2S complex was shown in Figure 5. IR spectral data of Schiff base and metal complexes were shown in Table 2.
| Ligand/complex | IR(cm-1) | |||
|---|---|---|---|---|
| n(O-H) | ν(C=N) | ν(M-N) | ν(M-O) | |
| (C15H11N3O3) | 3445 | 1627 | - | - |
| (C15H11N3O2S) | 3468 | 1615 | - | - |
| (C15H11CuN3O3) | 3427 | 1610 | 476 | 375 |
| [C15H11CuN3O2S] | 3422 | 1607 | 494 | 389 |
Table 2: Selected IR spectral data of the Schiff base and metal complexes.
Magnetic moment and UV-visible spectral analysis
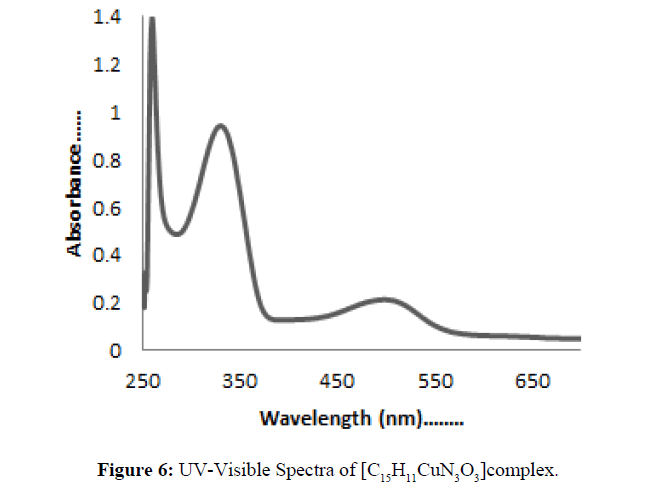
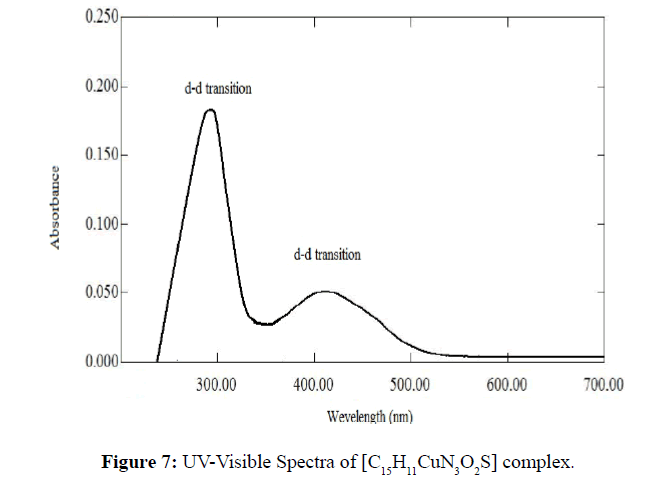
The spectra of both Schiff base ligand and their metal complexes [C15H11CuN3O3] and [C15H11CuN3O2S] were taken in DMSO. At room temperature magnetic moment value of Cu(II) complex HL1 and HL2 was found to 1.04 B.M and 1.10 B.M respectively, representing one unpaired electron per Cu(II) ion. The [C15H11CuN3O3] complex showed d-d transition at 350 and 500 nm (Figure 6). The [C15H11CuN3O2S] showed d-d transitions at 410 and 300 nm (Figure 7) which indicated the square planner structure of the complex.
Thermogravimetric analysis
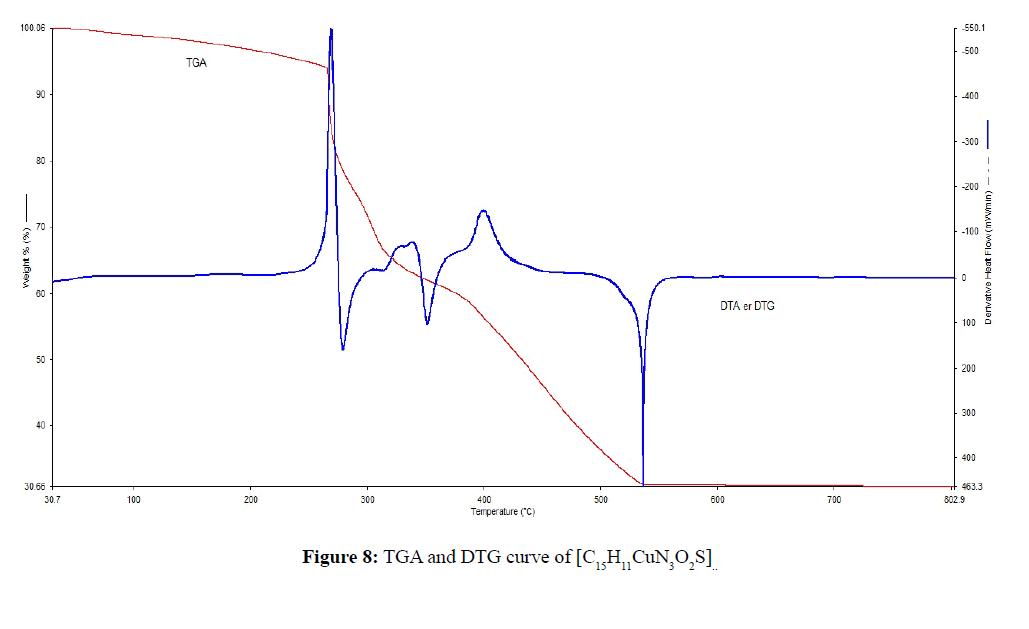
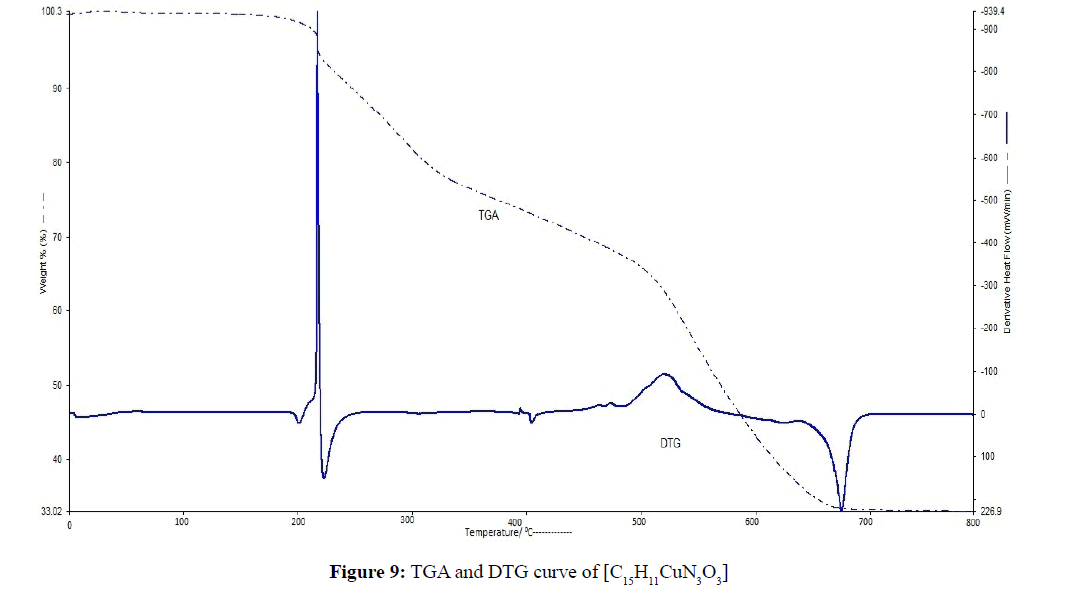
TGA was carried out for solid [C15H11CuN3O3] and [C15H11CuN3O2S] metal complexes under N2 flow. Thermal data of the complexes were shown in Table 3. The heating rate was suitably controlled at 30°C min-1 and the weight loss was measured from the ambient temperature up to 800°C. The TGA and DTG curve of [C15H11CuN3O2S] complex was shown in Figure 8, the curve indicated that the complex was decomposed into 3 main steps. Where, the 1st step involves the removal of C12H8O2 (calculated 53.25%, experimental 52.12% weight) at temperature range 180-320°C [23,24,28]. The part of complex -NH3S- was decomposed between temperature range 400-580°C (calculated 21.37%, experimental 20.25% weight) at 2nd Step of decomposition. In 3rd step the complex is completely decomposed and removed as CuO at above 670°C. The TGA and DTG curve of [C15H11CuN3O3] complex was shown in Figure 9. The curve indicated that the C15H9CuN3O3 complex was decomposed into 3 or 4 main steps. The major fragmentation occur at 280-330°C temperature range involves the decomposition of the part of complex C12H8O2 (calculated 55.75%, experimental 53.12% weight). The other part of complex -NH3O- was decomposed between temperature range 400- 540°C (calculated 24.09%, experimental 21.20% weight) at 2nd Step of decomposition. The complex is completely decomposed and removed as CuO at above 520°C.
| Complexes | Steps | Temperature Range/ °C | TG mass loss% calc./found |
Assignments |
|---|---|---|---|---|
| [C15H11CuN3O2S] | 1st 2nd 3rd |
180-320 400-580 ˃670 |
53.25/52.12 21.37/20.25 23.01/22.12 |
C12H8O2 NH3S- CuO |
| [C15H11CuN3O3] | 1st 2nd 3rd |
280-330 405-650 ˃580 |
55.75/53.12 24.09/21.20 26.33/24.45 |
C12H8O2 -NH3O- CuO |
Table 3: Thermal data of [C15H11CuN3O3] and [C15H11CuN3O2S] complexes.
Antibacterial activity
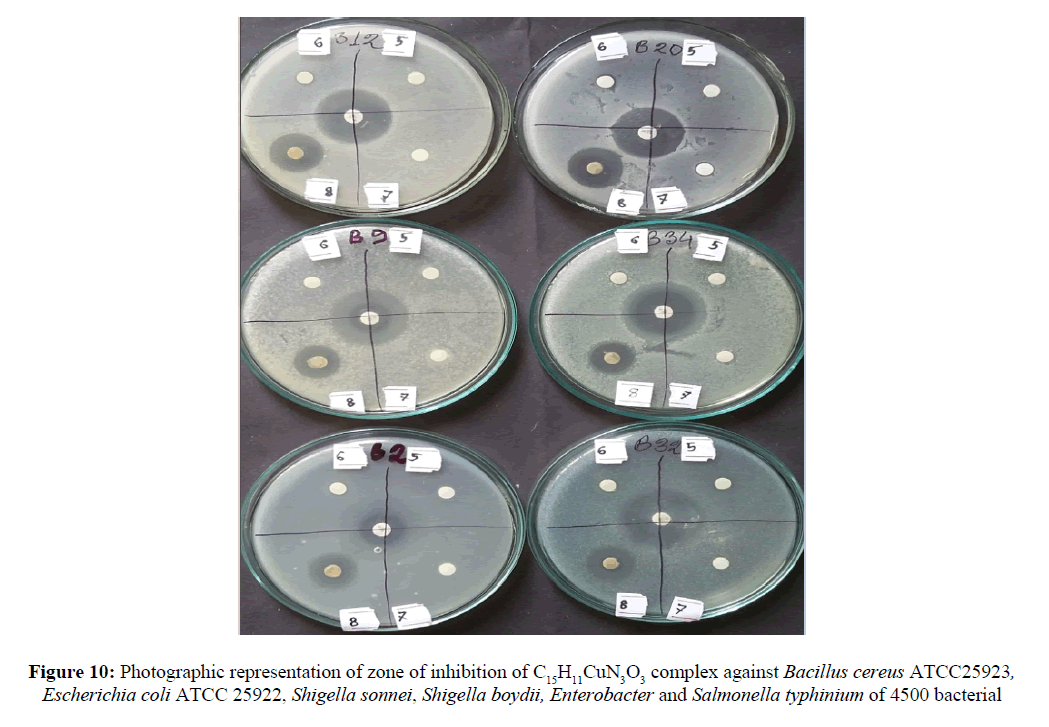
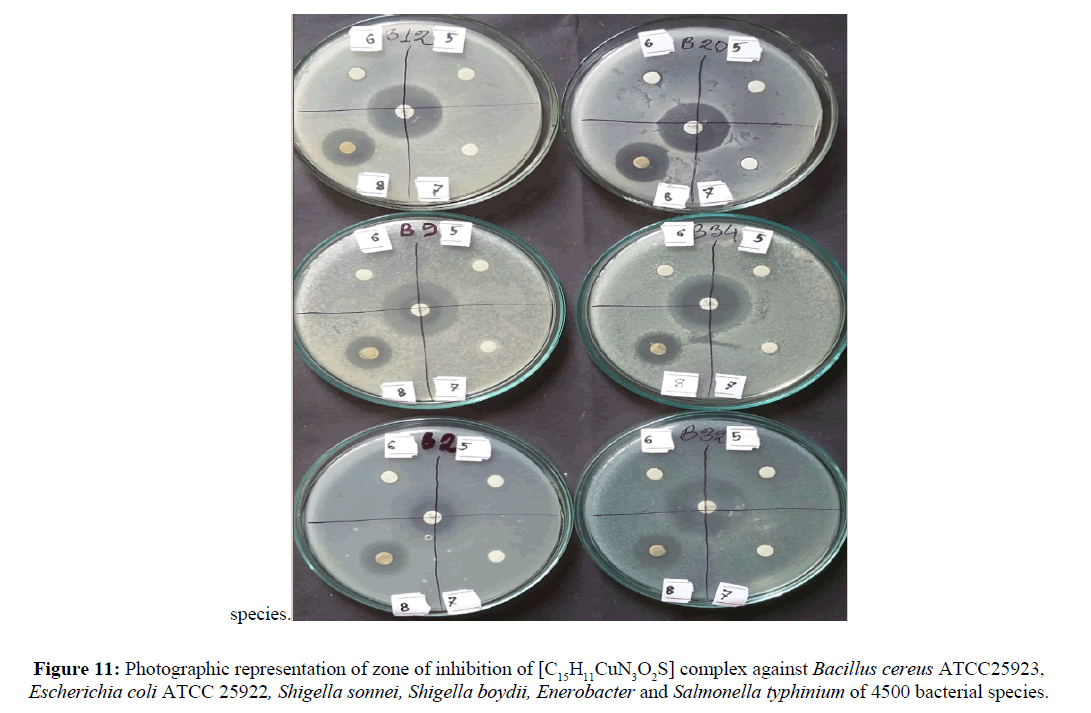
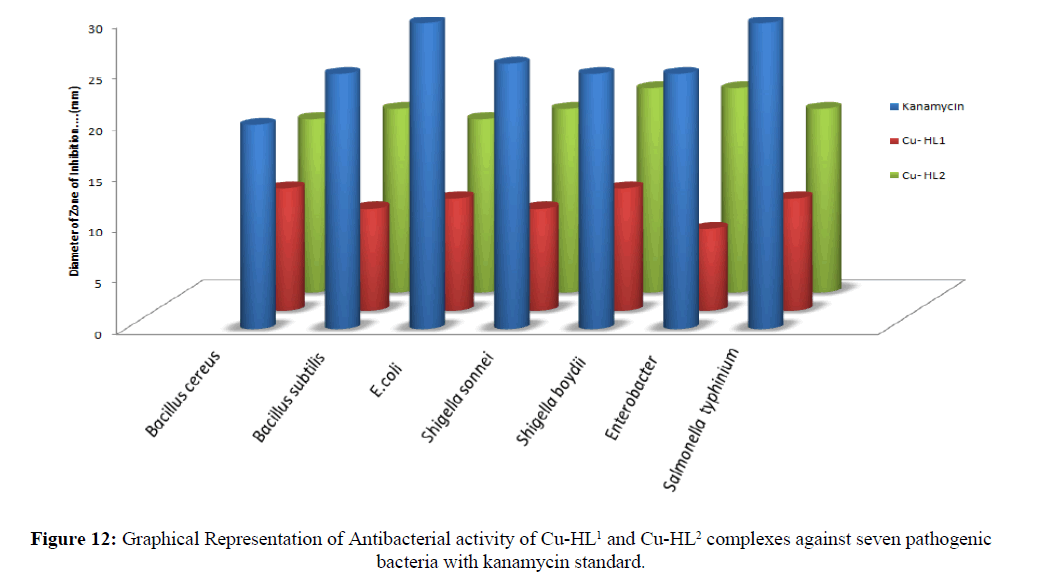
The prime objective of performing the antibacterial screening is to determine the susceptibility of the pathogenic microorganism to test the compound which, in turn is used to selection of the compound as a therapeutic agent. The free Schiff base ligand and their metal complexes were screened for their antibacterial activity against strains the Bacillus cereus ATCC25923, Escherichia coli ATCC 25922, Shigella sonnei, Shigella boydii, Enerobacter and Salmonella typhinium of 4500. The compounds were tested at a concentration of 50 μg/0.01 mL in DMSO solution using the paper disc diffusion method [29-31]. The susceptibility zones (Figures 10 and 11) were measured in diameter (mm) and the result are listed in Table 4. The susceptibility zones were the clear zones around the discs killing the bacteria. Copper(II) complex of the Schiff base ligand HL2 exhibited more inhibitory effects than that of Cu-HL1 complex on the growth of tested bacterial species. The graphical representation of antibacterial activity of Cu-HL1 and Cu-HL2 complexes against seven pathogenic bacteria with kanamycin standard was shown in Figure 12.
| Tested Bacteria | Diameter of zone inhibition(mm) of tested compounds | Kanamycin (30µg/disc) |
|||
| HL1 | HL2 | Cu- HL1 | Cu- HL2 | ||
| Bacillus cereus | 11 | 8 | 12 | 17 | 20 |
| Bacillus subtilis | 10 | 9 | 10 | 18 | 25 |
| E.coli | - | 8 | 11 | 17 | 30 |
| Shigellasonnei | 8 | 7 | 10 | 18 | 26 |
| Shigellaboydii | 9 | - | 12 | 20 | 25 |
| Enterobacter | - | 10 | 8 | 20 | 25 |
| Salmonella typhinium | 10 | 7 | 11 | 18 | 30 |
| DMSO control | - | - | - | - | 30 |
| Where, HL1=C15H11N3O3 and HL2= C15H11N3O2S | |||||
Table 4: Antibacterial screening activity of Schiff base and metal complexes.
Conclusion
In this paper we have explored the synthesis and coordination chemistry of Cu(II) complex with two new Schiff base ligand bis(2-hydroxybenzylidene)hydrazinecarboxamide and bis(2-hydroxybenzylidene)hydrazinecarbothioamide derived from the condensation reaction of salicylaldehyde with semicarbazide and thiosemicarbazide respectively. The physicochemical analysis indicated the formation of four coordinated metal complexes. IR spectral analysis indicated that N and O atoms were coordinated to central metal atom. Magnetic moment, UV-Visible and Thermogravimetic analysis confirmed the proposed structure of metal complexes. TGA analysis indicated that all the complexes are thermally stable up to 2000C. Biological activity revealed that the Cu- HL2 complex had more antibacterial activity as compared to Cu- HL1 complex. The metal complexes were more antibacterial activity than its free Schiff base ligand.
Acknowledgement
The authors are thankful to the Chairman Department of Chemistry, Rajshahi University, Rajshahi-6205, Bangladesh for providing the laboratory facilities.
Conflict of Interest
The authors have no conflict of interest to publish the article.
References
- Shahu R, Thakur DS, Kashyap P (2012) Schiff base: An overview of its medicinal chemistry potential for new drug molecule. Int J Pharm Sci Nanotech 5: 1757-1764.
- Liberta AE, West DX (1992) Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: current status. Biometal 5:121-126.
- Raja DS, Bhuvanesh NSP, Natarajan K (2011) Biological evaluation of a novel water soluble sulphur bridged binuclear copper(II) thiosemicarbazone complex. Eur J Med Chem 46: 4584-4594.
- Cory JG, Cory AH, Rappa G (1994) Inhibitors of ribonucleotidereductase comparative effects of amino- and hydroxy-substitutedpyridine-carboxaldehydethiosemicarbazones. BiochemPharmacol 48: 335-344.
- Li-Yan Lai, Zheng Liu, Guo-Cheng Han, Zhencheng Chen (2015) Synthesis, crystal structure and properties of three metal complexes based on a flexible Schiff base ligand. J ClustSci 26: 1845-1855.
- Yamada T, Ikeno T, Ohtsuka Y, Kezuka S, Sato M, et al. (2006) Manganese and cobalt 3-oxobutylideneaminato complexes: Design and application for enantioselective reactions. SciTechnolAdv Mater 7:184-196.
- Emayavaramban M, Kumar K, Mani P, Prabhakaran B,Muthuvel A (2014) Synthesis, complexation, spectral and antimicrobial study of some novel 5-bromofluorobenzaldehydeoxime and semicarbazone under ultrasonic irradiation. IJAC 2: 20-23.
- Khan SA, Asiri AM, Al-Amry K, Malik MA (2014) Synthesis, characterization, electrochemical studies, and In Vitro antibacterial activity of novelThiosemicarbazone and its Cu(II), Ni(II), and Co(II) Complexes. ScientificWorldJournal 2014:592375.
- AliAhmed MF, Yunus VM (2014) Microwave synthesis & antimicrobial activity of some Cu(II),Co(II),Ni(II) and Cr(III) complexes with Schiff base 2,6-pyridinedi carboxaldehydeThiosemicabazone. Orient J Chem30: 111-117.
- Kumari G, Kumar D, Singh CP, Kumar A, Rana VB (2010) Synthesis, characterization and antimicrobial activity of Trivalent metal Schiff base complexes. J Serb ChemSoc 75: 629-663.
- Shariar SMS, Jesmin M, Ali MM (2014) Antibacterial activities of some Schiff bases involving Thiosemicarbazide and Ketones. Intlettchemphysastron7: 53-61.
- Nasrin D, AshrafulAlam M, Hossain MN, Nazimuddin M (2013) Synthesis, characterisation and antimicrobial activity of Schiff Base metal complexes derived from S-benzyldithiocarbazate with 2-hydroxyacetophenone. Chemistry Journal 3: 13-19.
- Pahontu E, Julea F, Rosu T, Purcarea V (2015) Antibacterial, antifungal and in vitroantileukaemia activity of metal complexes with thiosemicarbazones. J Cell Mol Med 19: 865-878.
- Pandeya SN, Sriram D, Nath G, DeClercq E (1999) Synthesis antibacterial antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2- yl]thiosemicarbazide. Eur J Pharm Sci9: 25-31.
- YidlizM, Dulger B, Yancu SYK,Yanpici BM (2004) Synthesis and antimicrobial activity of bis (imido) Schiff bases derived from thiosemicarbazide with some 2-hydroxyaldehydes and metal complexes. J IndChemSoc 81: 7-12.
- Prasad S, Agarwal RK (2007) Cobalt (II) complexes of various thiosemicarbazones of 4-aminoantipyrine: Synthesis spectral thermal and antimicrobial studies. Transit Met Chem 33: 143-149.
- Parekh AK, Desai KK(2006) Synthesis and antibacterial activity of thiosemicarbazones. Indian J Chem 45: 1072.
- Murthy N, Dharmarajan TS (2006) Synthesis, characterization and biological activity of copper (II) complexes with phenylglyoxalbis-(thiosemicarbazones). Asian J Chem14:1325-1330.
- Ferrari MB, Capacchi S, Reffo G, Aelosi G (2005) Synthesis, structural characterization and biological activity of P-fluorobenzaldehydethiosemicarbazones and of a nickel complexes. J of InorgBiochem84: 89.
- Shih MH, Xu YY, Yang YS, Lin TT (2015) Syntheses of Nickel (II) complexes from novel semicarbazone ligands with chloroformylaryl hydrazine, benzimidazole and salicylaldehyde moieties. Molecules 20: 5184-5201.
- Chandra S, Raizada S, Verma R (2012) Synthesis, characterisation and biological activities of copper(II) complexes with semicarbazones and thiosemicarbazones. J Chem Pharm Res 4: 1612-1618.
- Raman N, Johnson Raja S, Joseph J, Raja J (2008) Designing, synthesis, spectral characterization of antimicrobial and DNA active tridentate Schiff base ligands and their complexes. J ChilChemSoc53: 1599-1604.
- Hossain MS, Islam MA, Zakaria CM, Haque MM, Mannan MA, et al. (2016) Synthesis, spectral and thermal characterization with antimicrobial studies on Mn(II), Fe(II), Co(II) and Sn(II) complexes of tridentate N,O coordinating novel Schiff Base Ligand. J Chem Bio PhySci6: 041-052.
- Hossain MS, Zakaria CM, Haque MM, Kudrat-E-Zahan M (2016) Spectral and thermal characterization with antimicrobial activity on Cr(III) and Sn(II) complexes containing N, O donor novel Schiff Base Ligand.Int J Chem Stud 4: 08-11.
- Refat MS, Ibrahim HK, Sowellim SZA, Soliman MH,Saeed EM (2009) Spectroscopic and thermal studies of Mn(II), Fe(III), Cr(III) and Zn(II) complexes derived from the ligand resulted by the reaction between 4-Acetyl Pyridine and Thiosemicarbazide.J InorgOrganometPolym19: 521-531.
- Al-Karawi AJM (2009) Synthesis and characterization of a new N2S2 Schiff base ligand and its complexes with nickel(II), copper(II) and cadmium(II) including the kinetics of complex formation. Transition Met Chem 34: 891-897.
- Alghool S, El-Halim HFA, El-sadek MA, Yahia I, Wahab L(2015) Mononuclear complexes based on reduced Schiff base derived from L-methionine, synthesis, characterization, thermal and in vitro antimicrobial studies. J Therm Anal Calorim121: 1309-1319.
- Haque MM, Kudrat-E-Zahan M (2015) Synthesis and characterization with antineoplastic, biochemical, cytotoxic, and antimicrobial studies of Schiff Base Cu(II) Ion Complexes. BioinorgChemAppl2015: 923087.
- Banu LA, Islam MS, Abdul Alim Al-Bari M, Kudrat-E-Zahan M (2015) Synthesis and characterization with antibacterial, antifungal, cytotoxicity studies on the Co(II),Ni(II) and Cu(II) complexes of tridentate ONO coordinating Schiff base ligand derived from heterocyclic amines. IJRAMR2: 0145-0148.
- Banu LA, Kudrat-E-Zahan M, Abul Bashar M, Haque MM, Quamruzzaman M, et al. (2015) Studies on synthesis and characterization with antimicrobial activity of mixed ligand coordinating co (ii) Complexes with phthalic acid and heterocyclic Amines.Int J Chem Stud 2: 38-41.
- Shiraj-U-Ddaula M, Anarul Islam M, Aktar S, Khairul Islam M, Abdul Alim Al-Bari M, et al. (2014) Synthesis, characterization and antimicrobial activity of Cd(II), Ni(II), Co(II) and Zr(IV) metal complexes of Schiff Base Ligand Derived from Diethylenetriamine and Isatin. Asian J Research Chem4: 619-621.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences