ISSN : 0976 - 8688
Der Pharmacia Sinica
Screening of In Vitro Membrane Stabilizing and Anti Atherothrombosis Activities of Different Fractional Extracts of Ludwigia Prostrata Roxb. ( Family: Onagraceae) Leaves
Shakhuat Hossain Tusher*, Irin Sultana and Mohi Uddin Chowdhury
Department of Pharmacy, Southern University Bangladesh, Bangladesh
- *Corresponding Author:
- Shakhuat Hossain Tusher
Department of Pharmacy, Southern University Bangladesh, Bangladesh
Tel: 01851635140
E-mail: shakhuattusher@gmail.com
Received Date:February 14, 2022, Manuscript No. IPDPS-22-12314; Editor assigned date: February 17, 2022, PreQC No . IPDPS-22-12314 (PQ); Reviewed date: March 03, 2022, QC No. IPDPS-22-12314 ; Revised date: April 18, 2022, Manuscript No.IPDPS-22-12314 (R); Published date: April 26, 2022, DOI: 10.36648/0976-8688/22.13.21
Citation: Tusher SH, Sultana I, Chowdhury MU (2022) Screening of In-Vitro Membrane Stabilizing and Anti-Atherothrombosis Activities of Different Fractional Extracts of Ludwigia Prostrata Roxb. (Family: Onagraceae) Leaves. Der Pharmacia Sinica Vol:13 No: 4
Abstract
The present study aimed to evaluate the in-vitro membrane-stabilizing activities and anti-atherothrombosis activities of the ethanolic, n-Hexane, and chloroform extracts of leaves of Ludwigia prostrata Roxb. Screening of in-vitro membrane-stabilizing activities was performed by hypotonic solution induced method where Acetylsalicylic acid used as a standard, significantly (p<0.05) inhibited hemolysis when compared to control. For instance, ethanolic, n-Hexane, and chloroform extracts inhibited 41.19%, 58.16%, and 44.35% respectively at the higher dose (500 μg/ml) used in this experiment compared with standard drug, Acetyl Salicylic Acid (ASA) inhibited hemolysis by 68.03% at the same dose. Screening for anti-atherothrombosis activities was carried out by the clot lysis method where Streptokinase (SK) was used as a standard. For instance, ethanolic, n-Hexane, and chloroform extracts 32.11%, 16.30%, and 37.36% respectively clot were lysed where show moderate activity compared with standard drug, Streptokinase (SK) inhibited hemolysis by 81.48%.
Keywords
Ludwigia prostrata Roxb; Anti-atherothrombosis; Membrane-stabilizing activities; Hypotonic solution; Clot lysis method
Introduction
Ever since history, in search of rescue for their malady, the folks explore for medication in nature. The beginnings of the healthful plants’ use were natural, as is that the case with animals [1]. Because at the time there wasn't sufficient information either concerning the reasons for the ails or concerning which factory and how it could be employed as a cure, everything was grounded on experience. In time, the explanations for the usage of specific healthful plants for the treatment of sure diseases were being discovered; so, the healthful plants’ usage bit by bit abandoned the empiric framework and has become supported onexplicatoryfacts. Until the arrival of iatrochemistry in the 16th century, shops had been the source of treatment and prophylaxis [2]. Ludwigia prostrata Roxb is a condiment erect, periodic, or short-lived imperishable? Stems are frequently red-pigmented, 10-60 cm altitudinous, frequently fanned, subglabrous. Petiole 4-25 mm; splint blade elliptic to hardly elliptic, 1-13×0.3-2.7 cm, rough or with many hairs on modes, side modes 8-12 per side, submarginal tone invisible, base hardly cuneate, apex acute [3].
In Bangladesh, it is mostly found in Chittagong, Chittagong Hill Tracks (Bandarban, Khagrachari, and Rangamati). It is grown in moist fields, roadside puddles, and near water. This plant is commonly known as Shayankura (Bangla), Taikosa (Tripura) and Gangku maichya (Chakma) [4]. The flowering and fruiting period is January-October. Generally, this plant is used as forage for domestic animals and Chakma is used to treat whooping cough by taking stem juice 3 times a day [5]. After the Chemical group test for the fractionated extracts (ethanolic, n-Hexane, and chloroform) of leaves of Ludwigia prostrata Roxb in my institute, I got alkaloids, glycosides, steroids, flavonoids, reducing sugar, gums, and amides.
This study aims to evaluate the in-vitro membrane-stabilizing activities and anti-atherothrombosis activities of the ethanolic, n-Hexane, and chloroform extracts of leaves of Ludwigia prostrata Roxb.
Materials and Methods
Identification and Collection of Plant Materials: Taxonomical identification of this plant was made by the experts of Bangladesh Forest Research Institute (BFRI) Herbarium, Chittagong, Bangladesh. The herbarium sheet was prepared following the standard procedure and specifications suggested by the expert of the institute. The sheet was signed by the taxonomist and preserved in the Pharmacognosy Laboratory, Department of Pharmacy, Southern University Bangladesh. Fresh leaves were collected from the agricultural fields of Hathazari, Chittagong, Bangladesh in December. The extraneous, undesired substances from the plant material were removed at two stages of processing of it.
Extraction of Plant Materials: To increase the surface area of the plant particles, these were crushed into coarse powders using a crushing mill after shade drying. Then these coarse powders of leaves weighing 350 gm were then placed in a soxhlet extractor (Quickfit, England) for continuous hot extraction with 1100 mL of absolute ethanol for almost 22 hours at 45°C temperature. After extraction, filtration was carried out and after evaporation of the solvent under the ceiling fan, a gummy concentrate was obtained which was designated as hot ethanolic crude extracts. The amount of crude extract from hot extraction was 28.55 gm. The extracts were weighed separately with the help of electronic and digital balance. Then the yield was determined by using the following formula:
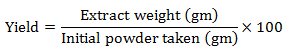
Hot ethanolic extract: % yield=8.1%
Fractionation of Crude Ethanolic Extracts: The hot ethanolic crude extract was undergone solvent-solvent partitioning using the protocol design by Kupchan and modified by Van Wagenen [6]. The crude ethanol extract (22.55 gm taken from 28.55 gm) was dissolved in DDW and then fractionated using a fractionating column with n-Hexane and subsequently with chloroform. Fractionation was done using 50 ml solvent each time until 150 ml n-Hexane and chloroform were used and each time after vigorous shaking the mixture was allowed to stand. Solvent layers were separated and decanted. The remaining extract was used as a fraction of ethanol.
Assessment of In-Vitro Membrane Stabilization Activity
Preparation of samples for treatment groups: For standard 5 mg acetylsalicylic acid was dissolved in 10 ml Double-Distilled Water (DDW) to get initial concentration (500 μg/ml) and then further diluted to get different concentrations (250 μg/ml and 125 μg/ml). To prepare iso-saline and hypo-saline, 900 mg and 500 mg NaCl were added to 100 ml of DDW respectively. For 10% RBCs suspension, fresh whole human 1 ml blood was collected and diluted with RBC diluting fluid up to 20 ml. Then 2 ml of this solution was diluted with 18 ml normal saline solution to get the 10% RBC suspension [7]. For test groups, 5 mg of different crude fractions were mixed with 10 ml volume of respective solvents viz. ethanol, n-hexane, and chloroform to get the stock solution and then further diluted as required.
Biological specimen for the experiment: Fresh whole human sodium citrated blood was collected and used readily to perform the work.
Experimental design: For this study, 25 clean centrifuge tubes were taken. Including three conc. six for standard, one for control, and other centrifuge tubes for each extract. The tubes were marked accordingly. 1 ml of 10% RBCs suspension was kept into all treatment tubes; 1 ml respective solvent (ethanol, n-Hexane, and chloroform) for the negative control, acetylsalicylic acid was added to the positive control tubes respectively. On the other hand for the test group, 1 ml of respective extract (required concentration) was mixed to the test group as marked. Then all the tubes were treated with 1 ml of hypotonic solution. The pH (7.4 ± 0.2) of the reaction mixtures was adjusted by phosphate buffer. All the reaction mixture’s centrifuge tubes were incubated in a water bath at 56°C for 30 min. At the end of the incubation, the tubes were cooled under running tap water. The reaction mixture was centrifuged at 2500 rpm for 5 min. After subsequent filtration, the absorbance of the supernatant fluids was spectrophotometrically measured at 660 nm. The test was repeated three times [8]. The percentage inhibition of hemolysis was calculated as follows:
% Inhibition of hemolysis=(Abs control–Abs sample)×100/Abs control
Assessment of Anti-Atherothrombosis Activity
Plant materials
Ethanolic, n-Hexane, and chloroform extracts of leaves of Ludwigia prostrata Roxb. For this purpose 2.5 mg of each extract was dissolved in 5 ml Double-Distilled Water (DDW) separately.
Preparation of controls: DDW and streptokinase were taken as negative control and positive control respectively.
Experimental design: In brief, 4 ml venous blood was drawn from healthy volunteers and distributed in five different groups (3 for extracts, 1 for reference standard, and 1 for negative control) of pre-weighed sterile microcentrifuge tubes (0.5 ml/tube) and incubated at 37°C for 45 min. After clot formation, serum was completely removed without disturbing the clot and each tube having clot was again weighed to determine the clot weight (clot weight=weight of clot containing tube–weight of tube alone). The standard tubes were treated with streptokinase (100 μl) and the test groups were treated with respective extracts of Ludwigia prostrata Roxb (100 μl). DDW was separately added to the negative control tubes. All the tubes were then incubated at 37°C for 90 minutes and observed for clot lysis. After incubation, released fluid was removed and tubes were again weighed to observe the difference in weight after clot disruption. The difference obtained in weight taken before and after clot lysis was expressed as the percentage of clot lysis [9].
% of clot lysis=weight of released clot×100/clot weight
Results and Discussion
In-vitro membrane stabilization activity
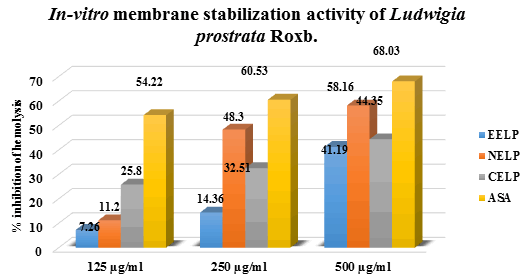
In the in-vitro membrane stabilization activity test as shown in (Table 1).
| Treatmentgroups | Dose(µg/ml) | %inhibition of hemolysis | SD | SEM | Totalanti-hemolysis count |
|---|---|---|---|---|---|
| Control(DDW) | --- | --- | 0 | 0 | 0 |
| Positivecontrol (ASA) | 500 | 68.03* | 0.0007 | 0.0007 | 68.03± 0.0007 |
| 250 | 60.53* | 0.002 | 0.002 | 60.53± 0.002 | |
| 125 | 54.22* | 0.002 | 0.002 | 54.22± 0.002 | |
| n-Hexaneextract | 500 | 58.16* | 0.001 | 0.001 | 58.16± 0.001 |
| 250 | 48.30* | 0.002 | 0.002 | 48.30± 0.002 | |
| 125 | 11.2 | 0.0007 | 0.0007 | 11.20± 0.0007 | |
| Chloroformextract | 500 | 44.35* | 0.021 | 0.021 | 44.35± 0.021 |
| 250 | 32.51* | 0.004 | 0.004 | 32.51± 0.004 | |
| 125 | 25.80* | 0.002 | 0.002 | 25.80± 0.002 | |
| Ethanolicextract | 500 | 41.19* | 0.013 | 0.013 | 41.19± 0.013 |
| 250 | 14.36 | 0.002 | 0.002 | 14.36± 0.002 | |
| 125 | 7.26 | 0.009 | 0.009 | 7.26± 0.009 | |
| ASA:Acetylsalicylic Acid; SD: Standard Deviation; SEM: Standard Error of Mean; *p<0.05. | |||||
Table 1: Spectrophotometric determination of membrane stabilization activity.
The ethanolic, n-Hexane, and chloroform extracts of leaves of Ludwigia prostrata Roxb. Showed mean inhibition of hemolysis of RBCs by 41.19%, 58.16%, and 44.35% respectively at the higher dose (500 μg/ml) used in this experiment. Whereas standard drug, Acetyl Salicylic Acid (ASA) inhibited hemolysis by 68.03% at the same dose. From the value of IC50, it can be predicted that ethanolic extract, n-Hexane extract, and chloroform extract of the plant possess moderate membrane stabilization activity in comparison with the standard drug, ASA as shown in (Table 2).
| Treatment groups | ASA | EE | NE | CE |
|---|---|---|---|---|
| IC50 (µg/ml) | 0.41 | 3.71 | 2.45 | 3.7 |
Table 2: Tabulation of IC50 for membrane stabilization activity.
In the membrane stabilization assay, the erythrocytes are challenged with totally different hemolytic stimuli like heat, diffusion shock, and free radicals. The heat-induced and hypo tonicity-induced hemolysis of erythrocytes is extensively used as a speedy, simple, economic, and sensitive tool in determining the anti-inflammatory drug property of medicine [10].
The present study was developed by bound modifications of the tactic claimed by Mizushima supported the in-vitro determination of the membrane instability caused by the management cluster and scrutiny it with the positive management and check cluster. A lot of the membrane in-stabilization effects, a lot of it'll cause hemolysis and therefore the measured absorbance is higher [11].
Anti-Atherothrombosis Activity
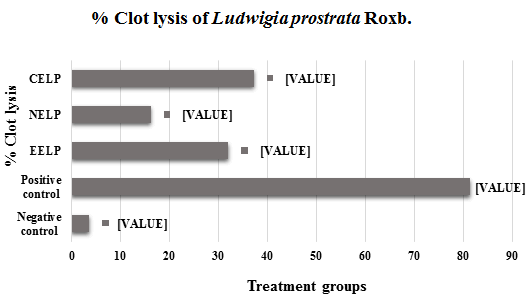
In the in-vitro thrombolytic activity test the addition of 100 μl Streptokinase (SK), the clots showed 81.48% clot lysis. On the other hand, when treated with 100 μl distilled water (negative control) showed clot lysis was 3.72%. After treatment of clots with 100 μl of ethanol, n-Hexane, and chloroform extracts of Ludwigia prostrata Roxb. The lysis was 32.11%, 16.30%, and 37.36% respectively when the concentration was 500 μg/100 μl as shown in (Table 3).
| Sample | Dose | Clot lysis (%) |
|---|---|---|
| Distilled Water (Negative control) | 100 µl | 3.72 ± 0.0017 |
| Streptokinase (15,00,000 U/Vial/10 ml) | 100 µl | 81.4832 ± 0.021 |
| EELP | 500 μg/100 µl | 32.11 ± 0.005 |
| NELP | 500 μg/100 µl | 16.30 ± 0.040 |
| CELP | 500 μg/100 µl | 37.36 ± 0.023 |
| Here, EELP: Ethanolic extract of Ludwigia prostrataRoxb. ; NELP: n-Hexane extract of Ludwigia prostrataRoxb. ; CELP: Chloroform extract of Ludwigia prostrataRoxb. | ||
Table 3: Anti-atherothrombosis activity of the fractional extracts of leaves of Ludwigia prostrata Roxb.
Atherosclerosis may be a diffuse method that starts early in childhood and progresses asymptomatically through adult life. Later in life, it's clinically manifested as Coronary Artery Disease (CAD), stroke, Transient Ischemic Attack (TIA), and Peripheral Arterial Disease (PAD). Crude biological elements possessing anti-thrombotic activity are studied mostly to seek out an attainable thanks to embodying them in life science (Figures 1 and 2).
Conclusion
Current medications are not fully free from adverse effects and it doesn't depend on how much it is effective on the body. So, undesired are yet to be minimized along with achieving higher efficacy. So, we should develop alternative ways to reduce adverse effects and make medications more safe and effective for the patients. Different extracts of leaves of the plant Ludwigia prostrataRoxb exhibited some promising activities in the above-mentioned tests. However further researches should be conducted to isolate the active compounds and to carry out subsequent clinical trials.
References
- Stojanoski N (1999) Development of health culture in veles and its region from the past to the end of the 20th century. PMC J 13:34
- Kelly K (2009) History of medicine. New York: Facts on file, pp. 29–50.
- Jiarui Chen, Peter c Hoch, Peter h Raven (2006) Chamerion. Flora of China 13:400-409
- Rahman MA, Uddin SB, Wilcock CC (2007) Medicinal plants used by Chakma Tribe in Hill Tracts districts of Bangladesh. Indian J Tradit Knowl 6:508-517
- Van-Wagenen BC, Larsen R, Cardellinall JH, Randazzo D, Lidert ZC, et al. (1993) Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J Org Chem 58:335-337
[Crossref] [Google scholar] [Indexed]
- Thenmozhi V, Elango V, Sadique J (1989) The anti-inflammatory activity of some Indian medicinal plants. Ancient science of life 8:258-261
- Shinde UA, Phadke S, Nair AM, Mungantiwar AA, Dikshit VJ, et al. (1999) Membrane stabilizing activity — a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Sciencedirect 70:251-257
[Crossref] [Google scholar] [Indexed]
- Sultana I, Noor MA, Barua J, Mahmood A, Das M, et al. (2012) In-vitro anti atherothrombosis activity of four Bangladeshi plants. Int J Green Pharm 4:1148-1153
[Crossref] [Google scholar] [Indexed]
- Kardile MV, Mahajan UB, Shaikh HM, Goyal SN, Patil CR (2016) Membrane stabilization assay for anti-inflammatory activity yields false-positive results for samples containing traces of ethanol and methanol. J Pharm Pharm Sci 5:493-497
- Y Mizushima, M Kobayashi (1968) Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 20:169-173
[Crossref] [Google scholar] [Indexed]
- Juan F Viles-Gonzalez, Valentin Fuster, Juan J Badimon (2004) Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. European heart journal 25:1197–1207
[Crossref] [Google scholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences