ISSN : 0976-8505
Der Chemica Sinica
Synthesis and Characterization of New Vic-dioximes and Their Nickel Complexes
Tülay ÇAM* and Gazi Ä°REZ
Department of Chemistry, Faculty of Arts and Sciences, Uludağ University, 16059-Bursa, Turkey
Abstract
In this study, four new vic-dioximes, anti-4-aminodiphenylaminoglyoxime (H2L1), anti-4-aminodiphenylaminophenylglyoxime (H2L2), anti-3-hydroxy-5-sulpho-2-naphthylaminoglyoxime (H3L3), and anti-3-hydroxy-5-sulpho-2-naphthylaminophenylglyoxime (H3L4), as well as their nickel complexes, were synthesized. The structures of these new compounds (both the ligands and complexes) were characterized by the following methods: IR, NMR, atomic absorption spectroscopy, elemental analysis, differential thermal analysis (DTA), and thermogravimetric analysis(TGA).
Keywords
Vic-dioximes, Nicel(II) complexes, Oxime complexes, Synthesis and characterization
Introduction
The chemistry of oxime/oximato metal complexes has been investigated since the time they were first synthesized. For example, Chugaev prepared nickel(II) dimethylglyoximato and recognized the five-membered chelate character of this complex [1]. Due to the presence of mildly acidic hydroxyl groups and slightly basic azomethine groups, vicdioximes are amphoteric ligands that form corrin-type square-planar, square-pyramidal, and octahedral complexes with transition metal ions, such as nickel(II), as the central atoms [2,3]. The exceptional stability and unique electronic properties of these complexes can be attributed to their planar structure, which is stabilized by hydrogen bonding [4]. Transition metal complexes of vic-dioximes have been of particular interest in biological model compounds and have been extensively investigated for their similarity to vitamin B12 [5,6]. Vic-dioximes have a strong tendency to form isomers. When the molecule is symmetrical, the following three forms listed in order of increasing stability are possible: syn-, anti-, and amphi-structures. However, there are some exceptions to this stability order. The dioxime anti-isomers are responsible for the formation of brightly colored chelating derivatives with nickel and other transition metal ions [7-11]. Especially of note is that the anti-isomer makes blood-red complexes with nickel ions.
This paper describes the synthesis and characterization of anti-4-aminodiphenylaminoglyoxime (H2L1), anti-4- aminodiphenylaminophenylglyoxime (H2L2), anti-3-hydroxy-5-sulpho-2-naphthylaminoglyoxime (H3L3), and anti-3- hydroxy-5-sulpho-2-naphthylaminophenylglyoxime (H3L4) as well as their nickel complexes.
Materials and Methods
Elemental analyses (C, H and N) were performed using a Costech model analyzer, and analyses of the metal were determined using a Varian Spectra 220-FS AAS spectrometer. 1H NMR, 13C NMR and IR spectra were obtained with a Varian Mercury Plus AS 400 spectrometer and a Thermo Nicolet 6700 FT/IR instrument, respectively. Melting points were measured on a BUCHI melting point B-540 apparatus. The pH values of the solutions were adjusted and controlled with a WTW inoLab pH Ion Level 2 pH meter. TGA curves were recorded with a SII Exstar 6200 (TG/ DTA) thermo balance.
The preparation of anti-chloroglyoxime and anti-chlorophenylglyoxime has been previously described [12-14]. All of the reagents used were purchased from Merck, Fluka or Sigma and were chemically pure.
Ligands
Anti-4-aminodiphenylaminoglyoxime (H2L1)
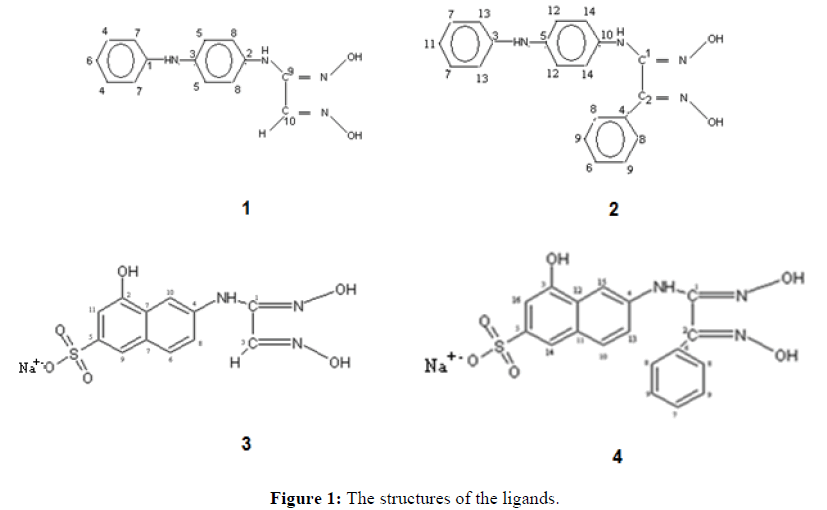
First, 4-aminodiphenylamine (5 mmol) was dissolved in ethanol and then pyridine (5 mmol). Then, anti-chloroglyoxime (15 mmol) was added to the solution before stirring for 72 h at room temperature and pressure. The pH of the solution was adjusted to 5.6 after 72 h. The precipitate was filtered and recrystallized in an alcohol-water mixture. Grey crystals were obtained (Figure 1).
Yield 50%, m.p. 172°C; IR (KBr): V=3186, 3387.1 cm-1 (VN-H), 3186 cm-1 (VO-H), 3088.8 cm-1 (VC-Harom), 2831.3 cm-1 (V C-H(aliph)), 1656.8 cm-1 (VC=N), 1013.5 cm-1, 985.6 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 11.4 (s, N-OH), 10.6 (s, N-OH), 7.9 (s, N-H), 7.8 (s, N-H), 7.2-6.7 (m, 9Harom), 7.4 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 145.5 (C1), 144.9 (C2), 134.7 (C3), 129.6 (C4), 122.2 (C5), 119.0 (C6), 118.6. (C7), 115.8 (C8), 142.9 (C9), 137.9 (C10) ppm.
Anal. calcd. (%): C, 62.22; N, 20.70; H, 5.19
Found, (%): C, 62.02; N, 20.94; H, 5.24
Thermal Decomposition of H2L1
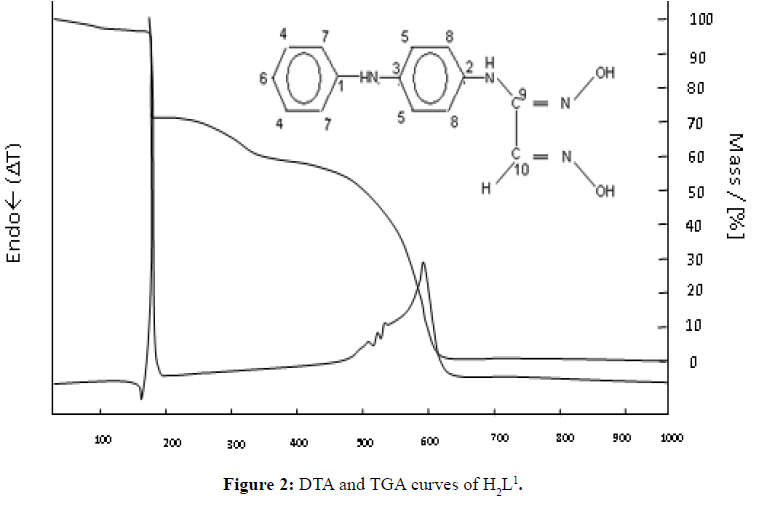
The thermal stability and decomposition of the ligands were investigated through thermal analysis (DTA) and thermo gravimetric analysis (TGA) under dry air flow at 25 to 1000ºC. The DTA and TGA curves obtained are shown in Figures 2-5.
For H2L1, in general, the mass loss occurred in three steps with the first being decomposition at 174.8ºC. All of the ligand was depleted at 900°C.
Anti-4-aminodiphenylaminophenylglyoxime (H2L2)
First, 4-aminodiphenylamine (7.5 mmol) was dissolved in THF, and then, triethylamine (7.5 mmol) was added dropwise. Anti-chlorophenylglyoxime (7.5 mmol) was added to the solution, which was then stirred for 72 h at typical room conditions. The evaporated, and the solid obtained was recrystallized in an alcohol-water mixture.
Yield 60%, m.p. 224.3°C; IR (KBr): V=3376.6 cm-1 (VN-H), 3000-3300 cm-1 (VO-H), 3022.1 cm-1 (VC-Harom), 2823.2 cm-1 (V C-H(aliph)), 1633.3 cm-1 (VC=N), 1037.5 cm-1, 1021.1 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 12.6 (s, N-OH), 12.3 (s, N-OH), 5.5 (s, N-H), 7.8 (s, N-H), 7.3-6.7 (m, 14Harom), 7.4 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 151.2 (C1), 150.0 (C2), 132.0 (C3), 131.9 (C4), 129.6 (C5), 129.4 (C6), 129.4 (C7), 128.5 (C8), 144.4 (C9), 136.0 (C10), 123.9 (C11), 119.5 (C12), 118.2 (C13), 116.2 (C14) ppm.
Anal. calcd. (%): C, 69.36; N, 16.18; H, 5.20
Found, (%): C, 70.01; N, 15.98; H, 5.14
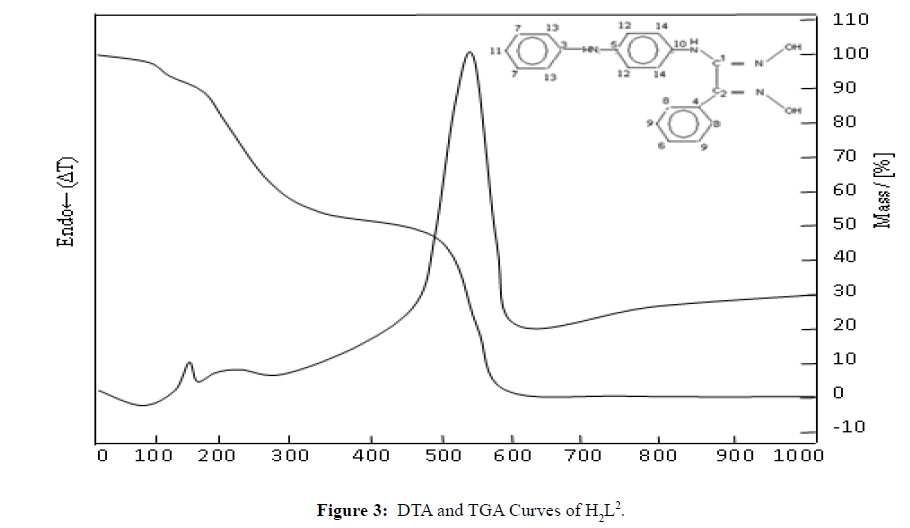
Thermal Decomposition of H2L2
The obtained DTA and TGA curves of the H2L2 ligand are shown in Figure 3. In general, the mass loss occurred over three steps: the first was decomposition at 149.3°C, the second was decomposition at 224.3°C, and the third was decomposition at 523.3°C. All of the ligand was depleted at 600°C.
Anti-3-hydroxy-5-sulpho-2-naphthylaminoglyoxime (H3L3)
First, 3-hydroxy-5-sulpho-2-naphthylamine (gamma acid, 1.0 mmol) was dissolved in 10 mL of an ethanol/water mixture (1:1), and then, NaHCO3 (1.0 mmol) was added dropwise. Anti-chloroglyoxime (1 mmol) was added to the solution, which was then stirred for 24 h at 0°C. The solvent of the solution was evaporated in an evaporator, and the remaining solid was dissolved in ethyl alcohol. The amine salt was precipitated by adding ether to the solution. The ligand was obtained by evaporating the solvent of the filtrate.
Yield 30%, m.p. 176.3°C; IR (KBr): V=3207.3 cm-1 (VN-H), 2962.1 cm-1 (VC-Harom), 2851.8 cm-1 (V C-H(aliph)), 1670.8 cm-1 (VC=N), 1044.5 cm-1, 972.1 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 12.4 (s, N-OH), 12.1 (s, N-OH), 9.2 (s, N-H), 7.7-7.0 (m, 5Harom), 7.8 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 164.3 (C1), 152.4 (C2), 145.8 (C3), 142.1 (C4), 130.4 (C5), 129.1 (C6), 125.2 (C7), 123.3 (C8), 115.4 (C9), 115.2 (C10), 106.8 (C11) ppm.
Anal. calcd. (%): C, 38.50; N, 11.23; H, 3.472; Na, 6.15
Found, (%): C, 38,88; N, 11,08; H, 3,53; Na, 6,03
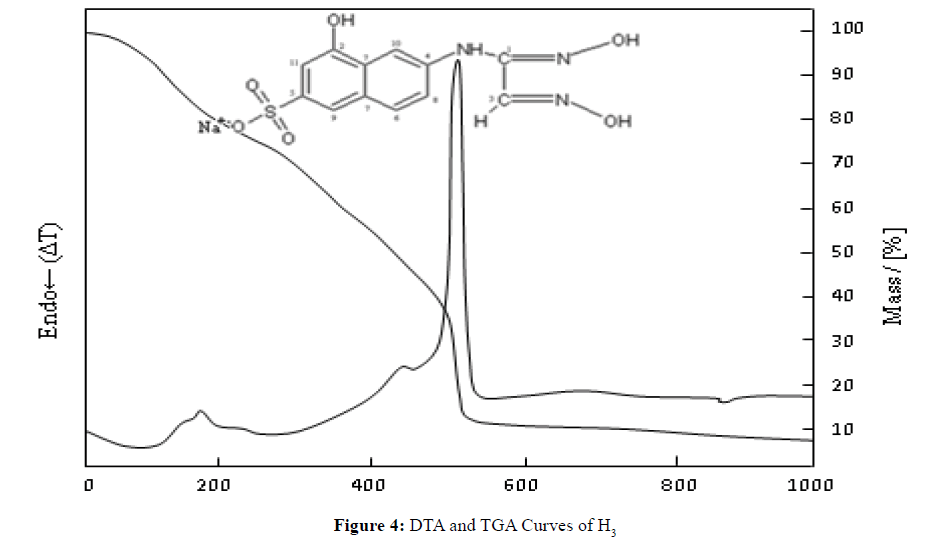
Thermal Decomposition of H3L3
The first mass loss was decomposition at 101.2ºC, the second loss was decomposition at 176.3ºC, and the third loss was decomposition at 510.7ºC. The mass loss up to 101.3ºC is caused by the loss of water in the structure, and this mass loss was determined to be 7.5% (theoretically 7.2%). These two values are consistent with each other and correspond to 1.5 moles of water. The mass left as a result of decomposition at 1000ºC is 7.1%. It is believed that Na2O will be left as a result of decomposition. Consequently, the theoretical amount left as a result of decomposition is 8.2%. This value is quite consistent with the empirical results (Na2O: m.p.: 1132ºC (dec)) [15].
Anti-3-hydroxo-5-sulpho-2-naphthylaminophenylglyoxime (H3L4)
First, 3-hydroxy-5-sulpho-2-naphthylamine (gamma acid, 1.0 mmol) was dissolved in 10 mL of a 1:1 ethanol/water mixture, and then, NaHCO3 (1.0 mmol) was added dropwise. Anti-chlorophenylglyoxime (1 mmol) was added to the solution, which was then stirred for 24 h at 0°C. The solvent of the solution was evaporated in an evaporator. The remaining solid was dissolved in ethyl alcohol, and the amine salt was precipitated by adding ether to the solution. The ligand was obtained by evaporating the solvent of the filtrate.
Yield 35%, m.p. 220.8°C; IR (KBr): V=3288.4 cm-1 (V N-H), 3052.0 cm-1 (V C-Harom), 2855.8 cm-1 (V C-H(aliph)), 1654.5 cm-1 (V C=N), 1068.1 cm-1, 1040.2 cm-1 (V N-O); 1H NMR (DMSO-d6, δ, 200 MHz): 12.2 (s, N-OH), 11.8 (s, N-OH), 9.4 (s, N-H), 7.4-7.1 (m, 5Harom), 7.6 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ): 153.0 (C1), 152.4 (C2), 147.8 (C3), 144.8 (C4), 136.7 (C5), 129.9 (C6), 129.6 (C7), 129.3 (C8), 129.0 (C9), 128.2
(C10), 125.2 (C11), 122.6 (C12), 115.2 (C13), 112.2 (C14), 110.0 (C15), 106.7 (C16) ppm.
Anal. calcd. (%): C, 51.06; N, 9.93; H, 3.30; Na, 6.20
Found, (%): C, 51.70; N, 10.05; H, 3.35; Na, 6.80
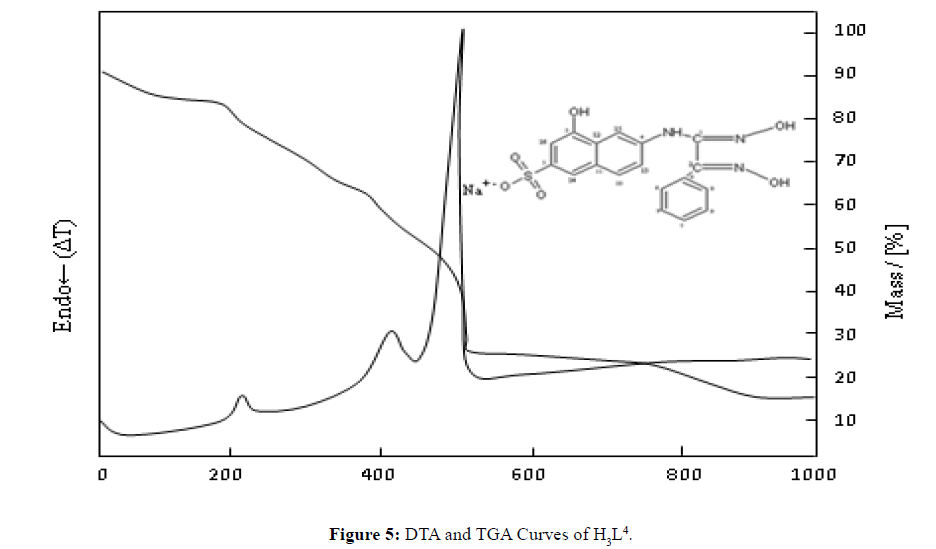
Thermal Decomposition of H3L4
The obtained DTA and TGA curves of the H3L4 ligand are shown in Figure 5. For the H3L4 ligand, in general, the mass loss occurred in four steps: the first was decomposition at 72.9°C, the second was decomposition at 220.8°C, the third was decomposition at 417.4°C and the fourth was decomposition at 508.4°C. The mass left as a result of decomposition at 1000°C was 12.6%. (Na2O: m.p.: 1132°C (dec)) [14].
Ni(II) complexes
To a solution of one of the ligands (5 mmol) in 10 mL of a 1:1 ethanol/water mixture, a solution of NiCl2∙6H2O (2.5 mmol) in 10 mL of 1:1 ethanol/water was added. After stirring for 24 h, the red complex was separated by filtration and recrystallized in an alcohol-water mixture.
Bis(anti-4-aminodiphenylaminoglyoxime)nickel(II) (NiL1):
Yield 30%, m.p. 260°C; IR (KBr): V=3379.7, 3353.4 cm-1 (VN-H), 3300.7 cm-1 (VO-H), 3047.2 cm-1 (VC-Harom), 1678.2, 1597.0 cm-1 (VC=N), 1163.3, 1106.0 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 14.5 (s, O-H----O), 9.4 (s, N-H), 8.2 (s, N-H), 7.0-6.7 (m, 18Harom), 7.2 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 148.4 (C1), 143.7 (C2), 130.2 (C3), 129.6 (C4), 125.5 (C5), 120.2 (C6), 117.5 (C7), 117.1 (C8), 141.5 (C9), 132.4 (C10) ppm.
Anal. calcd. (%): C, 54.63; N, 18.21; H, 4.55; Ni, 9.59
Found, (%): C, 54.97 ± 0.99; N, 17.72 ± 0.02; H, 4.28 ± 0.15; Ni, 9.22 ± 0.20
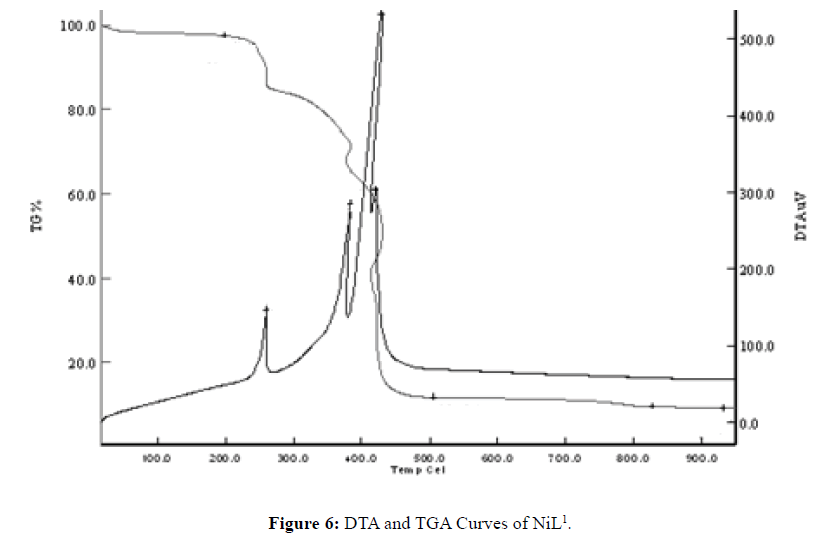
Thermal Decomposition of NiL1
The 2.5% mass loss between 0-199°C is caused by the loss of water in the structure. This loss corresponds to 1 mole of water. The mass left as a result of decomposition at 1000°C was 9.6%. It is believed that NiO is left as a result of decomposition. Consequently, the theoretical amount mass remaining after decomposition is 12.15%. This value is quite consistent with the empirical results (NiO: m.p.: 1955°C dec) (Figure 6) [14].
Bis(anti-4-aminodiphenylaminophenylglyoximato)nickel(II) (NiL2)
Yield 45%, m.p. 254.4°C; IR (KBr): V=3420.0 cm-1 (VN-H), 3338.7 cm-1 (VO-H), 3052.0, 3027.5 cm-1 (VC-Harom), 1668.3, 1593.2 cm-1 (VC=N), 1119.2, 1086.5 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 15.71 (s, O-H----O), 11.6 (s, N-H), 10.31 (s, N-H), 7.4-6.6 (m, 28Harom), 7.8 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 149.1 (C1), 148.0 (C2), 144.9 (C3), 137.9 (C4), 134.3 (C5), 132.0 (C6), 130.1 (C7), 129.6 (C8), 128.9 (C9), 128.1 (C10), 122.4 (C11), 119.4 (C12), 118.9 (C13), 115.7 ppm (C14)
Anal. calcd. (%): C, 64.09; N, 14.95; H, 4.53; Ni, 7.88
Found, (%): C, 64.47 ± 0.19; N, 14.95 ± 0.03; H, 4.33 ± 0.11; Ni, 7.69 ± 0.50
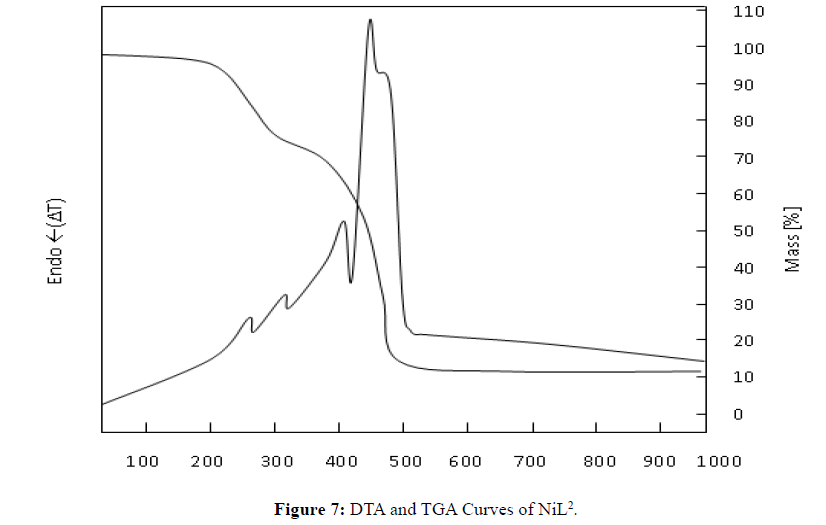
Thermal Decomposition of NiL2
In general, the mass loss occurred in two steps, and 89.8% of the complex was depleted at 1000°C. The remaining 10.2% is NiO. The theoretical amount left as a result of decomposition is 9.16% (Figure 7).
Bis(anti-3-hydroxy-5-sulpho-2-naphthylaminoglyoximato)nickel(II) (NiL3)
Yield 47%, m.p. 290.5°C; IR (KBr): V=3445.3, 3260.2 cm-1 (VN-H), 3084.7, (VC-Harom), 1599.2 cm-1 (VC=N), 1066.1 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 14.85 (s, O-H----O), 9.8 (s, N-H), 10.21 (s, O-H), 7.6-7.1 (m, 10Harom), 7.84 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 152.6 (C1), 147.5 (C2), 145.9 (C3), 135.7 (C4), 131.1 (C5), 129.9 (C6), 125.1 (C7), 123.6 (C8), 115.1 (C9), 114.2 (C10), 107.2 (C11) ppm.
Anal. calcd. (%): C, 36.09; N, 10.52; H, 2.98; Ni, 6.51
Found, (%): C, 35.94 ± 0.10; N, 10.85 ± 0.01; H, 2.72 ± 0.13; Ni, 6.68 ± 0.24
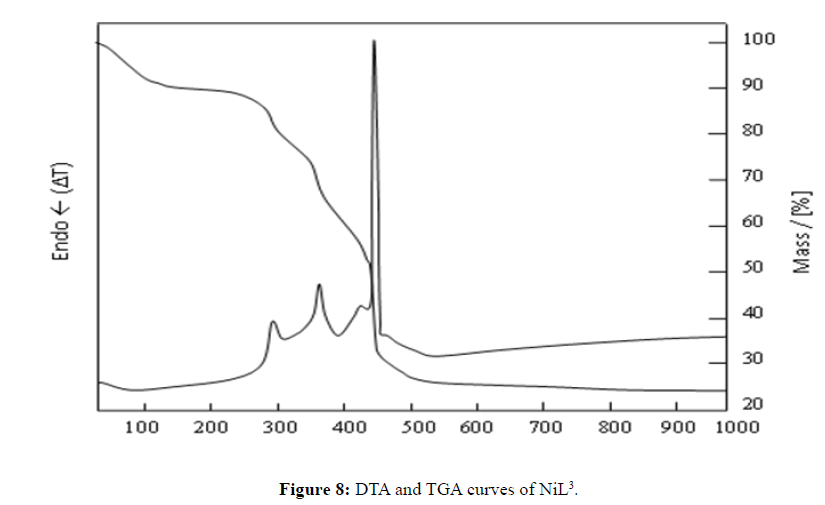
Thermal Decomposition of NiL3
According to the TGA and DTA graphs of the NiL4 complex, observed a water loss of 7.0% was observed at 78.0°C. Theoretically, the amount of water in the structure of the ligand is 6.7%. This value corresponds to 3 moles of water. In general, the mass loss occurred in three steps. The first was decomposition at 292.4°C, the second was decomposition at 362.7°C, and the third was decomposition at 445.8°C (Figure 8). The mass left at 1000°C as a result of decomposition is 22.2%. It is believed that NiO + Na2S is left as a result of decomposition. Consequently, the theoretical amount of mass left as a result of decomposition is 20.47%. This value is quite consistent with the empirical results (NiO: m.p.: 1955°C (en), Na2S: m.p.: 1172°C (en)) [14].
Yield 38%, m.p. 270.2°C; IR (KBr): V=3380.3 cm-1 (VN-H), 3051.7 cm-1 (VC-Harom), 1597.1 cm-1 (VC=N), 1127.0, 1093.4 cm-1 (VN-O); 1H NMR (DMSO-d6, δ, 400 MHz): 14.7 (s, O-H----O), 11.6 (s, N-H), 10.6 (s, O-H), 7.6-7.0 (m, 20Harom), 7.8 (s, N=C-H) ppm; 13C NMR (DMSO-d6, δ ): 152.9 (C1), 152.2 (C2), 147.7 (C3), 144.6 (C4), 136.6 (C5), 129.7 (C6), 129.6 (C7), 129.4 (C8), 128.8 (C9), 128.5 (C10), 125.4 (C11), 121.8 (C12), 114.9 (C13), 112.1 (C14), 110.7 (C15), 106.5 (C16) ppm.
Anal. calcd. (%): C, 47.89; N, 9.31; H, 5.76; Ni, 5.76; Na, 5.10
Found, (%): C, 48.04 ± 0.12; N, 9.17 ± 0.05; H, 2.53 ± 0.10; Ni, 5.17 ± 0.14; Na, 5.53 ± 0.14
Thermal Decomposition of NiL4
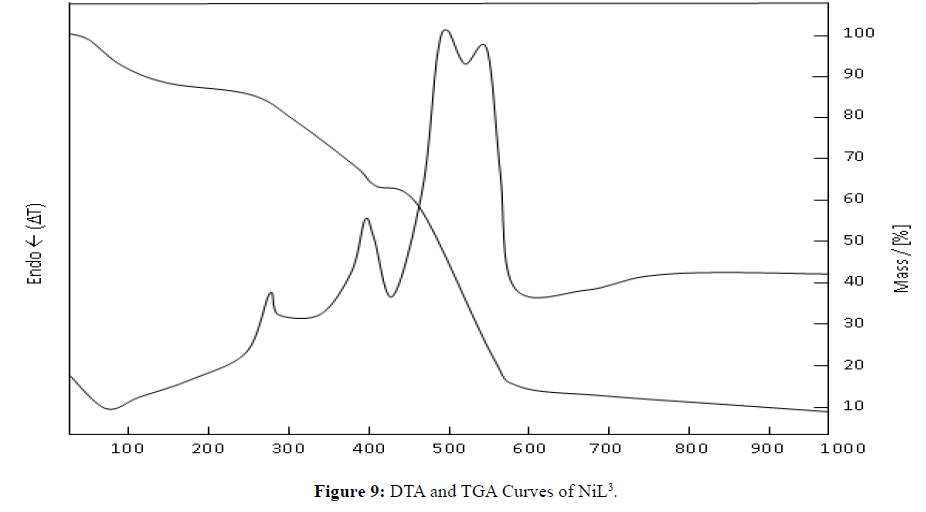
According to the TGA and DTA graphs of the NiL3 complex, it seems that the mass loss occurs by decomposition in the following three phases: at 274.2°C, 396.4°C and 497.4°C (Figure 9). The mass left as a result of decomposition at 1000°C is 8.9%. It is believed that NiO is left as a result of decomposition. Consequently, the theoretical amount left as a result of decomposition is 8.1%. This value is quite consistent with the empirical results (NiO: m.p.: 1955°C (dec), Na2O: m.p.: 1132°C) [14].
Results and Discussion
In the IR spectra of 1, 2, 3 and 4 ligands, a broad peak appears due to the presence of free O-H groups. In the spectrum of ligand 1, characteristic N-H peaks overlapped with the O-H peak. The bands due to the N-O and aromatic C-H vibrations were observed at 1013.5 cm-1, 985.6 cm-1 and 3088.8 cm-1. Additionally, the C=N stretching band characteristic of vic-dioximes was observed at 1656.8 cm-1. The N-H, N-O, aromatic C-H and C=N vibrations, which belong to 2 were observed at 3376.6 cm-1, 1037.5 cm-1, 1021.1 cm-1, 3022.1 cm-1 and 1633.3 cm-1. For 3, bands were observed at 3207.3 cm-1, 1044.5 cm-1, 972.1 cm-1, 2962.1 cm-1 and 1670.8 cm-1. Bands for 4 were observed at 3288.4 cm-1, 1068.1 cm-1, 1040.2 cm-1, 3052.0 cm-1, and 1654.5 cm-1.
In the 1H-NMR spectrum of the ligands, two different chemical shifts belonging to the N-OH protons of the oxime groups were observed for the anti-form, which shows that different substituents were attached to the oxime groups. The chemical shifts of the N-H protons were observed as singlets. The N-OH and N-H protons were also identified by D2O exchange. Chemical shift values of the C=N proton (a singlet at ~ 7 ppm) and the aromatic C-H protons (a multiplet at ~ 7.7 to 6.7 ppm) were observed. All 1H-NMR spectra support the proposed structures.
The 13C-NMR spectra of the ligands are consistent with the structures depicted in Figure 1.
The elemental analyses of 1, 2, 3 and 4 were found to agree with their formulas.
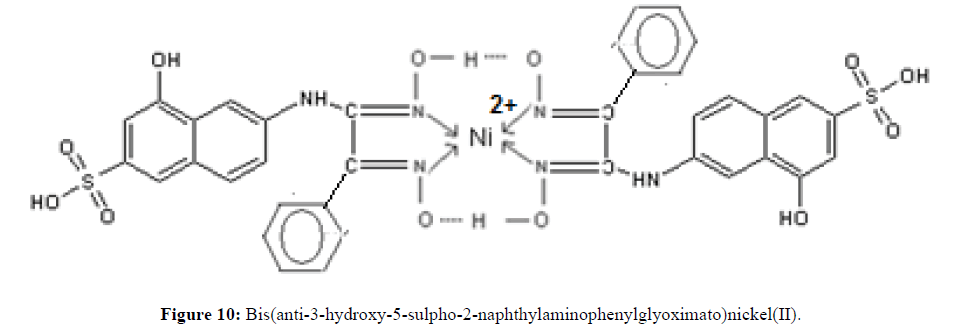
Mononuclear complexes were obtained with Ni(II). For each ligand, the Ni(II) ion is coordinated by the N and N’ atoms of each oxime group in the two ligand molecules as shown in Figure 10.
The IR spectra of all of the complexes were similar. The bands due to the N-O vibrations were observed between 1163 and 1066 cm-1. Aromatic C-H vibrations were observed at approximately 3000 cm-1. The C=N stretching band characteristic of vic-dioximes was observed between 1678.2 and 1593.2 cm-1.
In the 1H NMR spectra of the nickel (II) complexes, the bridging hydrogen atoms were observed as single D2O exchangeable peaks at approximately 14 ppm. The signals from the N-H protons of NiL1, NiL2, NiL3, and NiL4 appeared at 9.4 and 8.2; 11.6 and 10.3; 9.8; and 11.6 ppm, respectively, and disappeared upon the addition of D2O. The signals from the O-H protons of NiL3 and NiL4 appeared at 10.21 and 10.6 ppm, respectively. No signals attributable to O-H protons were observed for the NiL1 and NiL2 complexes.
Conclusion
As a result of the characterization studies, the structures of the obtained ligands were determined,
For H2L1, C14H13N4O2; For H2L2, C20H18N4O2;
For H3L3, NaC12H10N3O6S•1.5H2O; For H3L4, NaC18H13N3O6S
The structures of the obtained coordination compounds were determined.
For NiL1; Ni(C14H13N4O2)2.H2O; For NiL2: Ni (C20H17N4O2)2;
For NiL3: (Na2[Ni(C12H9N3O6S)2].3H2O); For NiL4: (Na2[Ni(C18H13N3O6S)2].3H2O).
References
- Chugaev LA (1909) Russ Physicochem soc, 41, 184.
- Smith PA (1967) The Chemistry of Open-Chain Organic Nitrogen Compounds. WA Benjamin: New York 2.
- Gok Y, Kantekin H, Degirmencioglu I (1993) The synthesis and characterization of novel dioximes and their heteronuclear complexes containing crown ether moieties. Polyhedron 12: 2097-2104.
- Brown BG (1973) Prog Inorg Chem 18, 17.
- Chakravorty (1974) A Structural chemistry of transition metal complexes of oximes. Corrd Chem Rev 13: 1.
- Schrauzer GN, Windgassen RJ (1999) Cobalamin model comppounds. Preparation and reactions of substituted alkyl-and alkenylcobaloximes and biochemical implications. J Am Chem Soc 89: 1999.
- Gök Y, Demirbaş A (1989) The synthesis and complex formation of dibenzo [e,k]-2,3-Bis(Hydroxyimino)-1,4-Diaza-7,10-Dithia-2,3,8,9-Tetrahydrocyclododecine. Synth React Inorg Met Org Chem 19: 681-698.
- Gül A, Bekaroğlu Ö (1983) Syntheses of NN′-bis(4′-benzo[15-crown-5])diaminoglyoxime and its complexes with copper(II), nickel(II), cobalt(II), cobalt(III), palladium(II), platinum(II), and uranyl(VI). Dalton Trans 2537-2541.
- Durmuş M, Ahsen V, Luneau D, Pécaut J (2004) Synthesis and structures of morpholine substituted new vic-dioxime ligand and its Ni(II) complexes. Inorg Chim Acta 357: 588-594.
- Duman S, Sekerci M (2010) The synthesis and characterization of some metal complexes derived from 1,3-dioxalane groups and vic-dioxime ligands. IJNES 4: 23-28.
- Sarıkavakli N, İrez G, Yıldız S (1997) Synthesis and complex formation of the structural isomers of N-(Phenyl)aminoglyoximes and N-(Benzyl)aminoglyoximes. Chimica Acta Turcica 25: 105-112.
- Ponzio G, Baldrocco F (1930) Ricerche Sulle Diossime. Gazz. Chim. Ital 60: 415-419.
- Brintzinger H, Titzmann R (1952) Notiz eingine halogenierte aliphatische oxime. Chem Ber 85: 344.
- Burakevich JV, Lore AM, Volpp GVV (1971) Phenylglyoxime separation, characterization, and structure of three ısomers. J Org Chem 36: 5-8.
- Lide DR (2003-2004) Handbook of chemistry and physics. 84th edn., CRC Press: NewYork.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences