ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Characterization, Antibacterial, Antifungal Screening and Cytotoxic Activity Of Schiff Base Nickel(II) Complexes with Substituted Benzylidine Aminobenzoic Acid
PG and Research Department of Chemistry, Seethalakshmi Ramaswami College, Tiruchirappalli-620002, Tamil Nadu, India.
Abstract
The synthesis, characterisation and antimicrobial study of Ni(II) complexes from Schiff base ligands L1 and L2 are recorded in this study. The ligand L1, 2-((2-hydroxy-3-methoxybenzylidene)amino) benzoic acid obtained by the condensation of o-vanillin and 2-aminobenzoic acid and the ligand L2, 2-((2-hydroxybenzylidene) amino)benzoic acid is obtained by the condensation of salicylaldehyde and 2-aminobenzoic acid. The ligands and the complexes were characterised by molar conductance, vibrational, electronic, 1H-NMR spectrum and redox sudies. The molar conductance of these complexes convey the non-ionic character of the complexes. The spectral data show the composition of the metal complexes to be ML (L is the Schiff bases L1 and L2).Antibacterial as well as antifungal activities of these complexes of Ni(II) with the Schiff bases have been performed by disc diffusion method.The evaluated highly biologically active [Ni(L2-2H)(H2O)3].H2O complex shows excellent cytotoxicity towards (MG-63) cancer cell line.The results of the characterisation and biological activities are elaborately discussed in this paper.
Keywords:
Schiff base ligands, Ni(ii) complexes, 2-aminobenzoic acid, IR, Antibacterial, Antifungal activity, Cytotoxic activity.
Introduction
A coordination compounds or metal complex might be defined as central metal atom attached with the sheath of ions or molecules [1]. One of the more interesting aspects of transition metal is their ability to form coordination compounds. Such compounds are formed between a metal ion and a molecule with one or more unshared electron pairs called a ligand [2]. Coordination compounds are of great importance in biological system. A number of catalysts used in the chemical industry make use of coordination compounds [3]. Schiff base is a compound with a functional group that contains a carbon nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group not hydrogen. The synthesis of Schiff base ligands and their metal complexes have been extensively studied because of their interesting biological activities. They have been reported to be useful in medicine and catalyst. They are known to exhibit potent antibacterial, antiulcer and analgesic activities [4]. In addition some Schiff bases show pharmacologically useful activities like anticancer, anti-tuberculosis and antioxidant activities [5]. In this study, we have synthesised the ligands 2-((2-hydroxy-3-methoxybenzylidene)amino)benzoic acid (L1) from o-vanilin and 2-amino benzoin acid, and 2-((2-hydroxybenzylidene) amino)benzoic acid (L2) from salicylaldehyde and 2-amino benzoic acid. The Ni(II) complexes of these ligands were synthesised and it was characterised by colorimetry, thin-layer chromatography, IR, 1H-NMR, UV, ESR, molar conductance and magnetic susceptibility measurements. The in vitro antimicrobial activity of the free Schiff bases and its Ni(II) complexes were investigated.
Experimental
Materials and methods
The reagents such as o-vanillin, salicylaldehyde, 2-aminobenzoic acid, nickel chloride (NiCl2.6H2O) and the solvents like DMSO, DMF were used as such. The commercially available ethanol was dried over anhydrous quicklime for one day, filtered and distilled before use and ether was dried over sodium bits and then distilled by circulating ice cold water. The molar conductivity of the complexes isolated was measured in 10-3 M solutions of the Ni(II) complexes in dimethylformamide(DMF) using a Elico conductivity bridge and a dip type conductivity cell.
Melting points of the ligands and the Ni(II) complexes were determined by using the finely powdered sample in a fused capillary using Elico melting point apparatus. The IR spectra of the free Schiff bases and its Ni(II) complexes were taken using KBr pellets on Perkin Elmer RXI spectrometer in the region 4000 cm-1 to 400 cm-1. The electronic spectra of the ligands and the complexes in UV-Visible region of 190 nm to 1110 nm were taken using Perkin Elmer Lambda 35 spectrometer provided with quartz cells. The magnetic susceptibility of the complex at room temperature was measured by Gouy method using PICO K11 GAUSS Gouy balance. Proton NMR spectra was taken for the ligands and the complexes using the instrument, Bruker 400 MHz FT-PMR spectrometer. Electrochemical cyclic voltammetry studies for the ligands and the complexes were carried out on Multichannel VersaSTAT-II. The antimicrobial studies of the Schiff bases and the complexes were carried out by disc diffusion method in Periyar College of Pharmaceutical Sciences, Tiruchirappalli, TamilNadu, India.
Synthesis of the Schiff base ligands
The Schiff base ligand L1 was synthesised mixing the equimolar quantity of alcoholic solution of 2-aminobenzoic acid (0.01 mol, 1.37 g) and o-vanillin (0.01 mol, 1.52 g). The mixture was magnetically stirred for 1 hour. This solution was evaporated to remove the solvent over vacuum and cooled at room temperature. A brick red coloured precipitate obtained is filtered and washed with ether several times. It is recrystallized from hot ethanol and the melting point was noted (Yield=75%, Melting point=99oC).
The Schiff base ligand L1 was synthesised mixing the equimolar quantity of alcoholic solution of 2-aminobenzoic acid (0.01 mol, 1.37 g) and o-vanillin (0.01 mol, 1.52 g). The mixture was magnetically stirred for 1 hour. This solution was evaporated to remove the solvent over vacuum and cooled at room temperature. A brick red coloured precipitate obtained is filtered and washed with ether several times. It is recrystallized from hot ethanol and the melting point was noted (Yield=75%, Melting point=99oC).
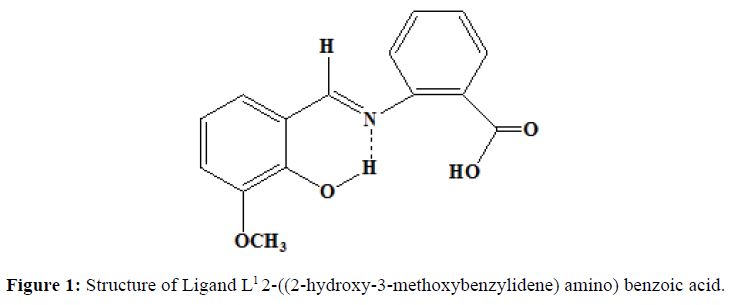
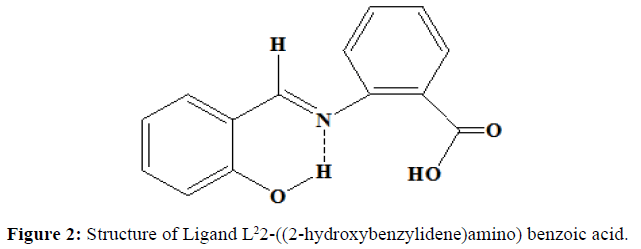
Similarly, the ligand L2 was synthesised by the mixing of the alcoholic solution of 2-aminobenzoic acid (0.01 mol, 1.37 g) and salicylaldehyde (0.01 mol, 1.065 ml). The mixture was stirred magnetically for half an hour. The solution is left as such at room temperature. The orange coloured precipitate obtained is filtered and washed with ether several times. It is recrystallized from hot ethanol. Melting point was noted. (Yield=70%, Melting point=190oC). Proposed structure of the ligands are shown in the (Figures 1 and 2).
Synthesis of the Ni(II) complexes
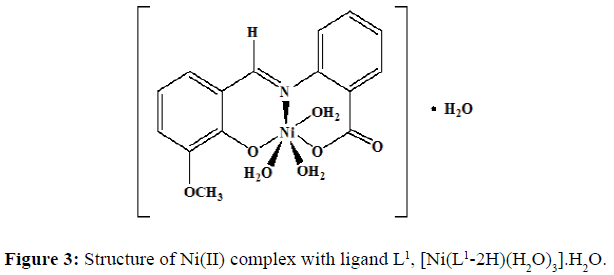
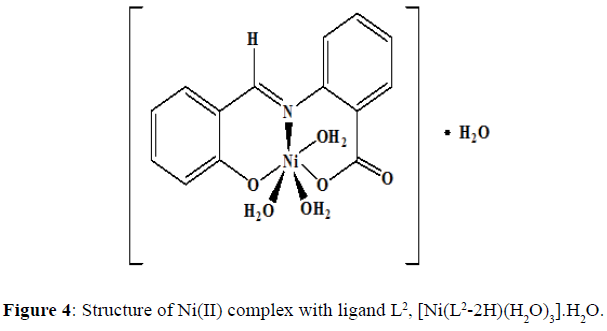
The complexes of nickel(II) with the Schiff base ligands derived from 2-aminobenzoic acid and o-vanillin/ salicylaldehyde was synthesized by mixing hot ethanolic solution of the ligand (0.002 mol, L1=0.5420 g and L2=0.4820 g) and an ethanolic solution of the Ni(II) chloride (0.002 mol, 0.4754 g). The mixture was refluxed in a water bath for 4-5 hours and the pH of the solution was maintained by the buffer solution. The precipitate obtained was filtered and washed with hot ethanol several times to remove excess metal ion. The precipitate was then dried and stored in a desiccator over anhydrous CaCl2 under vacuum. The complex of Ni(II) chloride with the Schiff base L1 was obtained as a fluorescent green solid (yield=67%) with a melting point of 218°C, and the dirty white complex of Ni(II) with the Schiff base L2 was obtained (yield=69%) and the melting point of this complex was noted to be 245°C.Proposed structure of the Ni(II) complexes with the ligands L1 and L2 are shown in the (Figures 3 and 4) respectively.
Purity of the Ni(II) complexes and the ligands L1 and L2 were tested by thin-layer chromatography.
Results and Discussion
Physical characteristics and analytical data of Schiff bases and their Ni(II) complexes were given in the (Table 1). The Schiff base ligands and the Ni(II) complexes are stable in the room temperature. The complexes are soluble in DMSO and DMF, whereas insoluble in ether.
Molar conductance studies
The molar conductance (Table 1) of 10-3m solution of the complexes in DMF has been measured. For [Ni(L1-2H) (H2O)3].H2O,it has been found to be 12.3 x 10-3 ohm-1cm2mol-1 and 17.9 x 10-3 ohm-1cm2mol-1 for [Ni(L2-2H)(H2O)3]. H2O complex. This shows the non-electrolytic behaviour of both the complexes [6]. This accounts that in both the complexes the metal ions are coordinated with water molecules and present inside the coordination sphere.
| S. No | Compounds | Molecular formula | Colour of the compound | Melting point°C | Molar conductance ´ 10-3 ohm-1 cm2 mol-1 |
|---|---|---|---|---|---|
| 1 | Ligand (L1) | C15H13NO4 | Brick red | 99 | - |
| 2 | Ligand (L2) | C14H11NO3 | Orange | 190 | - |
| 3 | [Ni(L1-2H)(H2O)3].H2O | C15H19NO8Ni | Fluorescent green | 218 | 12.3 |
| 4 | [Ni(L2-2H)(H2O)3].H2O | C14H17NO7Ni | Dirty White | 245 | 17.9 |
Table 1: Physical properties and analytical data of Schiff base ligands and their Ni(II) complexes
IR spectra
The strong band at 1602 cm-1 in the IR spectra of the ligand (L1) confirming the presence of imine group. This band corresponds to >C=N stretching frequency ν>C=N. Another broad band at 3449 cm-1 show the characteristic frequency of hydrogen bonded νO-H. The IR spectra of the ligand (L2) shows a band at 1616 cm-1 which corresponds to >C=N stretching frequency, ν>C=N. The broad band at 3435 cm-1 shows the characteristic frequency of hydrogen bonded νO-H. The FT-IR spectra of the free ligands with those of the complexes were compared (Table 2) in order to find out the binding modes of the ligand to the M(II) centres. The ligands used in the present investigation contains three possible donor sites phenolic oxygen (Ar-O), azomethine nitrogen (-C=N) and carboxyl oxygen (-C-O).The azomethine band in ligand (L1) at 1602 cm-1 is shifted to 1595 cm-1 in [Ni(L1-2H)(H2O)3].H2O, complex, suggesting the coordination through >C=N– group [7]. The azomethine band in Ligand (L2) at 1616 cm-1 is shifted to 1596 cm-1 in Ni(II) complex suggesting the coordination through >C=N– group. The ligand is coordinated to the M(II) ion through azomethine nitrogen and this results in the reduction of electron density on the azomethine ‘N’ atom and thus lowers the ν(>C=N-) absorption frequency after complexation by 7 cm-1 and 20 cm-1 in both the complexes.
| S.No | Name of the microorganism | (L1) | (L2) | [Ni(L1-2H)(H2O)3].H2O | [Ni(L2-2H)(H2O)3].H2O |
|---|---|---|---|---|---|
| 1 | Staphylococcus aureus | 12 (++) |
14 (++) |
17 (+++) |
27 (+++) |
| 2 | Klebsiella aerogenes | 12 (++) |
14 (++) |
20 (+++) |
22 (+++) |
| 3 | Aspergillus niger | 12 (++) |
14 (++) |
12 (++) |
16 (+++) |
Table 2: Biological activity of Schiff bases and their Ni(II) complexes
The appearance of a new non-ligand bands at 422 cm-l and 658 cm-l which are assignable to ν(M-O) and ν(M-N) respectively are further evidence for the coordination of the N and O atoms of the Schiff base with the metal ion in both the complexes. The coordination of water molecule is indicated by the presence of band at 3303 cm-l for both the complexes [8]. A overlapped band in the region of 1602-1616 cm-l assigned to νC-O stretching frequency in Schiff bases are shifted to lower frequency in the range 1541-1542 cm-l in both the metal complexes indicates the involvement of oxygen atom of hydroxyl group of –COOH group in bonding with metal ions. The νC-O (phenolic) stretching frequency of ligands in the region 1362-1368 cm-l are shifted to a lower frequency region in the range of 1219-1244 cm-l in the complexes indicates the bonding through phenolic oxygen.
Electronic spectra and magnetic susceptibility measurements
The electronic spectra of the ligand (L1) exhibits 3 absorption maxima. The band observed at 275 nm (36364 cm-1) may be due to Π-Π* transitions of the aromatic part of ligands. The other two bands at 315 nm (31746 cm-1) and 356 nm (28089 cm-1) could be assigned to n–Π* transition associated with azomethine linkage [9]. Similarly, the electronic spectra of the ligand (L2) shows 2 absorption maxima. The band observed at 275 nm (36364 cm-1) may be due to Π-Π* transitions of the aromatic part of ligands. Another band observed at 330 nm (30303 cm-1) could be assigned to n-Π* transition associated with azomethine linkage.
The Ni(II) complex with ligand (L1) show absorption bands at 253 nm (39525 cm-1), 268 nm (37313 cm-1), 337 nm (29674 cm-1) and 440 nm (22727 cm-1). The first two bands at 39525 cm-1 and 37313 cm-1 are due to intraligand transitions. The band observed at 29674 cm-1 may be attributed to M→L charge transfer transition. The band at 22727 cm-1 may be due to the reason of 3Ag2 (F) → 3T2g(P) d-d transition [10]. The Ni(II) complex with ligand (L2) is dirty white in colour and it exhibits four absorption bands at 259 nm (38610 cm-1), 269 nm (37174 cm-1), 322 nm (31055 cm-1), 406 nm (24630 cm-1) and 520 nm (19231 cm-1). The first two bands at 38610 cm-1 and 37174 cm-1 are due to intraligand transitions. The band observed at 31055 cm-1 is because of M→L charge transfer transition. The band at 24630 cm-1 and 19231 cm-1 may be due to 3T1(F)→3T1(P) and 3A2g(F) →T1g(P) d-d transitions. This suggests the octahedral geometry around both Ni(II) complexes.
The magnetic moment values (μeff= 2.81BM and μeff=2.80BM) for Ni(II) complexes are indicative of two unpaired electrons per Ni(II) ion and are also consistent with the octahedral geometry for Ni(II) complexes.
1H NMR spectra
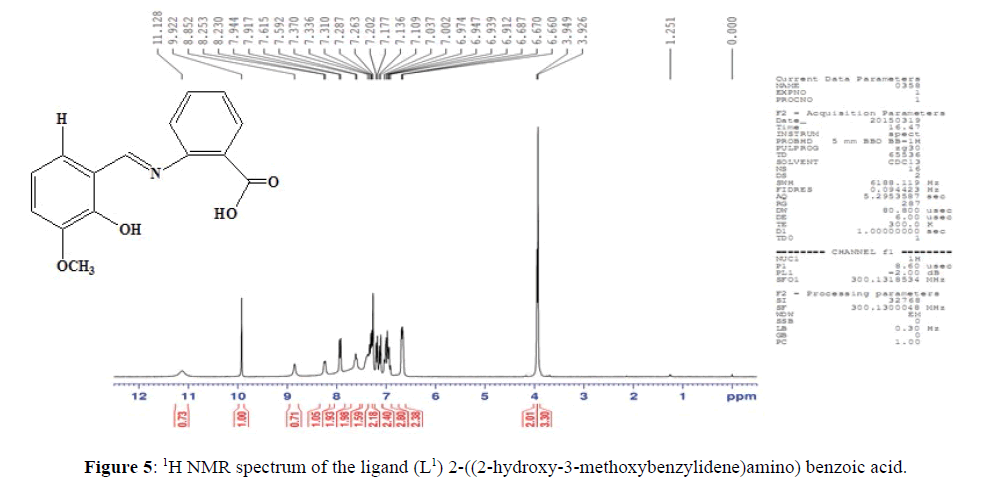
The NMR spectrum of ligand (L1) (Figure 5), shows a signal at δ=8.85 ppm which corresponds to the azomethine protons of the ligand suggesting the presence of >C=N– linkage [11]. The multiplet which extends from δ=6.67–7.94 ppm corresponds to the seven protons present in the aromatic ring. The signal at δ3.95 ppm may be allocated to the methoxy proton in L1. The signal at δ=9.92 ppm confirms the presence of hydroxyl proton. The signal at δ=11.13 ppm corresponds to the hydroxyl proton of –COOH group.
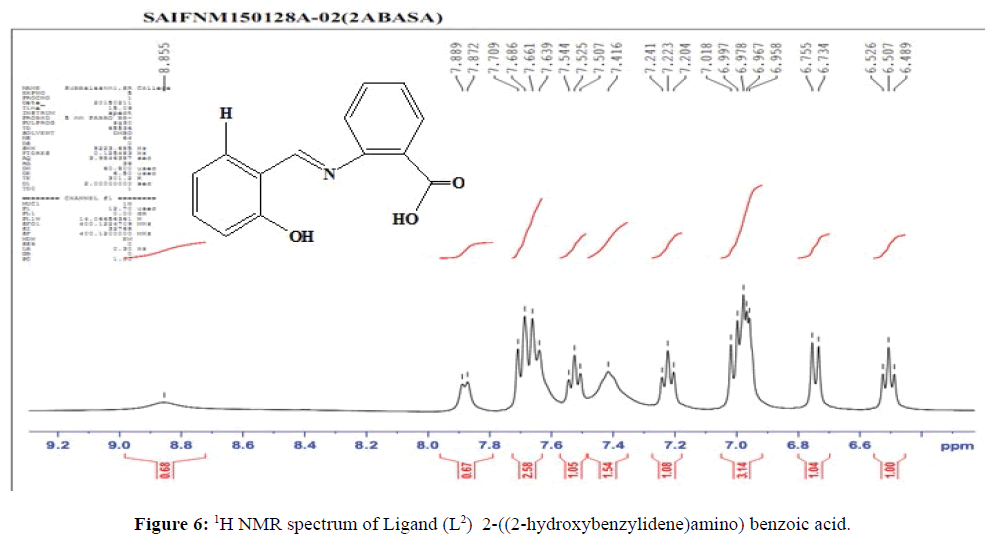
The NMR spectrum of the ligand (L2) (Figure 6), shows a signal at δ=8.86 ppm which corresponds to the azomethine protons of the ligand suggesting the presence of (>C=N–) linkage. The multiplet which extends from δ=6.49–7.89 ppm corresponds to the eight protons present in the aromatic ring. The presence of hydroxyl proton is confirmed by the signal at δ=10.27 ppm. The signal at δ=10.71 ppm corresponds to the hydroxyl proton of –COOH group.
In the NMR spectrum of the complexes of Ni(II), the absence of the peaks in the region δ10.0 ppm and δ11.0 ppm, indicates the removal of protons from the phenolic –OH group and carboxylic –COOH group and the involvement in bonding [12]. The peak at δ8.8 ppm in the ligands is shifted to downfield, it outlines the participation of this group in the complexation. From the data, it can be evident that the bonding of the Ni(II) with the ligands L1 and L2 is through the phenolic oxygen, carboxylate oxygen and azomethine group.
Redox behaviour
The important parameters are calculated from cyclic voltammogram of the complexes : Cathodic peak potentials (Epc), anodic peak potentials (Epa) versus glassy carbon electrode (GCE), the difference between the first oxidation and reduction processes (ΔEp) and cathodic vs anodic peak current ratio (ipa / ipc).
The redox process of [Ni(L1-2H)H2O)3].H2O is quasi – reversible in nature, characterized by large peak to peak separation (ΔEp) of 350 mV. The ratio of anodic and cathodic current (ipa/ipc<1) confirms the quasi-reversible process [13]. The observed cyclic voltammogram for [Ni(L2-2H)H2O)3].H2O is characterized as irreversible with oxidative response of Epa=100 mV.
Antimicrobial activities of the complexes
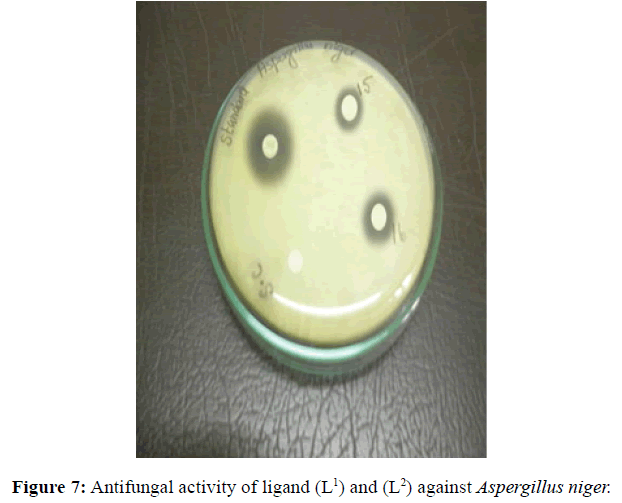
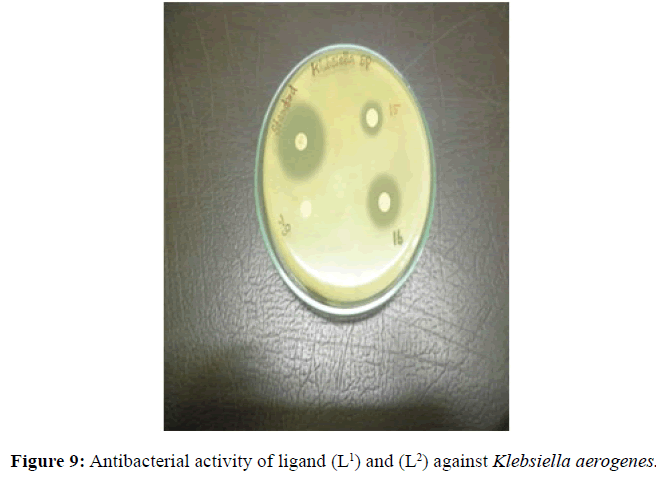
Antifungal and antibacterial studies of Schiff base ligands L1 and L2 and their Nickel(II) complexes have been tested by disc diffusion technique [14]. The gram positive and gram negative bacterial organisms such as Staphylococcus aureus (gram positive), Klebsiella aerogenes (gram negative) and Aspergillus niger (fungi) are used to find out the antimicrobial activity (Figures 7-10).
The results were formulated with standard drug Ciprofloxacin (5 μg/disc) for bacteria and Nystatin (100 units/disc) for fungi. The synthesised complexes showed a remarkable biological activity against bacteria and fungi as indicating in (Table 2). The Nickel(II) complexes was observed to be more active compared with the ligands against all pathogens. The antimicrobial results evidently shows that the activity of the Schiff-base ligands are pronounced when coordinated to the metal ions especially [Ni(L2-2H)(H2O)3].H2O shows higher antibacterial activity against Staphylococcus aureus. Thus, overall potency of the free ligand is enhanced on coordination with metal ion.
The overall order of anti-microbial activity is
[Ni(L2-2H)(H2O)3].H2O>[Ni(L1-2H)(H2O)3].H2O>L2 >L1
The enhancement in antimicrobial activity of the Ni(II) complexes as compared to free Schiff base may be explained on the basis of Tweedy’s chelation theory [15]. According to Tweedy’s chelation theory, chelation reduces the polarity of the metal ion mainly because of the partial sharing of the positive charge with the donor groups present in the ligand and possibly the electron delocalization within the whole chelate ring system thus formed during coordination.
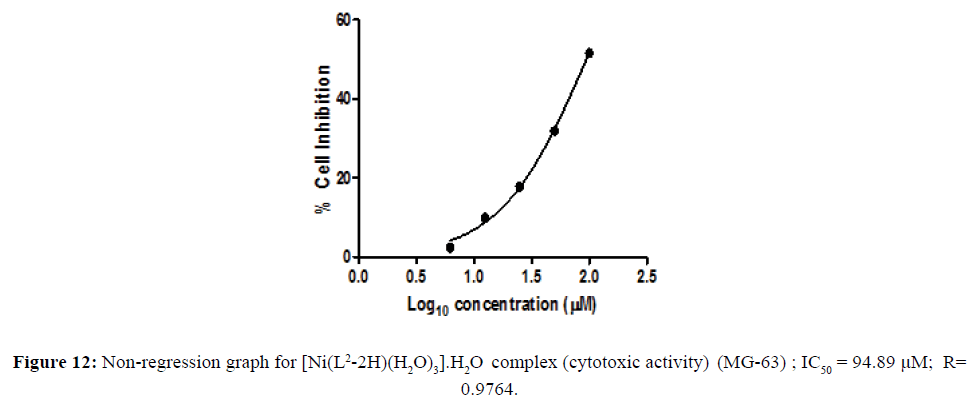
Cytotoxic activity of [Ni(L2-2H)(H2O)3].H2O complex
The metal complexes may also be a vehicle for activation of the ligand as the cytotoxic agent. The in vitro cytotoxicity of the newly-synthesized complexes with remarkable antimicrobial activity were carried out in the human tumor cell lines. Cell line namely human osteosarcoma cell line (MG-63) were assayed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay [16,17] . The concentration of the compounds at % cell Inhibition growth (IC50) was calculated. Cytotoxicity activity of the complex shown in the Figure 11. The concentration of the metal complex at 50% cell growth was inhibited and IC50was calculated is shown in the (Table 3). The IC50 values of the complex were assigned from the dose- survival curves obtained from the MTT assay. Ni(II) complex was observed to be more effective than cis platin in human osterosarcoma cell line (Figure 12). The better cytotoxicity activity of the complex against (MG -63) may play a significant role in metallodrug formulation in the field of bioinorganic chemistry.
Figure 11: Anticancer activity of [Ni(L2-2H)(H2O)3].H2O against human osteosarcoma cell line (MG -63).
| [Ni(L2-2H)(H2O)3].H2O  against MG -63 |
||
|---|---|---|
| Conc (µM) | % Cell Inhibition | |
| 6.25 | 2.310803 | |
| 12.5 | 9.936453 | |
| 25 | 17.79318 | |
| 50 | 31.88908 | |
| 100 | 51.58868 | |
Table 3: In vitro cytotoxicity assay
Conclusion
With the support of the results obtained, the following conclusions can be drawn. The ligand is acting as a tridentate ligand leading to the formation of 1:1 metal ligand complex. The measured molar conductance value indicates the non-electrolytic nature of the complex. The ligands are coordinated to Ni(II) with the nitrogen atom of the azomethine group, oxygen atom of the phenolic OH group and oxygen atom of the carboxyl group and they are confirmed from IR spectra. The UV visible and magnetic moment values signify an octahedral environment around the nickel(II) ion. Both the complexes possess electrochemical behaviour.
From the above conclusions, an octahedral structure has been suggested to both Ni(II) complexes. The ligand L1 and L2 and their Nickel (II) complexes have been tested against both bacterial and fungal pathogens. From the observed biological and anticancer activities, it is inferred that the Schiff base complexes possess more activity than the corresponding ligands.
References
- Lee JD (1996) Concise Inorganic Chemistry, Chapman and Hall Ltd, London.
- David WO, Gillis HP, Campion A (2008) Principles of Modern Chemistry, Thomson Brooks, USA.
- Muhammad H, Chohan ZH (2012) Synthesis, spectral characterization and biological studies of transition metal(II) complexes with triazole Schiff bases. Appl Organometal Chem 27: 36-44.
- Mehmet G, Mehmet S, ˙Ismet B, (2012) Synthesis, characterization, and antimicrobial activity of a new pyrimidine Schiff base and its Cu(II), Ni(II),Co(II), Pt(II), and Pd(II) complexes. Turk J Chem 36: 189-200.
- Parashar, RK, Sharma RC, Mohan G (2005) Biological activity of some Schiff bases and their metal complexes. Biol Trace Elem Res. 23:145-50.
- Valarmathy G, Subbalakshmi R (2013) Synthesis, characterization and antimicrobial screening of Co(II), Ni(II),Cu(II), Mn(II) and Zn(II) complexes with schiff base derived from 2-Sulphanilamidopyridine and 2-Hydroxy-3-methoxybenzaldehyde. Asian J Chem 25:2077-2079.
- Phatak P, Jolly VS, Sharma KP (2000), Synthesis and biological activities of some new substituted arylazo schiff bases. Orient J Chem 16:3.
- Omar BI, Mahmoud AM, Moamen SR (2014), Nano sized schiff base complexes with Mn(II), Co(II), Cu(II), Ni(II) and Zn(II) Metals : Synthesis, Spectroscopic and Medicinal Studies. Can Chem Trans 2: 108-121.
- Gomathi V, Selvameena R (2013), Synthesis, spectroscopic, electrochemical and biological studies of novel Schiff base complexes derived from 4-(3-ethoxy-2-hydroxybenzylideneamino)-N-(pyridin-2-yl)benzenesulfonamide. Main group chem 12: 275- 284.
- Anant P, Devjani A (2011) Application of Schiff bases and their metal complexes-A Review J Teach Res Chem 2004, 11, 39.
- Rama I, Selvameena R (2015) Synthesis, structure analysis, anti-bacterial and in vitro anti-cancer activity of new Schiff base and its copper complex derived from sulfamethoxazole. J of Cheml Sci 127: 671-678.
- Guo Z, Xing R, Liu S, Yu H, Wang P (2005) Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorg Med Chem 13: 1573-1577.
- Mabbot GA (1983) An introduction to cyclic voltammetry J Chem Educ 60: 252-706.
- Indian Pharmacopoeia. 1996, IIA, 105.
- Dharmaraj N, Viswanathanmurthi P, Natarjan K (2001), Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transit Metal Chem 26: 105-109.
- Navarra M, Celano M, Maiuolo J, Schenone S, Botta M, et al. (2010) Antiproliferative and pro-apoptotic effects afforded by novel Src-kinase inhibitors in human neuroblastoma cells. BMC Cancer 10: 602.
- Kenan B, Nevin T, Ahemet S, Naki C, Synthesis (2018), structural characterization and biological activities of metal(II) complexes with Schiff bases derived from 5-bromosalicylaldehyde: Ru(II) complexes transfer hydrogenation. Journal of Saudi Cheml Soci, June 2018 (In press).

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences