ISSN : 2347-5447
British Biomedical Bulletin
Sub-Chronic Oral Toxicity Study of Kushta Qalai (A Unique Herbo-mineral Unani formulation) in Wistar Rats
Showkat A Dar1,2, Seema Akbar1*, Khalid Ghazanfar1, Mariya Hamdani1, Tazeen Nazir1, Akbar Masood2, Masood S Mir3, Khalid M Siddiqui4 and Showkat A Ganie2*
1Regional Research Institute of Unani Medicine, Srinagar, J&K, India
2Department of Biochemistry, University of Kashmir, Srinagar, J&K, India
3Sher-e-Kashmir University of Agriculture Science & Technology-Kashmir, Shuhama Alustang, J&K, India
4Central Council for Research in Unani Medicine, New Delhi, India
- *Corresponding Author:
- Seema Akbar
Regional Research Institute of Unani Medicine
Srinagar, J&K
India
E-mail: seemaakbar@hotmail.com
Showkat A Ganie
Department of Biochemistry
University of Kashmir
Srinagar, J&K
India
E-mail: showkat_ganie786@yahoo.com
Received date: ;June 14, 2016 Accepted date: July 18, 2016; Published date: July 30, 2016
Citation: Dar SA, Akbar S, Ghazanfar K, Hamdani M, Nazir T, et al. (2016) Sub-Chronic Oral Toxicity Study of Kushta Qalai (A Unique Herbo-mineral Unani formulation) in Wistar Rats. Br Biomed Bull 4: 293.
Copyright: © 2016 Dar SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Kushta Qalai, a Herbo-mineral effective formulation of Unani medicine used for the treatment of male sex disorders has as yet not been evaluated for pre clinical toxicity study. The objective of the study was to investigate the Sub-Chronic oral toxicity study of Kushta Qalai Drug as well as Crude material (the un-detoxified form) in young, healthy Albino rats (Wistar Strain) of both the sexes in order to know their adverse effects, if any, and also to check the scientific validity of detoxification method. Methods: The study was conducted as per OECD-408 Guideline, during which the test substance was administered orally to the rats at the limit dose of 1000mg/kg, and its sub fractions 500 and 250mg/kg body wt., daily for 90 days. Overnight fasted rats were sacrificed on the 91st day and blood sample for Haematological and Biochemical parameters were collected. At the end, the tissues were collected and subjected to histopathological examination by a veterinary pathologist. Results: The results showed that, there were no statistically significant changes in the general behaviour, body weight gain, feed & water consumption, haematological parameters and biochemical parameters between test substance treated rats and control groups. Also there were no gross histopathological changes in vital organs of rats except the test substance treated rats showed degeneration of liver and kidney tissues, which were confirmed by the histopathological examination. Conclusion: The drug Kushta Qalai showed impairment at 1000mg/kg body weight while as the Crude material showed at all the three dose levels, indicating that the method of detoxification used in preparing the Kushta reduces the elements of toxicity.
Keywords
Sub-chronic toxicity; Qalai; Biochemical parameters; Hematological parameters; Histopathology
Introduction
The word Kushta owes its origin to a Persian word “Kushtan” which means to kill or conquered [1]. Kushta is the finest powder form of the Unani medicinal preparations obtained by the calcinations of metal, mineral and animal drugs. These drugs, by special process are calcined in closed crucibles and in pits of different sizes, having varying number of cow dung cakes and with different intensity of heat. According to the traditional healers these calcination techniques used in Kushta preparation are specialized processes wherein herbal juices are incorporated during preparation of ash [2]. It is claimed by the Traditional healers that these techniques increases the efficacy of drug and also either completely remove the elements of toxicity at all or downgrade them to the level where the drug can be safely used [3-5]. Kushta (Calcined product) is easily absorbed in the human body which accounts for its high efficacy [6]. The preparation of Kushta results in higher efficacy of the medicine and after its entry into the body, it discharges its curative role promptly and effectively. The composition of Kushta Qalai as mentioned in the National Formulary of Unani Medicine, 2008 is shown in Table 1.
| Ingredients | English/Scientific name | Quantity |
|---|---|---|
| Qalai | Stannum (TIN) | 250 gm |
| Phitkari | Aloe/Aloe vera | 125 gm |
| Aab-e-Gheekwar | Alum/potassium alum | 50 ml |
Table 1: Constituents of crude KushtaQalai.
Special method of preparation
Before making Kushta Qalai, Qalai and Phitkari are cleaned and purified. After this the drugs are ground in a pestle and mortar (Kharal) with Aab-e-Gheekwar for a specified period of time. Thereafter, small cakes of varying sizes and thickness are made. These cakes are well dried in the shade and are put in earthen discs and the process of Gil-e-Hikamat is followed and the whole apparatus is dried. After this a pit is dug in an open space. Half the pit is filled with cowdung cakes. The apparatus (sealed earthen discs) is now placed in the pit and the remaining space is filled with more cowdung cakes which are then ignited. After the calcinations is over, the pit is allowed to cool completely, the apparatus is removed and the contents, thus obtained, are again powdered with Aab-e-Gheekwar as many times till the proper fineness and the quality of Kushta Qalai is obtained [6]. In Unani system of medicine Kushta Qalai is used for the treatment of diseases like spermatorrhoea, excessive nocturnal emission, premature ejaculation, impotency, increases sex vigour and density of semen [7-9]. As per Unani medicine classical books the drug Kushta Qalai is very much efficacious, used for the treatment of male sexual disorders [10-12]. The ingredients of Kushta Qalai are Qalai, Aloevera and Phatkari. The Qalai (Tin) is one of the most popular metal known for its use since Vedic period [13]. Qalai is silver like metal, which is soft than the gold but harder than the lead, malleable and sparingly ductile with little elasticity [14]. It is used internally in the form of Kushta. Qalai is known by diverse vernacular names like rasas in Arabic, urziz in Persian, rang in Hindi, vanga in Sanskrit and Qalai in Guajarati. There are two varieties of QalaiKhuraka and mishraka only the Khuraka being acceptable for therapeutic applications [15]. The Qalai tin was grinded with heavy duty wiley mill to make the fine powder which was used. Aloevera (Aloe bardadensis) is an important component of Vrihani Gutika which is considered as the drug of highest potency useful in the treatment of sexual dysfunction [16]. Phatkari (Alum) is an important mineral origin drug of Unani medicine [17]. Since there is limited scientific data available about the difference between the calcinated material and crude material to determine the effectiveness of detoxification method adopted while preparation of Kushta’s. In this study, an effort has been made to scientifically validate these methods and also try to intensify the adverse effects of the crude materials used in preparation of Kushta’s.
Material and Methods
Test item (Drug and crude material)
The drugs and crude ingredients of Kushta Qalai were procured from the registered Unani drug dealer M/s. Ellahi Dawakhana, Unani Ayurvedic Medicines, Habak, Naseem Bagh. The manufacturer of the drug of Kushta Qalai is Hamdard Laboratory India (P Certified) Mg. Lic No U-212/78. All the crude material was identified by Dr. M.A. Wajid (Unani Pharmacologist), Ex. Deputy Director, RRIUM, Srinagar. The drug and crude material powder was mixed with RO water to make the suspension for oral administration.
Experimental animals and maintenance
Young adult Albino Wistar rats of either sex were procured from IIIM, Jammu. These rats were monitored closely during the quarantine and acclimatization period [18]. A detailed veterinary examination was conducted on the rats prior to and at the end of the acclimatization and quarantine period of 14 days. The five rats of same sex were housed in a polypropylene cage under standard environmental conditions i.e., temperature of 20-250°C with 12 hour light/dark cycle and had a free access to pelleted feed procured form Pronow Agro Industries, New Delhi and RO water ad libitum. The use of animals in the study was approved by the Institutional Animal Ethics Committee (IAEC) of Regional Research Institute of Unani Medicine, Srinagar which is registered with the Committee for the Purpose of Control and Supervision of experiments on Animals (CPCSEA, India) with registration No. 927/GO/C/06/C PCSEA.
Experimental design
The doses were selected according to Organization of Economic Co-operation and Development guideline (OECD guideline- 408) [19]. One highest dose of 1000 mg/kg body weight was selected with the aim of inducing some toxic effects but not death or severe suffering and also two sub fractions i.e., 500 mg and 250 mg were selected with the aim to check any dose related response at the lowest dose level. The rats were randomly divided into 14 groups as shown in Table 2 (7 male and 7 female groups) each group consisted of 5 rats. All the animals were closely observed for the first 1 to 4 hours after dosing to examine any adverse toxic signs, behavioral changes etc. The body weight of the rats was evaluated weekly. Feed and water consumption/rat/24 hours were recorded before dosing and then weekly up to 13 weeks. On the 91st day, after overnight fasting, all the animals were sacrificed by withdrawing blood in a syringe from the dorsal vena cava after opening the abdomen under ISOFLURANE anesthesia. 2 ml of blood was collected in EDTA vacutainer for hematological studies and 3 ml in red top vacutainer for serum biochemistry. All the animals were dissected to check macroscopic examination of the body organs. The vital organs such as liver, lung, kidney, adrenal gland, pancreas, spleen, brain, ovary/testes and heart were collected to determine the relative organ weight followed by grossing for the collection of tissues to evaluate Histopathological studies.
| Group No. | Male/Female | Description | Dose for 90 days (Daily in the morning) |
|---|---|---|---|
| Group I | Male | Control | RO water (Vehicle only) |
| Group II | Drug Treated | 1000 mg/kg body wt. | |
| Group III | Drug Treated | 500 mg/kg body wt. | |
| Group IV | Drug Treated | 250 mg/kg body wt. | |
| Group V | Crude Treated | 1000 mg/kg body wt. | |
| Group VI | Crude Treated | 500 mg/kg body wt. | |
| Group VII | Crude Treated | 250 mg/kg body wt. | |
| Group VIII | Female | Control | RO water (Vehicle only) |
| Group IX | Drug Treated | 1000 mg/kg body wt. | |
| Group X | Drug Treated | 500 mg/kg body wt. | |
| Group XI | Drug Treated | 250 mg/kg body wt. | |
| Group XII | Crude Treated | 1000 mg/kg body wt. | |
| Group XIII | Crude Treated | 500 mg/kg body wt. | |
| Group XIV | Crude Treated | 250 mg/kg body wt. |
Table 2: Description of rat groups.
Dosage of Kushta Qalai in Humans: 125 mg a day orally with water [6]. This amounts to about 2 mg/kg/day in human subjects. Table 3 shows the corresponding dose level in rats. Curry et al., 2011 has previously calculated interspecies dose conversion from rats weighing 100 gm body weight to humans with 60 kg body weight. The equivalent surface area dosage conversion factor from rat to humans was 1/720.
| Particulars of Study | Sub-Chronic toxicity study |
|---|---|
| Dose level | 1000, 500 and 250mg/kg body weight (Corresponding to 70X, 35X and 17.5X the extrapolated dose in rats) |
| Dosing Schedule | Repeated daily dosing by oral route |
| Period of Observation | 90 Days |
| Observation Parameters | See Text |
Table 3: Corresponding dose level in rats.
Biochemistry parameters
Biochemical parameters were studied in serum obtained after centrifugation of blood at 2000 RPM for 15 minutes on the same day of the rat sacrifice. Biochemical parameters were determined on fully automatic biochemistry analyzer (XL- 640 TRANSASIA) using ERBA kits.
Liver function tests- aspartate aminotransferase AST, alanine aminotransferase ALT, alkaline phosphatase ALP, Total bilirubin, total protein, and albumin, and kidney function tests- blood urea, serum uric acid and serum Creatinine were done. In addition to this other Metabolic function tests such as blood glucose, serum Cholesterol and serum Triglyceride were estimated.
Haematological parameters
Haematological parameters were analyzed in freshly collected blood in blue top vacutainer containing EDTA anticoagulant. The blood was gently mixed with the EDTA anticoagulant coated on the tube walls. Haematological parameters were determined on fully automatic haematological analyzer (Sysmex XT- 2000 iV Sysmex Corporation Japan) having animal version software.
Haematological parameters such as Haemoglobin Conc. WBC count, RBC count, haematocrit, Mean Corpuscular Volume, Mean Corpuscular Haemoglobin Concentration, Mean Corpuscular Haemoglobin, Platelate Count, differential leukocyte count – Neutrophil%, Lymphocyte%, Monocyte%, Eosinophil% and basophil%, and Reticulocyte count were studied.
Histopathology
Tissue samples were collected from the organs of control as well as treated male/female rats. The tissue collected from the organs such as liver, lung, kidney, adrenal gland, pancreas, spleen, brain, ovary/testes and heart ware numbered for identification and then transferred to tissue cassettes (SS) to enable fixation in 10% formalin for 3 to 5 days followed by the tissue processing which was carried on Automatic tissue processor Model No. 1020 (LIECA make Germany).
The tissue processing included dehydration in graded isopropyl alcohol, clearing in xylene I & II, impregnation in paraffin wax and finally tissue blocks was prepared on paraffin block maker Model No. 1150 H+C (LIECA make Germany).
Section cutting of tissue blocks was done using microtome (YARCO) to the thickness of 3–5 microns. The tissue sections were fixed on the slide by heat technique following by the staining, Haematoxylin and Eosin stain were used.
The staining was done with Automatic slide stainer (THERMO MAKE Germany). After staining the tissue section were covered with cover slip using DPX mountant to prevent any damaging of tissue section which was done on Auto cover slipper Model No. CV 5030 (LIECA make Germany).
The stained tissue sections were examined under microscope 10x and 40x objective to check the adverse effects of drug on cell morphology as well as on the cell organelles.
Statistical analysis
All results are expressed as mean ± standard deviation. Comparison of all results on body weight, feed & water consumption, haematological value, biochemical values, were performed using one way analysis of variance (ANOVA) method using statistical software Graph Pad Prism version 6.07. Probability of 0.05 or small (p = 0.05) was used as the criterion of significance.
Results
Average mean body weight
The rats treated with Kushta Qalai drug and crude material at the dose of 1000, 500 and 250 mg/kg of body weight were found to grow and gain body weight normally and there was no any adverse effect found in the body weight gains. Figure 1a-1d shows the body weight of rats in sub-chronic oral toxicity study.
Mean feed and water consumption
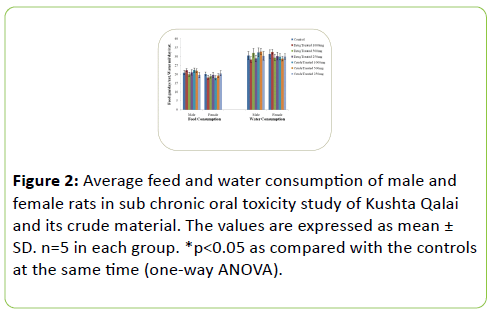
Relative the average feed and water consumption of both treated groups were found to be unaffected by the Kushta Qalai drug and crude material as there were no statistically significant changes in the average feed and water consumption when compared with the respective controls as shown in Figure 2.
Relative organ weight of male and female rats in sub-chronic oral toxicity study (Gram/100 gram of body weight)
The relative organ weight of male and female rats in subchronic oral toxicity study were found unaltered by the Kushta Qalai drug and crude material when comparing treated rats with the respective controls as shown in Table 4a and 4b.
| Organ | Male | ||||||
|---|---|---|---|---|---|---|---|
| Control | Drug Treated 1000mg | Drug Treated 500mg | Drug Treated 250mg | Crude Treated 1000mg | Crude Treated 500mg | Crude Treated 250mg | |
| Brain (gm) | 0.602 ±0.12 | 0.584 ±0.11 | 0.633 ±0.15 | 0.608 ±0.10 | 0.623 ±0.12 | 0.613 ±0.13 | 0.600 ±0.14 |
| Spleen (gm) | 0.295 ±0.12 | 0.297 ±0.14 | 0.318 ±0.10 | 0.282 ±0.12 | 0.277 ±0.08 | 0.277 ±0.09 | 0.312 ±0.11 |
| Right adrenal (gm) | 0.012 ±0.008 | 0. 011 ±0.006 | 0.013 ± 0.007 | 0.012 ±0.009 | 0.014 ±0.008 | 0.010 ±0.008 | 0.012 ±0.009 |
| Left adrenal (gm) | 0.011 ±0.007 | 0.012 ±0.008 | 0.013 ±0.007 | 0.012 ±0.006 | 0.013 ±0.09 | 0.010 ±0.008 | 0.011 ±0.007 |
| Heart (gm) | 0.282 ±0.09 | 0.303 ±0.07 | 0.262 ±0.08 | 0.338 ±0.06 | 0.312 ±0.08 | 0.312 ±0.09 | 0.299 ±0.07 |
| Lung (gm) | 0.539 ±0.11 | 0.554 ±0.14 | 0.535 ±0.15 | 0.494 ±0.16 | 0.503 ±0.13 | 0.499 ±0.12 | 0.600 ±0.13 |
| Right kidney (gm) | 0.293 ±0.11 | 0.319 ±0.13 | 0.289 ±0.14 | 0.318 ±0.12 | 0.321 ±0.16 | 0.321 ±0.11 | 0.314 ±0.10 |
| Left kidney (gm) | 0.268±0.11 | 0.307 ±0.12 | 0.278 ±0.13 | 0.303 ±0.10 | 0.312 ±0.12 | 0.312 ±0.13 | 0.302 ±0.14 |
| Right testis (gm) | 0.426±0.15 | 0.481 ±0.12 | 0.397 ±0.12 | 0.395 ±0.11 | 0.397 ±0.11 | 0.394 ±0.10 | 0.433 ±0.13 |
| Left testis (gm) | 0.411±0.13 | 0.454 ±0.12 | 0.376 ±0.10 | 0.385 ±0.15 | 0.383 ±0.14 | 0.393 ±0.10 | 0.400 ±0.12 |
| Liver (gm) | 2.804 ±0.55 | 3.309 ± 0.54 | 2.761 ±0.43 | 2.809 ±0.59 | 2.824 ±0.61 | 2.744 ±0.38 | 2.833 ±0.40 |
| Pancreas (gm) | 0.188±0.05 | 0.171 ±0.07 | 0.151 ±0.07 | 0.177 ±0.04 | 0.192 ±0.05 | 0.192 ±0.05 | 0.180 ±0.08 |
Table 4a: Relative organ weight of male rats in sub-chronic oral toxicity study of KushtaQalai and its crude material.
| Organ | Female | ||||||
|---|---|---|---|---|---|---|---|
| Control | Drug Treated1000mg | Drug Treated500mg | Drug Treated 250mg | Crude Treated 1000mg | Crude Treated 500mg | Crude Treated 250mg | |
| Brain (gm) | 0.600 ±0.11 | 0.624 ±0.10 | 0.610 ±0.14 | 0.575 ±0.13 | 0.659 ±0.12 | 0.661 ±0.10 | 0.580 ±0.14 |
| Spleen (gm) | 0.333 ±0.10 | 0.300 ±0.11 | 0.321 ±0.09 | 0.321 ±0.14 | 0.301 ±0.11 | 0.341 ±0.10 | 0.320 ±0.12 |
| Right adrenal (gm) | 0.014 ±0.009 | 0.016 ±0.008 | 0.012 ± 0.007 | 0.011 ±0.007 | 0.013 ±0.009 | 0.014 ±0.006 | 0.012 ±0.008 |
| Left adrenal (gm) | 0.012 ±0.008 | 0.014 ±0.009 | 0.010 ±0.007 | 0.009 ±0.008 | 0.011 ±0.009 | 0.012 ±0.008 | 0.010 ±0.008 |
| Heart (gm) | 0.288± 0.10 | 0.311 ± 0.11 | 0.300 ±0.12 | 0.300 ±0.12 | 0.265 ±0.08 | 0.310 ±0.10 | 0.280 ±0.09 |
| Lung (gm) | 0.620 ±0.13 | 0.640 ± 0.16 | 0.624 ± 0.15 | 0.592 ±0.11 | 0.620 ±0.15 | 0.568 ±0.16 | 0.588 ±0.13 |
| Right kidney (gm) | 0.298 ± 0.12 | 0.279 ± 0.13 | 0.302 ± 0.12 | 0.325 ±0.13 | 0.325 ±0.10 | 0.286 ±0.11 | 0.305 ±0.10 |
| Left kidney (gm) | 0.274 ±0.11 | 0.270 ±0.12 | 0.289 ± 0.13 | 0.311 ± 0.13 | 0.309 ±0.10 | 0.267 ±0.11 | 0.289 ± 0.13 |
| Right Ovary (gm) | 0.025 ± 0.009 | 0.024 ± 0.008 | 0.025 ±0.009 | 0.022 ±0.007 | 0.023 ±0.008 | 0.018 ±0.008 | 0.021 ±0.009 |
| Left Ovary (gm) | 0.021 ±0.008 | 0.020 ±0.007 | 0.020 ± 0.007 | 0.018 ±0.006 | 0.021 ±0.008 | 0.017 ±0.006 | 0.019 ±0.007 |
| Liver (gm) | 3.019 ± 0.55 | 2.894 ± 0.39 | 3.347 ± 0.57 | 2.727 ±0.44 | 3.458 ±0.58 | 2.812 ±0.47 | 2.976 ±0.42 |
| Pancreas (gm) | 0.173 ± 0.07 | 0.169 ±0.06 | 0.192 ± 0.04 | 0.209 ±0.05 | 0.209 ±0.06 | 0.201 ±0.07 | 0.200 ±0.09 |
Table 4b: Relative organ weight of female rats in sub-chronic oral toxicity study of KushtaQalai and its crude material.
Biochemistry parameters
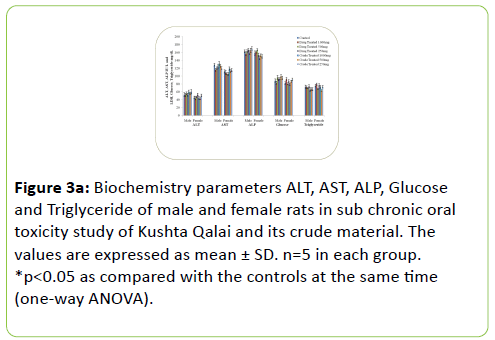
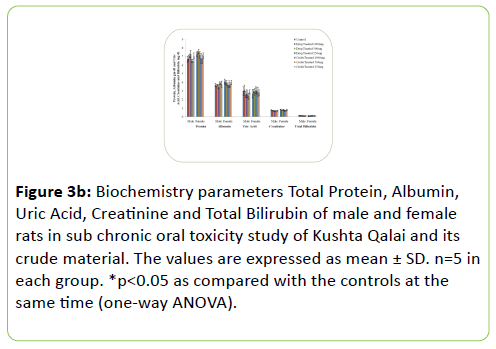
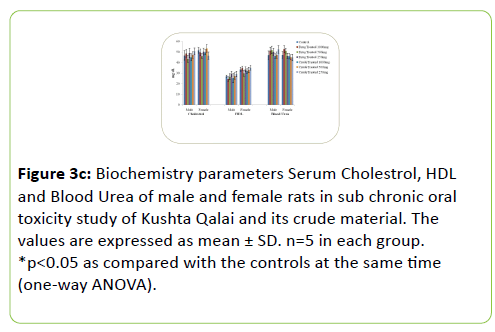
The results of Biochemical parameters of both the treated rat groups did not show any statistically significant change when compared with the controls groups as shown in Figure 3a-3c.
Figure 3a: Biochemistry parameters ALT, AST, ALP, Glucose and Triglyceride of male and female rats in sub chronic oral toxicity study of Kushta Qalai and its crude material. The values are expressed as mean ± SD. n=5 in each group. *p<0.05 as compared with the controls at the same time (one-way ANOVA).
Figure 3b: Biochemistry parameters Total Protein, Albumin, Uric Acid, Creatinine and Total Bilirubin of male and female rats in sub chronic oral toxicity study of Kushta Qalai and its crude material. The values are expressed as mean ± SD. n=5 in each group. *p<0.05 as compared with the controls at the same time (one-way ANOVA).
Figure 3c: Biochemistry parameters Serum Cholestrol, HDL and Blood Urea of male and female rats in sub chronic oral toxicity study of Kushta Qalai and its crude material. The values are expressed as mean ± SD. n=5 in each group. *p<0.05 as compared with the controls at the same time (one-way ANOVA).
Haematology parameters
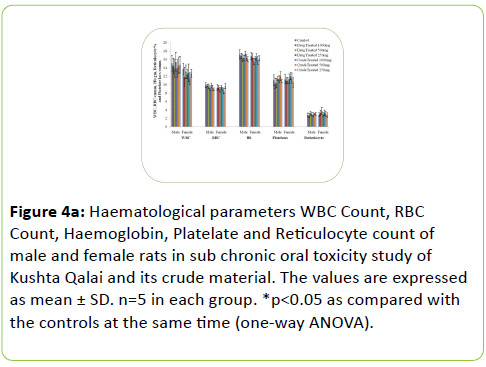
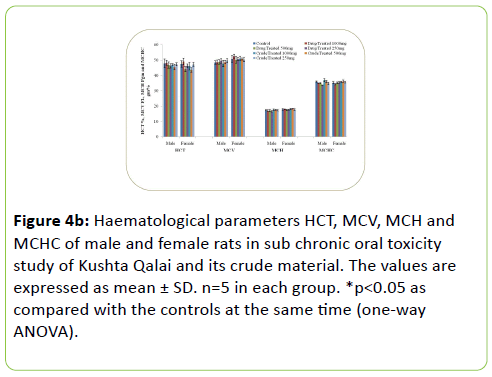
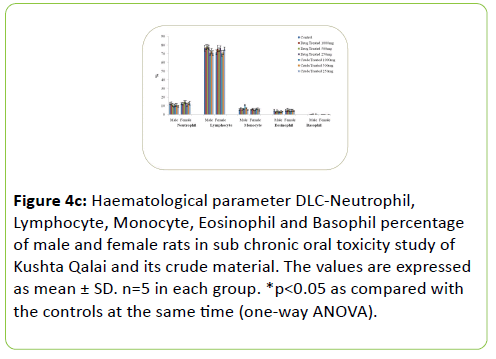
The results of Haematological parameters of the drug and crude material treated males and females rats did not show statistically significant change in the values when compared with the respective controls. Figure 4a-4c shows the haematological parameters of rats.
Figure 4a: Haematological parameters WBC Count, RBC Count, Haemoglobin, Platelate and Reticulocyte count of male and female rats in sub chronic oral toxicity study of Kushta Qalai and its crude material. The values are expressed as mean ± SD. n=5 in each group. *p<0.05 as compared with the controls at the same time (one-way ANOVA).
Figure 4c: Haematological parameter DLC-Neutrophil, Lymphocyte, Monocyte, Eosinophil and Basophil percentage of male and female rats in sub chronic oral toxicity study of Kushta Qalai and its crude material. The values are expressed as mean ± SD. n=5 in each group. *p<0.05 as compared with the controls at the same time (one-way ANOVA).
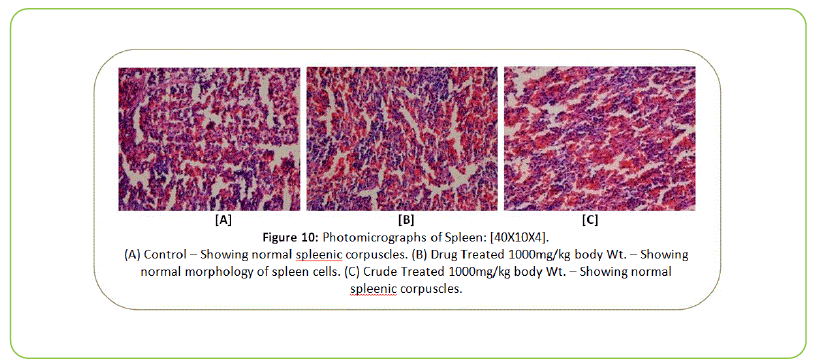
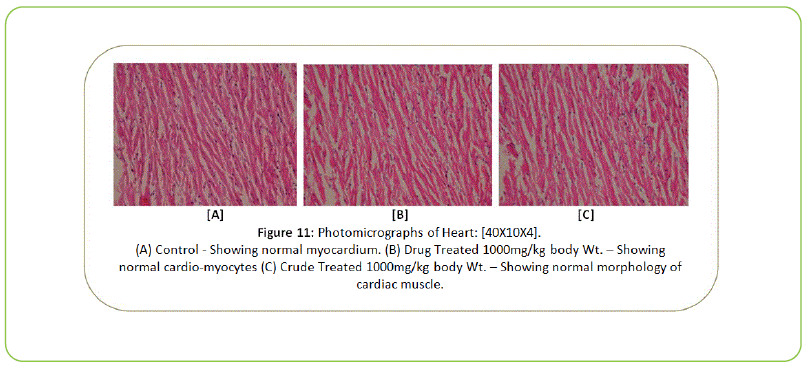
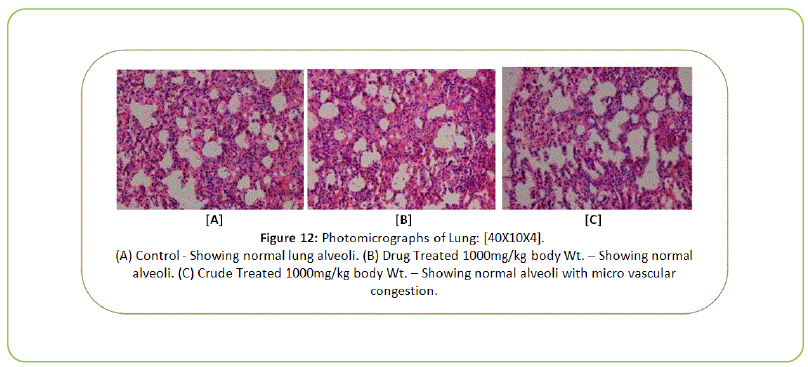
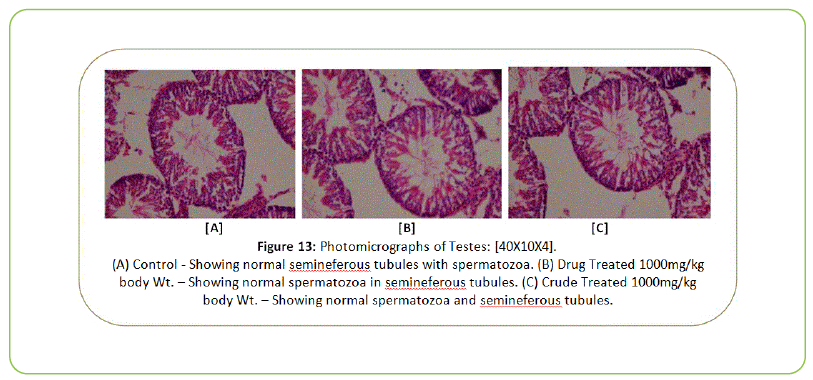
Histopathology
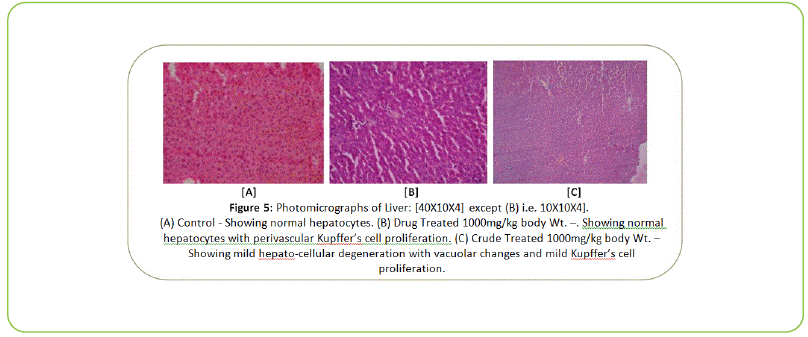
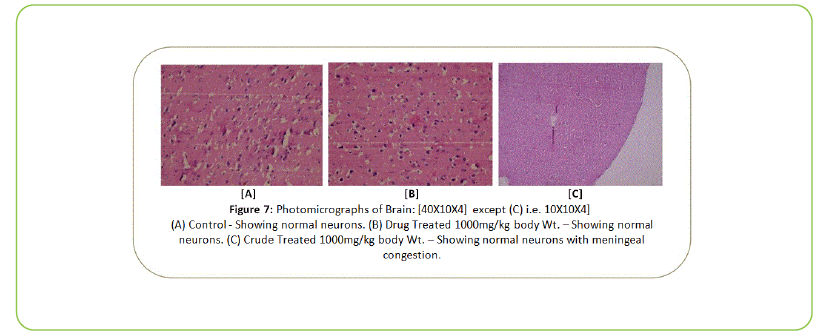
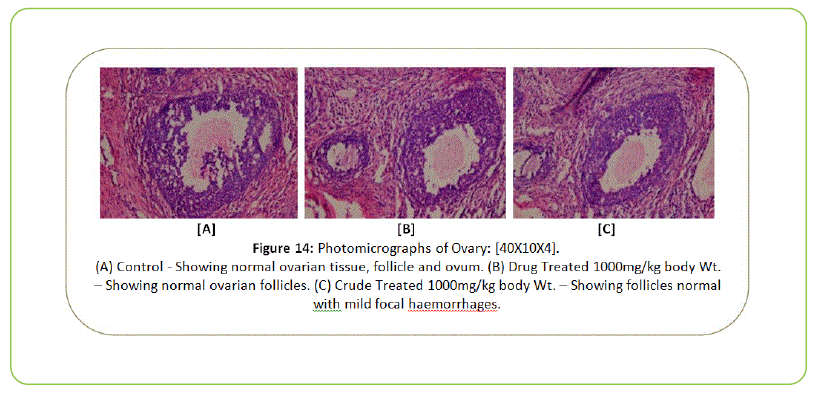
The Histopathological examination of vital organs such as Liver, Kidney, Lung, Pancreas, Spleen, Adrenal Gland, Testes/ Ovaries, Heart and Brain shows normal architecture and did not reveal any pathology after the oral administration of Kushta Qalai and its crude material for 90 days as shown in Figure 5-14, except there were mild degeneration in liver and kidney tissues of crude material treated rats as shown in Figure 5 and 6. Effect of drug on the histology of tissues/organs in male and female rats in sub-chronic oral toxicity study of Kushta Qalai [20].
Figure 5: Photomicrographs of Liver: [40X10X4] except (B) i.e. 10X10X4]. (A) Control - Showing normal hepatocytes. (B) Drug Treated 1000mg/kg body Wt. –. Showing normal hepatocytes with perivascular Kupffer’s cell proliferation. (C) Crude Treated 1000mg/kg body Wt. – Showing mild hepato-cellular degeneration with vacuolar changes and mild Kupffer’s cell proliferation.
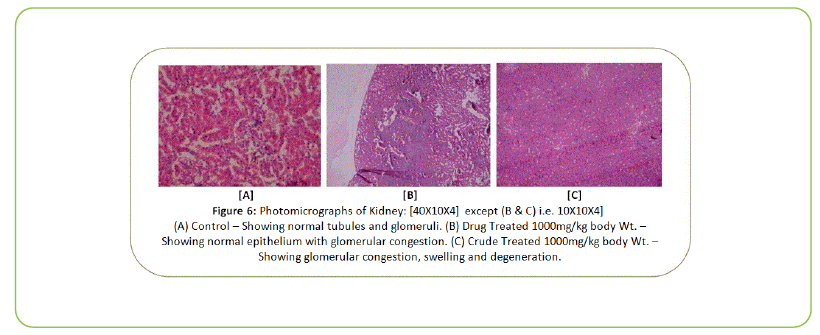
Figure 6: Photomicrographs of Kidney: [40X10X4] except (B & C) i.e. 10X10X4] (A) Control – Showing normal tubules and glomeruli. (B) Drug Treated 1000mg/kg body Wt. – Showing normal epithelium with glomerular congestion. (C) Crude Treated 1000mg/kg body Wt. – Showing glomerular congestion, swelling and degeneration.
Discussion
World Health Organization survey indicates that about 70-80% of the world’s population rely on non-conventional medicine mainly of herbal source in their primary healthcare [21]. The growing number of herbal drug users around the globe and lack of scientific data on the safety profile of herbal products make it necessary to conduct toxicity study of herbal products [22]. Therefore, the safety of these products has become an importance issue [23]. An increase in the number of cases due to poisoning by herbal drugs makes their pharmacovigilance and toxicity studies all the more imperative to ensure their safe use and to protect public health [24]. In view of the above facts the principle goal of sub-chronic oral toxicity study of Kushta Qalai and its crude material, are to identify and characterise the specific organ/parameter that may be affected, after repeated oral administration for 90 days and also to check the validity of detoxification method adopted while preparation of Kushta.
In this study, the body/organ weight of Treated rats did not show any significant changes after oral administration of Kushta Qalai drug and crude material at the dose level of 1000, 500 and 250 mg/kg body weight for 90 days when compared with the Control rats. The change in body/organ weight is simple and sensitive indicator of toxicological studies [25-27]. The Haematological parameters reveal anomalies in body metabolic process. The haematological parameters furnish important information of the body response to injury, deprivation and stress. The haematological parameters remained unaltered between test substances treated rats and Control rats. The biochemical parameters were performed to check the liver, kidney, lipid and glycemic profile of the Kushta Qalai drug and crude material treated rats. In this study, the LFT (AST, ALT, ALP) of Treated rats did not show any significant change when compared with the respective Control rats. The KFT (Blood Urea, Serum Creatinine and Uric Acid) of Treated rats did not show any significant change when compared with the respective Control rats and also the other metabolic parameters of the Treated rats were also at par with the Control rats. The Histopathological examination of the vital organ did not reveal any morphological changes after oral administration of Kushta Qalai drug and crude material for 90 days at the dose level of 1000, 500 and 250 mg/kg body weight, except in liver and kidney mild degenerative changes were seen in case of Kushta Qalai drug Treated rats at the dose level of 1000 mg/kg body weight and at all the three dose levels of crude material. The glomeruli congestion and swelling was also seen in the kidney tissue due to overburden/ overload of excretion/reabsorption as shown in Figure 6. It may be noted that the Kushta Qalai drug is normally orally administered to the human subjects at the dose level of 125 mg/kg body weight per day for a period of 30 days. The aim of these animal toxicity studies was to unmask the damaging potential of the drug by very high and several doses were used to establish dose relatedness of the adverse effects.
Conclusion
The result therefore suggests that the Kushta Qalai drug was potentially safe for oral consumption at the therapeutic dose level however it was found to cause impairment in the liver and kidney tissues at the dose level of 1000 mg/kg body weight (70X, X is the extrapolated therapeutic dose in rats) while as the crude material was found to be potentially cause degenerative changes at all the three dose levels due to the increase hepatic overburden in detoxification/removal process of crude material. This study indicates that the method of detoxification adopted by the Unani System of Medicine while preparation of Kushta is scientifically affective in removing/decreasing the elements of toxicity.
Acknowledgements
The authors thank the Director General, Central Council for Research in Unani Medicine, New Delhi for his interest in the study, guidance, administrative sport. The authors also thank the Department of Science and Technology, Govt. Of India, New Delhi for funding and establishing a high quality research facility at RRIUM, Srinagar. The authors also thank to Dr. Sudhir Srivastava, Consultant, DST Project, for his technical guidance. The authors are also grateful to Mr. Ashaq Ahmad, Mr. Bashir Ahmad and Mr. Shafiq Ahmad for their support in carrying out the experimental work and maintenance of animal house.
Conflict of interest statement
We declare that we have no conflict of interest.
References
- Said M (1997) Hamdardpharmacopoiea of eastern medicine New Delhi. Sri Satguru Publications: 223-300.
- Aziz N, Gilani AH, Rindh MA (2002) Kushta(s) unique herbo-mineral preparations used in south Asian traditional medicine. Medical Hypothesis 59: 468-474.
- Chopra RN, Chopra IC, Handa KL, Kapur LD (1982) Chopra’s indigenious drugs of india. 2nd ed. Calcutta Academic Publisher: 461-464.
- Wahid MA, Kabeeruddin (1992) Kitab-ul-taqleesilm-e-kushta-e-jat (urdu) new delhi. DarulKutubulMasseh: 157-158.
- Bajaj S, Vohora SB (2000) Anti-Cataleptic Anti-anxiety and anti-depressant activity of gold preparations used in indian system of medicine. Indian Journal of Pharmacology 32: 339-346.
- National Formulary of Unani Medicine (2008) Part V: 55, 57.
- Said M (1970) hamdard pharmacopoeia of eastern medicine Second impression Karachi. Hamdard Academy: 232-234.
- Sadananda S. MotilalBanarasidas (1998) Rasa Tarangini. New Delhi, India 18: 39-42.
- El-Eswed, Bassam I (2002) Lead and Tin in Arabic Alchemy (Arabic Sciences and Philosophy). Cambridge University Press, US: 139-153.
- QarabadeenJadeed (2005) New Delhi. CCRUM: 181.
- Hkm Abdul Hafeez (2005) SanatulTahlees New Delhi. CCRUM: 135-137.
- HkmMohdKabeer-u-din BayaazKabeer, Hyderabad. Hikmat Book Depot 1: 117.
- Jani JH, Patgiri BJ, Ravi SB, Prajapati PK (2009) The role of media in the preparation of VangaBhasma. Jour of Res in Ayurveda 30: 211-216.
- NadkarniKM (1976) Indian Materia Medica:116.
- Tariq M, Rahman K, Chaudhary SS, Imtiyaz S (2014) Formulation and characterization of a traditional Unani formulation: KushtaQalai. International journal of pharmcaceutical sciences and research 5: 171-177.
- Charak Samhita (1998) Shloka 24-32. II. Varanasi: ChaukhambhaOrientalia, chap II, Qtr 1, section 6, Chikitsasthanam: 37.
- Mariyam R, Wajeeha B (2015)A review of medicinal aspect of alum in Unani medicine and scientific studies. World Journal of Pharmaceutical Research 4: 929-940.
- Capdevila S, Giral M, Ruiz JL, de la Torre, Russell RJ, et al. (2007) Acclimatization of rats after ground transportation to a new animal facility. Laboratory Animals 41: 255.
- OECD (1998) OECD Guidelines for testing of chemicals. Test no. 408:Repeated dose 90 days oral toxicity study in Rodents.
- Curry SH, Decory HH, Gabrielsson J (2011) Phase I: the first opportunity for extrapolation from animal data to human exposure. In: Edwards LD, Fox AW, Stonier PD, eds. Principles and practise of pharmaceutical medicine. 3rd ed. West Succex: Wiley Blackwell: 85-98.
- Hariharan S, Suba V, Murugesan M (2012) Toxicological assessment of Siddha herbomineral preparation KASHA GulanthagaMathirai in animal models. International journal of Pharma and Bio Sciences 4: 646-656.
- Saad B, Azaizeh H, Abu-Hijleh G, Said O (2006) Safety of traditional Arab herbal medicine.Evid based complement Alternat Med 3: 433-439.
- WHO guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems. Geneva, Switzerland: World Health Organisation; 2004.
- Zhou J, Ouedraogo M, Qu F, Duez P (2013) Potential genotoxicity of traditional Chinese medicinal plants and phytochemicals: an overview. Phytoter Res.
- Winder AC, Lembke LA, Stephens MD (1969). Arthritis Rheumatism 12: 472-482.
- KlassenCD (2007) Casarett and Doull’s Toxicology: the basic science of poisons. (7th edn.) Mc Graw Hill.
- Yavasoglu A, Karaaslan MA, Uyanikgil Y, Sayim F, Ates U, et al. (2008) Toxic effects of anatoxin-a on testes and sperm counts of male mice. Experimental and toxicological pathology 60: 391-396.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences