Solid State Fermentation of Non-Edible Oil Seed Cakes for Production of Proteases and Cellulases and Degradation of AntiNutritional Factors
1Department of Chemistry, St. Stephen’s College, University of Delhi, Delhi, India
2Center for Rural Development and Technology, Indian Institute of Technology Delhi, New Delhi, India
3Metagenomics & Secretomics Research laboratory, Department of Botany, Dr. Harisingh Gour University (A Central University), Sagar, India
- *Corresponding Author:
- Dr. Ashwani Kumar
Assistant Professor, Department of Botany
Metagenomics & Secretomics Research Laboratory
Dr. Harisingh Gour University (A Central University)
Sagar, Madhya Pradesh 470003, India
Tel: 917697432012
E-mail: ashwaniiitd@hotmail.com
Received Date: January 22, 2018; Accepted Date: February 09, 2018; Published Date: February 15, 2018
Citation: Gupta A, Sharma A, Pathak R, Kumar A, Sharma S (2018) Solid State Fermentation of Non-Edible Oil Seed Cakes for Production of Proteases and Cellulases and Degradation of Anti-Nutritional Factors. J food Biotechnol Res. Vol.2 No.1:4
Abstract
In the present scenario of depleting energy resources and search for eco-friendly and economically viable alternatives, bio-diesel has gained worldwide attention. Non-edible oils over edible oil hold the potential to act as sustainable sources for the production of bio-diesel. Removal of oil left behind a major part of the seed material as seed cake. The latter is generally regarded as waste because of its anti-nutritional content. In the present study, two de-oiled seed cakes, Madhuca indica (mahua) and Jatropha curcus (jatropha) were used for the growth of Paecilomyces variotii, to produce proteases and cellulases, respectively. These enzymes are already known to have considerable industrial importance. At an initial moisture content of 50% and temperature 30°C, maximum protease activity of 52.5 U/g and cellulase activity 27.3 U/g was recorded from A. niger and P. variotii respectively. HPLC studies showed 67.9-71.5% degradation of saponins, the major anti-nutritional factors present in mahua cake. This study proved beneficial for management of oil seed cakes via solid state fermentation, producing enzymes as value added products.
Keywords
Cellulases; Jatropha cake; Mahua cake; Proteases; Saponins
Introduction
The world population is increasing at a great pace [1]. Conservation of fossil fuels and utilization of renewable and nontraditional sources of energy have become the prime needs of the hour [2-5]. In order to establish a sustainable future, biofuel production from non-edible oils has gained worldwide consensus [6,7]. Extraction of oil from seeds generates large quantities of seed cake. The presence of several anti-nutritional and toxic components in these de-oiled cakes limits their use as quality fertilizers or animal feeds, rendering most of it as a waste [8,9]. Management and disposal of these cakes therefore becomes a major problem, posing serious environmental threats [10]. However, these cakes are a rich source of nutrients, viz., protein, sugars, etc. [11-15]. Nutrient composition of these cakes makes them noteworthy to be explored for their potential to produce various enzymes, antibiotics, vitamins and antioxidants, etc. possessing vide industrial as well as biotechnological applications [16]. The present paper attempts to address the management of two de-oiled seed cakes, Madhuca indica (mahua) and Jatropha curcus (Jatropha), via solid state fermentation (SSF), producing proteases and cellulases as value added products.
Mahua and jatropha are tropical plants, belonging to the family Sapotaceae and Euphorbiaceae, respectively. They are extensively cultivated in Asian and Australian continents for their oil bearing seeds. Mahua and jatropha oils have been extensively used for biodiesel production [2]. After the expulsion of oil from its seed, ~60% of the material is left as de-oiled seed cake [8]. These seed cakes are rich in nutrients but contain several antinutritional factors, viz., saponins and tannins in mahua cake (MC) and curcin, phorbol esters, saponins, phytate, curcalonic acids, flavanoids, vitexine and isovitexine in jatropa cake (JC) [8,17,18].
Aspergillus niger and Paecilomyces variotii have been found to be few of the important protease and cellulase producers, respectively [19,20]. Fungi used in the present study were selected on the basis of their ability to grow on MC and JC, respectively. Proteases are industrially important enzymes that possess wide applications in industries, biotechnology, medicinal and research fields [21]. Lignocellulolytic enzymes such as cellulases and xylanases play a critical role in depolymerization of structural carbohydrate polymer into monomeric sugars. Cellulases play significant role in winery, laundry, brewery, textiles and paper industries [22,23]. Increased demand of proteases and cellulases in agro-industrial applications has put a pressure on the production system of these enzymes [24]. Recently, oil seed cakes of coconut, palm, sesame, olive, linseed, soybean, mustard, cotton, etc. have been evaluated for their potential to support enzyme production [25]. However, to the best of our knowledge, very few studies have been carried out with MC and JC [26,27]. It is thus imperative to utilize these low cost organic wastes to make bioconversion processes more economical and feasible.
Materials and Methods
Procurement of cake and their characterization
MC and JC were procured from village Pratapgarh, U.P., India. The moisture and ash contents in the cakes were estimated by heating them in an oven (100°C) and muffle furnace (550°C), respectively, for a period of about 24 h, followed by subsequent cooling, till constant weights were achieved [28]. CHNS analyzer (CHNOS Elementar, Vario EL III model) was used to calculate the nitrogen content, which when multiplied by 6.25, gave the crude protein content of the cakes [29]. Fat content was estimated by soxhlet extraction using hexane as the solvent AOAC and total soluble sugars by the anthrone method [28,30]. The amount of available potassium and available phosphorous was estimated using flame photometer (128, Systronics) and spectrophotometer (UV-Vis, Perkin Elmer), respectively. Cellulose, hemicelluloses and lignin were estimated using standard protocols [31].
Active components, saponins, in MC, were extracted using methanol followed by partitioning into the butanol phase [32]. Repeated methanol extractions were followed to completely remove saponins from MC, giving saponin free detoxified mahua cake (MC) [33]. Saponins were analyzed using HPLC (Agilent 1260 Infinity) equipped with a Lichrocart RP-18 column (Merck, 250 × 4.6 mm, 5 μm) and a photodiode array detector (λmax= 214 nm). Solvent system used was methanol : deionized water (6.5:3.5; v/v) at a flow rate of 0.5 mL/min.
Phorbolesters from JC were extracted using methanol-water (9:1) mixture in a soxhlet apparatus. The combined methanolwater extracts was concentrated in a rotary evaporator and further extracted repeatedly using diethyl ether. The combined ether layers were washed with water and evaporated to give the crude yield [18].
Procurement of cultures and growth assays
The pure cultures of A. niger and P. variotii were collected from ITCC, IARI, New Delhi, India. These cultures were maintained on PDA slants at 28°C and regularly sub cultured. The fungal spores were harvested by washing these slants with 0.1% tween 80.
To test the growth of these fungi, experiments were performed with PDA and seed cakes, as the control and test growth medium, respectively. Autoclaved PDA and mahua/jatropha cake (containing agar) were poured in sterilized petridishes (9 cm diameter) under aseptic conditions in a laminar flow and allowed to solidify. A 5 mm disc of each culture (5 day old) was placed using a sterilized cork borer at centre of the solidified medium (PDA/cake) in a petridish. The dishes were incubated at 30°C and radial diameters were measured after 5-7 days, till full growth in control was achieved.
Enzyme production under solid state fermentation (SSF)
SSF of MC and JC to study enzyme production by above mentioned fungi was done as follows. 5 g seed cake (50% moistened with distilled water) was taken in 150 mL Erlenmeyer flask and autoclaved at 121°C and 15 psi for 20 min. After cooling, flasks were inoculated with 0.5 mL spore suspension (containing approximately 107 spores/mL) of the respective fungi and incubated at 30°C under static condition for specific time intervals.
For protease extraction, water containing 0.1% tween 80 was added (1:5; w/v) to the fermented substrate and homogenized for 1 hour (Orbitek, Scigenics Biotech, India; 30°C, 200 rpm). The suspension so obtained was centrifuged (10,000 rpm) at 4°C for 15 min [31]. Protease activity in the supernatant was determined with the help of casein substrate. 1 mL of supernatant was mixed with 5 mL of casein solution (0.6% casein in 50 mM NaOHGlycine buffer, pH 10), and the mixture was incubated at 50°C for 20 minutes. 5 mL of trichloroacetic acid (TCA) mixture (0.11 M TCA + 0.22 M CH3COONa + 0.33 M CH3COOH) was subsequently added to stop the reaction. After half an hour, the reaction mixture was filtered using Whatman paper No. 1. Spectroscopic measurement at 280 nm gave the absorbance of the filtrate. One unit of protease activity was defined as the amount of enzyme required to produce one microgram of tyrosine per minute under the conditions described above. Protease activity using detoxified mahua cake (MC) was also determined in the same manner as above. The aqueous extracts (supernatants) obtained above from each flask were analyzed for the presence of saponins as mentioned above.
Cellulases were extracted by adding 25 mL citrate buffer (0.05 M, pH 4.8) to the fermented substrate, followed by constant shaking at 200 rpm for half an hour. Subsequently, the suspension was centrifuged (10,000 rpm) at 4°C for 15 min. The clear supernatant was retained for cellulase (Fpase) activity [34]. One unit of cellulase activity was defined as the amount of enzyme required to liberate 1 μmol of reducing sugar (glucose/xylose) per ml of crude filtrate per minute under standard assay conditions described above.
Statistical analysis
The experiments were performed in triplicates and the data has been reported in terms of average value and standard deviation (SD). One way analysis of variance (ANOVA) was performed using SPSS for windows (version 18.0). p values ≤ 0.05 were considered statistically significant as per Duncan’s multiple range test (DMRT).
Results and Discussion
Characterization of seed cakes
The composition of two cakes has been summarized in Table 1. The seed cakes were found to be rich in nutrients, especially nitrogen and protein but contained significant amounts of antinutritional factors (toxins), viz., 16.70% saponins and 1.2 mg/g phorbolesters in MC and JC, respectively.
Table 1: Composition of mahua seed cake (MC) and jatropha seed cake (JC).
| Constituents (%) | Mahua cake (MC)(Mean ± SD) | Jatropha cake (JC)(Mean ± SD) |
|---|---|---|
| Moisture | 7.81 ± 0.23 | 7.5 ± 0.23 |
| Total solublesugars | 50.05 ± 1.23 | 12.25 ± 0.4 |
| Proteins | 19.68 ± 1.27 | 24.6 ± 1.29 |
| Oil | 5.31 ± 0.75 | 7.31 ± 0.75 |
| Ash | 5.82 ± 0.03 | 6.42 ± 0.03 |
| Celluloses | 18.60 ± 1.57 | 18.53 ± 0.37 |
| Hemicelluloses | 12.30 ± 1.78 | 22.56 ± 0.42 |
| Lignin | 4.30 ± 0.62 | 10.30 ± 0.62 |
| Nitrogen | 3.15 ± 0.02 | 3.93± 0.4 |
| Phosphorous | 0.65 ± 0.08 | 1.43 ± 0.12 |
| Potassium | 1.24 ± 0.05 | 1.26 ± 0.2 |
| Toxins | 16.70 ± 4.28 (Saponins) | 1.2± 0.3*(Phorbolesters) |
| *mg/g |
Growth of fungi on oil seed cakes
The growth of A. niger and P. variotii was monitored on MC and JC, respectively. Table 2 lists the radial growth diameters and spore counts of A. niger and P. variotii, when grown on MC and JC as growth mediums, respectively. It was seen that the cakes positively supported the growth of respective fungi. These cakes are rich in sugars, proteins and fats, and therefore it was considered worthwhile to explore them as a substrate for solid state fermentation (SSF) for the production of proteases and cellulases.
Table 2: Growth of Aspergillusnigerand Paecilomycesvariotiion mahua cake and jatropha cake, respectively.
| Fungus | Radial diameter (Mean ± SD; cm) | Spore count (×106/mL) |
|---|---|---|
| Aspergillusniger | 8.8 ± 0.05a | 68.5 ± 4.0b |
| Paecilomycesvariotii | 8.9 ± 0.04a | 15.7 ± 7.0a |
Note: Means sharing a different superscript in each column are significantly different from each other (p≤ 0.05) using DMRT
Production of enzymes using SSF
In SSF, microbial growth and product yield is critically affected by initial moisture content of the medium. If the moisture levels are low, the solubility of nutrients in the substrate is decreased. On the other hand, if moisture content is high, it leads to reduction in porosity, enhanced aerial mycelium formation, along with loss of particle structure [21]. Therefore, rationale for using 50% moisture was to use the optimum moisture concentration to start with the experiment [19]. The inoculum size is also a crucial factor that affects the biomass growth and yield. At lower levels of the inoculum, the biomass production is decreased, whereas larger sized inoculums suffer from scarcity of nutrients required for their growth. Hence, 1 mL of the inoculum containing approximately 107 spores of each fungi was used for our study [21]. Temperature was chosen to be 30°C. Using the above mentioned experimental conditions, maximum protease activity of 52.5 U/g and cellulase activity 27.37 U/g were recorded from A. niger and P. variotii, on second and fourth day of SSF, on MC and JC, respectively. Thereafter, the enzyme activity decreased in both the cases (Table 3).This might be due to depletion of nutrients available to the fungi or inactivation of the enzyme by other constituents. Many authors have reported protease activity from different species of Aspergillus grown on a variety of solid substrates. The value of maximum protease activity obtained in our study, viz., 52.5 U/g from A. niger, using MC as the substrate, is greater than the values reported by Sandhya et al. who achieved highest protease activity of 31.2 U/g and 3 U/g from A. oryzae during SSF of wheat bran and rice bran substrates, respectively and Sumantha et al. who reported total protease activity of 11.78 U/g from A. oryzae grown on a mixed substrate of wheat bran and coconut oil cake (3:1), at 48 h of fermentation period at 30°C [21,35]. However, Paranthaman et al. reported A. niger protease activity between 44.7-67.7 U/g using different varieties of rice brokens [36]. Rajmalwar & Dabholkar observed that Aspergillus sp. gave a maximum protease activity of 107.66 U/mL on soybean seed cake followed by 76.04 U/mL on sesame seed cake, after 72 hr of incubation period [37]. Cellulase (Fpase) activities by different microorganisms on a variety of substrates have also been reported by various authors. The value of cellulase activity obtained in our study is greater than that reported by Sukumaran et al. who achieved cellulase (Fpase) activity of 22.8 U/gds from Trichoderma ressei RUT C30 on wheat bran at a moisture content of 57% and 30°C temperature [38]. Latifan et al. who recorded cellulase activity of 1.1635U/g and 2.134 U/g from T. reesei QM9414 and T. reesei MCG77, respectively, on wheat bran at a moisture content of 70%, pH 5, and 30°C temperature and by Mekala et al. who reported activity of 25.6 Fpase U/gds from Trichoderma reesei RUT C30 using sugar cane baggase as a substrate at 33°C after 67 hr [39,40].
Table 3: Enzyme production by Aspergillusnigerand Paecilomycesvariotiion mahua cake and jatropha cake, respectively.
| Fungus | Oil seed cake | Enzyme | Enzyme activity (Mean ± SD;U/g) | ||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |||
| A. niger | Mahua cake | Proteases | 49.7 ± 0.5a | 52.5 ± 0.8a | 48.6 ± 1.2a | 48.5 ± 0.7a | 45.4 ± 0.5a |
| P. variotii | Jatropha cake | Cellulases | Not detected | 4.8 ± 0.2b | 11.8 ± 0.2b | 27.3 ± 0.5b | 7.7 ± 0.6b |
Note: Means sharing a different superscript in each column are significantly different from each other (p≤ 0.05) using DMRT
It was seen that MC supported higher production of proteases as compared to cellulase production from JC. This might be due to differential nature of nutrient supply from the cake(s). Since these de-oiled seed cakes contained some anti-nutritional factors, it was interesting to study the effect of detoxified cakes on the enzyme production.
Saponin free detoxified mahua seed cake (MC) was tested as a growth medium for A. niger. Table 4 compares the protease activity of A. niger when grown on raw and detoxified mahua cake, under the same experimental conditions. It was observed that detoxified cake significantly (p ≤ 0.05) decreased the activity of the fungus. Despite differences in nutrient composition of raw and detoxified mahua cake, a major difference was present in their saponin content. It was therefore necessary to monitor the saponin content in MC during its fermentation.
Table 4: Protease production by Aspergillusnigeron raw and detoxified mahua cake.
| Mahua cake | Protease activity (Mean ± SD; U/g) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Raw cake | 49.7 ± 0.5a | 52.5 ± 0.8a | 48.6 ± 1.2a | 48.5 ± 0.7a | 45.4 ± 0.5a |
| Detoxified cake | 20.2 ± 0.5b | 22.6 ± 0.6b | 24.5 ± 0.8b | 19.2 ± 1.1b | 18.9 ± 0.4b |
Note: Means sharing a different superscript in each column are significantly different from each other (p≤ 0.05) using DMRT
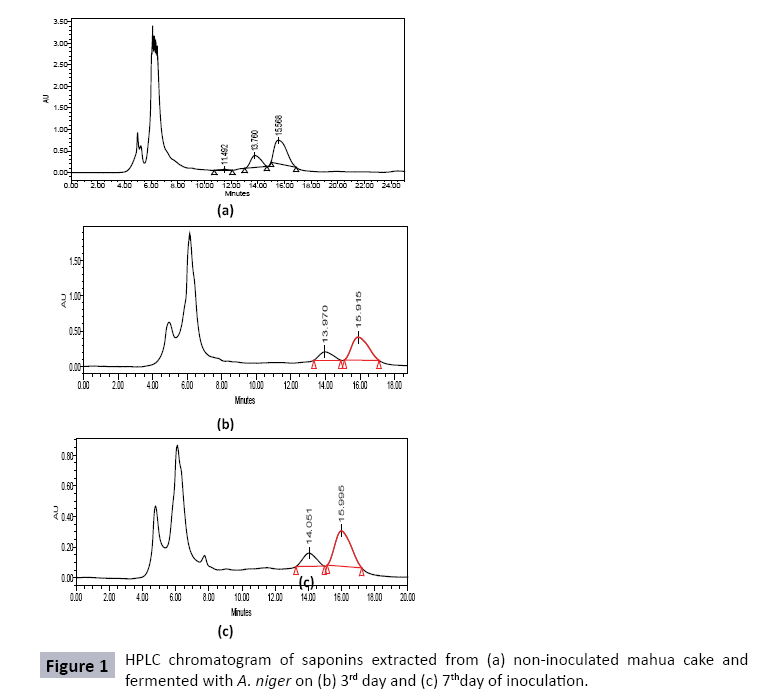
Saponins were extracted from MC inoculated with A. niger, each day, for upto 7 days, and compared with saponins extracted from uninoculated mahua cake (control). HPLC chromatogram of mahua saponins gave three peaks with retention times 10.536, 12.887 and 13.572 minutes. However, HPLC chromatograms of saponins extracted from mahua cake fermented with A. niger, on third and seventh day of inoculation, showed the presence of only two major peaks, slightly shifted (Rt : 13.676-14.051 min and 15.481-15.995 min) (Figure 1). Table 5 shows the time course profile of mahua saponins degraded by A. niger during SSF of MC. It can be seen that degradation of 54.2-71.5% in major peak 1, and 52.2-67.9% in major peak 2 was obtained during seven days of fermentation of MC by A. niger. It was envisaged that degradation of saponins might have been due to enzymatic action, especially, by glycosidases, produced by the fungus [41]. Thereafter, these degraded saponins might have been utilized as nutrient sources promoting fungus growth and enzyme production [27]. Such observations can be supported by literature as well. de Barros et al. reported that white rot fungi Bjerkandera adusta and Phlebia rufa, significantly reduced (91% and 97%, respectively) the phorbol esters content below the toxicity levels in JC [42]. Joshi and Khare reported that Pseudomonas aeruginosa could complete degrade phorbol esters during SSF of JC under optimized conditions [27].
Table 5: Time course profile of saponin degradation by Aspergillusniger.
| Fermentation time (days) | Saponin degradation by A. niger (Mean ± SD; %) | |
|---|---|---|
| Major peak 1 | Major peak 2 | |
| 0 | 0.0 ± 0.00a | 0.0 ± 0.00a |
| 1 | 14.3 ±0.06b | 18.3 ±0.05b |
| 3 | 54.2±0.05c | 52.2±0.07c |
| 5 | 67.4±0.06d | 66.2±0.04d |
| 7 | 71.5±0.04e | 67.9 ±0.06e |
Note: Means sharing a different superscript in each column are significantly different from each other (p≤ 0.05) using DMRT
References
- VyasP, Kumar A, Singh S (2018) Biomass breakdown : A review on pretreatment, instrumentations and methods. Front Biosci 10: 155-174.
- Kumar A, Sharma S (2008) Anevaluation of multipurpose oil seed crop for industrial uses (Jatrophacurcas L.): A review. Ind Crops Prod 28: 1-10.
- Kumar A, Sharma S (2011) Potential non-edible oil resources as biodiesel feedstock: An Indian perspective. Renew Sustain Energy Rev 15: 1791-1800.
- Kumar A, Kumar K, Kaushik N, Sharma S, Mishra S (2010) Renewable energy in India: Current status and future potentials. Renew Sustain Energy Rev 14: 2434-2442.
- Kothari R, Pandey A, Ahmad S, Kumar A, Pathak VV, et al. (2017) Microalgal cultivation for value-added products: a critical enviro-economical assessment. 3 Biotech 7:243.
- Rakshit KD, Darukeshwara J, Rathina Raj K, Narasimhamurthy K, Saibaba P, et al. (2008) Toxicity studies of detoxified Jatropha meal (Jatrophacurcas) in rats. Food ChemToxicol 46: 3621-3625.
- Sharma A, Sharma S, Yadav S, Naik SN (2014) Role of Karanjadeoiled cake based medium in production of protease and fatty acids by Paecilomyceslilacinus 6029. J Biosci Bioeng118: 270-271.
- Gupta A, Sharma S, Vijay VK (2011) Utilization of Non-traditional biomass for biogas production. In: 19th European Biomass Conference and exhibition, Berlin, Germany. pp. 6-10.
- Gupta A, Chaudhary R, Sharma S (2012) Potential applications of mahua (Madhucaindica) biomass. Waste and Biomass Valorization 3: 175-189.
- Sadaf A, Khare SK (2014) Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiledJatrophacurcas seed cake and its application in xylooligosachharide synthesis. BioresourTechnol 153: 126-130.
- Chaturvedi S, Kumar A (2012) Bio-diesel waste as tailored organic fertilizer for improving yields and nutritive values of Lycopercicumesculatum (tomato) crop. J Soil Sci Plant Nutr 12: 801-810.
- Chaturvedi S, Kumar A, Singh B, Nain L, Joshi M, et al. (2013) Bioaugmented composting of Jatropha de-oiled cake and vegetable waste under aerobic and partial anaerobic conditions. J Basic Microbiol 53: 327-335.
- Gupta A, Kumar A, Sharma S, Vijay VK (2013a) Comparative evaluation of raw and detoxified mahua seed cake for biogas production. Applied Energy 102:1514-1521.
- Kumar A, Sharma S, Mishra S, Dames JF (2013) Arbuscularmycorrhizal inoculation improves growth and antioxidative response ofJatrophacurcas (L.) under Na2SO4 salt stress. Plant Biosyst An Int J Deal with all Asp Plant Biol 149: 260-269.
- Singh NB, Kumar A, Rai S (2014) Potential production of bioenergy from biomass in an Indian perspective. Renew Sustain Energy Rev 39: 65-78.
- Ramachandran S, Singh SK, Larroche C,Soccol CR, Pandey A(2007) Oil cakes and their biotechnological applications - A review. BioresourTechnol 98: 2000-2009.
- Makkar HPS, Aderibigbe AO, Becker K (1998) Comparative evaluation of non-toxic and toxic varieties of Jatrophacurcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem 62: 207-215.
- Verma M, Pradhan S, Sharma S, Naik SN, Prasad R (2011) Efficacy of karanjin and phorbol ester fraction against termites (Odontotermesobesus). IntBiodeteriorBiodegrad 65: 877-882.
- Chutmanop J, Chuichulcherm S, Chisti Y, Srinophakun P (2008) Protease production by Aspergillusoryzae in solid-state fermentation using agroindustrial substrates. ChemTechnol 1018: 1012-1018.
- Arora K, Sharma S, Krishna SBN, Adam Jamila K, Kumar A (2017) Non-edible oil cakes as a novel substrate for DPA production and augmenting biocontrol activity of Paecilomycesvariotii. Front Microbiol8: 1-12.
- Sandhya C, Sumantha A, Szakacs G, Pandey A (2005) Comparative evaluation of neutral protease production by Aspergillusoryzae in submerged and solid-state fermentation. Process Biochem 40: 2689-2694.
- Ferreira G, Boer CG, Peralta RM (1999) Production of xylanolytic enzymes by Aspergillustamarii in solid state fermentation. FEMS MicrobiolLett 173: 335-339.
- Howard RL, Abotsi E, Jansen van Rensburg EL, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. African J Biotechnol 2: 602-619.
- Song JM, Wei DZ (2010) Production and characterization of cellulases and xylanases of Cellulosimicrobiumcellulans grown in pretreated and extracted bagasse and minimal nutrient medium M9. Biomass and Bioenergy 34: 1930-1934.
- Germano S, Pandey A, Osaku CA, Rocha SN, Soccol C (2003) Characterization and stability of proteases from Penicillium sp. produced by solid-state fermentation. Enzyme MicrobTechnol 32: 246-251.
- Krishnan T, Chandra AK (1982) Effect of oilseed cakes on alpha-amylase production by Bacillus licheniformis CUMC305. Appl Environ Microbiol 44: 270-274.
- Joshi C, Khare SK (2011) Utilization of deoiledJatrophacurcas seed cake for production of xylanase from thermophilicScytalidiumthermophilum. BioresourTechnol 102: 1722-1726.
- AOAC (1995) Official methods of analysis(16thedn.). Association of Official Analytical Chemists, Washington DC, USA.
- Gothwal R, Gupta A, Kumar A, Sharma S, Alappat BJ (2012) Feasibility of dairy waste water (DWW) and distillery spent wash (DSW) effluents in increasing the yield potential of Pleurotusflabellatus (PF 1832) and Pleurotussajor-caju (PS 1610) on bagasse. 3 Biotech 2: 249-257.
- Scott, TA, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25: 1656-1661.
- Thimmaiah SK (2006) Standard methods of biochemical analysis. KalyaniPublishers, New Delhi. p. 534.
- Lalitha T, Seshadri R, Venkataraman LV (1987) Isolation and properties of saponins from madhucabutyraceaseeds. J Agric Food Chem 35: 744-748.
- Gupta A, Sharma S, Saha S, Walia S (2013b) Yield and nutritional content of Pleurotussajorcaju on wheat straw supplemented with raw and detoxified mahua cake. Food Chem 141: 4231-4239.
- Ghose TK (1987) Measurement of cellulase activities. Pure ApplChem 59: 257-268.
- Sumantha A, Fontanille P, Larroche C, Pandey A (2008) Exploration of fungal spores as a possible storehouse of proteolytic biocatalysts. World J Microbiol Biotechnol 24: 28970-2901.
- Paranthaman R, Alagusundaram K, Indhumathi J (2009) Production of Protease from Rice Mill Wastes by Aspergillusniger in Solid State Fermentation. J Agric Sci 5:308-312.
- Rajmalwar S, Dabholkar PS (2009) Production of protease by Aspergillussp using solid-state fermentation. African J Biotechnol 8: 4197-4198.
- Sukumaran RK, Singhania RR, Mathew GM, Pandey A (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew Energy 34: 421-424.
- Latifian M, Hamidi-Esfahani Z, Barzegar M (2007) Evaluation of culture conditions for cellulase production by two Trichodermareesei mutants under solid-state fermentation conditions. BioresourTechnol 98: 3634-3637.
- Mekala NK, Singhania RR, Sukumaran RK, Pandey A (2008) Cellulase production under solid-State fermentation by trichodermareesei RUT C30: Statistical optimization of process parameters. Appl Biochem Biotechnol 151: 122-131.
- Carmona AT, Whigtman RH, Robina I, Vogel P (2003) Synthesis and glycosidase inhibitory activity of 7-deoxycasuarine. Helv Chim Acta 86: 3066-3073.
- de Barros CRM, Ferreira LMM, Nunes FM, Bezerra RMF, Dias AA, et al. (2011) The potential of white-rot fungi to degrade phorbol esters of Jatrophacurcas L. seed cake. Eng Life Sci 11: 107-110.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences