Regulatory Peptides: Exchange across the Blood-Brain Barrier
1Mechinkov Northwestern State Medical University, St. Petersburg, Russia
2Kirov Military Medical Academy, St. Petersburg, Russia
3Sechenov Institute of Evolutionary Physiology and Biochemistry, St. Petersburg, Russia
- *Corresponding Author:
- Alexander T Maryanovich
Mechinkov Northwestern State Medical
University, St. Petersburg, Russia.
Tel: +7 9531658430
E-mail: atm52@mail.ru
Received date: March 16, 2017; Accepted date: April 13, 2017; Published date: April 28, 2017
Citation: Maryanovich AT, Kormilets DY, Polyanovsky AD. Regulatory Peptides: Exchange across the Blood-Brain Barrier. J Psychol Brain Stud. 2017, 1:5.
Copyright: © 2017 Maryanovich AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Regulatory peptides produced by endocrine cells of the digestive tract and other peripheral organs affect the brain and thereby elicit multiple CNS-dependent effects. For example, more than two dozen of gut peptide hormones are involved in the transmission of satiety signals, opening up alluring prospects of creating anorexigenic drugs. Nevertheless, these agents, known for almost four decades, have not so far received wide application in clinical medicine. Such a protracted delay in application suggests a need for a better understanding of the theoretical conceptualizations underlying peptide regulation. Our mini review attempts to bridge the gap between theory and practice.
Keywords
Blood-brain barrier; Regulatory peptides; Transport; Appetite suppressants
Introduction
Regulatory peptides produced by endocrine cells of the digestive tract and other peripheral organs affect the brain and thereby elicit multiple CNS-dependent effects. For example, more than two dozen of gut peptide hormones are involved in the transmission of satiety signals, opening up alluring prospects of creating anorexigenic drugs. As potential medicinal agents, regulatory peptides have important advantages: (a) eventually, they all break down to amino acids, making overdose impossible, (b) short regulatory peptides can be taken perorally.
Nevertheless, these agents, known for almost four decades, have not so far received wide application in clinical medicine. Such an inconsistency suggests a need for a better understanding of the theoretical conceptualizations underlying peptide regulation.
The same regulatory peptides -gastrin, secretin, cholecystokinine and many others— are produced both by endocrine cells of the gut mucosa and neurons of the brain. These peptides are implicated in humoral regulation in the digestive organs and the brain, respectively. However, the relationship between central and peripheral regulatory peptides, such as intestinal and cerebral cholecystokinines, or peripheral and cerebral secretins, is still unclear. Is there a synergistic relationship between these 'peripheral' and 'central' peptides, or do they represent two distinct and independent regulatory systems? If a cooperative relationship does exist, how is it realized if the brain is separated from the periphery by a peptide-impermeable (or poorly permeable) blood-brain barrier (BBB)? Answering these questions can better inform the choice of strategies used in developing new medicines.
The passability of peptides across the BBB
The passability of peptides across the BBB, i.e. their ability to pass through the BBB, depends on such properties as the size, flexibility, conformation, biochemical properties of constituent amino acids and spatial arrangement of the molecules. Peptide structure determines to some extent such properties of the molecule as the degree of binding it to plasma proteins, peptidase resistance, absorption by nontarget tissues, excretion rate, and affinity for transporters [1]. By a very rough estimate [2], the passability of peptides across the BBB is two-three orders of magnitude lower than for glucose and two orders higher than for albumin.
Passive influx of peptides
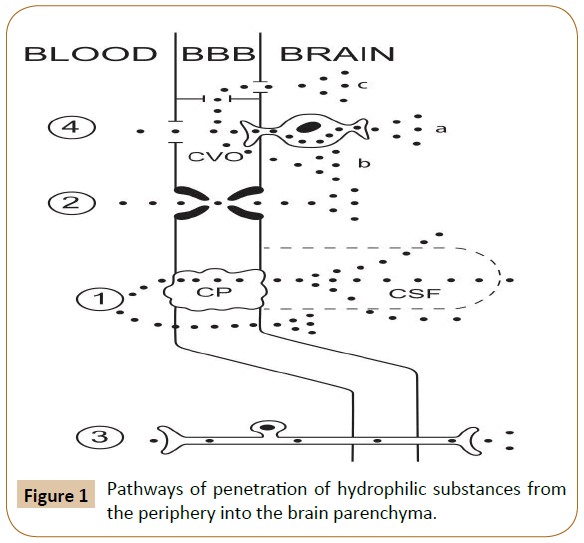
Substances with low (but not zero) lipophilicity can penetrate from the blood to the brain in small amounts via simple diffusion (Figure 1). Pathways of penetration of hydrophilic substances from the periphery into the brain parenchyma. Proven pathways: 1) simple diffusion to the brain parenchyma, directly or via the choroid plexus and cerebrospinal fluid; 2) active transport; 3) intra-axonal transport along afferent fibers. Putative pathways: 4) via the circumventricular organs and then (a) by intra-axonal transport, (b) across the ependyma into the cerebrospinal fluid, or (c) by bulk flow to the regions not protected by the ependyma. (BBB: blood-brain barrier; CP: Choroid Plexus; CVO: Circumventricular Organ; CSF: Cerebrospinal Fluid).
Figure 1: Pathways of penetration of hydrophilic substances from the periphery into the brain parenchyma.
A variant of such penetration is the pathway: blood → choroid plexus → ependymal of the cerebral ventricles → cerebrospinal fluid. "Theoretically, there is also what is termed as the "functional leak", where the ependymal cells are linked by tight junctions around the circumventricular organs and the permeable ependymal lining the ventricles join [3].
Dipeptides, glycylphenylalanine (Gly-Phe) and glycylleucin (Gly-Leu), are absorbed by the brain at about the same rate as sucrose, i.e. insignificantly and slower than their constituent amino acids, singly. Dipeptides do not suppress the absorption of their constituent amino acids [4], indicative of the mechanism of passive influx.
The rate of passive penetration of a peptide from the blood into the brain is directly proportional to such its properties as: (a) lipophilicity (being quite low); (b) arterial blood concentration (in a free state); and (c) resistance to enzymatic degradation. It is also inversely proportional [5] to the: (a) polarity of a molecule (e.g., in the presence of -COOH, -OH or -NH2 groups — the surface of the molecule is very important in terms of how the molecule passes through the BBB, since there is an interaction between the molecule surface charge and the cells of the BBB) and (b) binding to plasma proteins.
The surface of the molecule is very important in terms of how the molecular passes through the BBB since there is an interaction between the charge of the surface molecule and the cells of the BBB. In general, major restrictors for molecules of hydrophilic substances, such as peptides, for passing through the BBB are the number and strength of intermolecular bonds that hold water molecules [6]. Therefore, the factors that reduce the passability are: (a) dipolarity; (b) polarizability; and (c) hydrogen bonding potential. The passability is promoted by (a) small size of a molecule and (b) molar refraction. Importantly, the role of lipophilicity far exceeds that of all other factors. Yet, August Krogh (winner of the Nobel Prize in Physiology or Medicine in 1920) maintained that creating new pharmaceutical preparations which are able to penetrate into the brain should be guided, not by the charge of a molecule, but by its solubility in lipids [5]. Simple diffusion was shown to drive into the brain the following regulatory peptides:(a) dipeptide cyclohistidylproline cyclo(His- Pro) [7] and tripeptide thyrotropinreleasing hormone [8]; (b) some opioid peptides [9]; and (c) amylin (number of amino acids, n=37) [7] and nesfatin-1 (n=82) [10].
Active influx of peptides
Peptide transporter-2 (PEPT2) mediates the influx of di- and tri- peptides [11]. A peptide transport system is not identical to a peptide-specific receptor. Thus, Tyrmelanotropin releaseinhibiting factor-1 and met-enkephalin are transported by the same peptide transporter system (PTS-1) but have different receptors [12].
The systems for active transport from the blood to the brain have been described for various regulatory peptides, including: (a) ghrelin [13]; (b) urocortin I [14,15]; (c) corticotropinreleasing hormone [16]; (d) leptin [17]; (e) pituitary adenylate cyclaseactivating polypeptide [18,19]; (f) thyrotropin-releasing hormone [20,21]; (g) insulin [7,22]; and (h) opioid peptides. Nevertheless, as exemplified by ghrelin, the role of peptides penetrating across the BBB via active transport is dubious: the ghrelin penetration rate is too small to create a peptide concentration in the brain that is sufficient for its binding to specific receptors GHS-R [23-25].
Quantitative assessment of peptide influx
The degree of peptide passability is positively associated with the quantitative assessment of the peptide lipophilicity (octanol coefficient or octanol water partition coefficient, Kow) and negatively correlated with the molecular weight, percentage of unbound peptide, and various kinds of charges of a molecule Oldendorf technique. William Oldendorf (1925−1992) was the first to announce in 1961 the possibility of neurovisualization. His lesser known contribution to neurology was the proposition in 1970 of the method for quantitative assessment of BBB permeability, based on determining the brain uptake index (BUI). To do this, the substance tagged with a radioactive label was injected by bolus dosing into the carotid artery with animal decapitated the label concentration was then measured in brain tissue and compared to the reference level, the superheavy water (3H2O) passability [2,26], using 14C-antipyrine with a zero passability as a reference substance. Oldendorf and his colleagues adapted this method to assess the transport of amino acids [27] and peptides [28] to the brain.
In assessing the peptide influx, the Oldendorf's method theoretically has a drawback typical for similar methods: the passage of the radioactive label to the brain does not necessarily mean the passage of the whole molecule; peptide molecule can undergo peptidase-induced degradation, after which the label, alone or coupled to a peptide fragment, penetrates into the brain. Nevertheless, the Oldendorf method has been widely used until very recently [29].
The attempts to experimentally establish the percentage of peptide absorbed by the brain from the blood yielded quite high values: 2-3% for enkephalins and 1% for the thyrotropinreleasing hormone [30]. It is more likely that penetration of peptides from the blood to the brain parenchyma via simple diffusion is evaluated in fractions of a percentage. For example, 0.18% of peptide YY injected intravenously [31] and about 0.40 % of natriuretic peptide A injected into the carotid artery [32] pass through the BBB in this way. A whole-molecule penetration? Results of experiments with intracarotid injections of delta sleep-inducing peptide (DSIP), conducted in the late 1970s by researchers in the lab of Andrew Schally (winner of the Nobel Prize in Medicine in 1977) at Tulane University indicated that small amounts of regulatory peptides can penetrate across the BBB as whole molecules. Initial evidence was only indirect [33] and included findings that: (a) the brain/blood ratio of radioactive label concentrations was higher for labeled DSIP than for labeled insulin and (b) the brain DSIP concentration rises too fast (within first seconds after peripheral peptide injection) and lasts too short (about 1 min) to be explained by activation of the intracerebral synthesis of DSIP.
Shortly after that, more reliable evidence appeared. The use of highly specific antibodies to DSIP, targeted at the sequence fragment of no less than eight amino acids, showed that DSIP passes to the brain as a whole molecule [34]. Later, it was demonstrated that this is true not only for oligopeptides, but also for polypeptides with a molecular weight of 16−18 kD, specifically for interleukins IL-1alpha, IL-1beta, IL-6, tumor necrosis factoralpha and leptin [35].
Efflux of peptides
Release of peptides in and through the hypophysis — are classic pathways of releasing peptide hormones from the brain (hypothalamus) into the blood via: (a) the hypophyseal portal system to the adenohypophysis, apart from the wellknown releasing (corticotropin-releasing hormone, luteinizing hormone-releasing hormone, growth hormone-releasing hormone, thyrotropin-releasing hormone, prolactinreleasing peptide) and inhibitory (somatostatin, melanotropin releaseinhibiting factor-1) hormones, as well as pituitary adenylate cyclase-activating polypeptide, which is also discharged into the bloodstream [36,37] and (b) the axons of hypothalamic neurons from which two cyclic nonapeptides, vasopressin and oxytocin, are released anterogradely into the neurohypophysis. Nonselective release of peptides with excessive liquid. Vascular plexuses produce and secrete into the ventricles of the human brain roughly 500 ml of cerebrospinal fluid per day, while the total volume of the ventricular system is about 3.5 times smaller (~140 ml). Excessive liquid is periodically flushed down a hydrostatic pressure gradient into the dural venous sinuses through the arachnoid villi, which serve as valves. This provides a continuous "flushing" of brain tissues by cerebrospinal fluid [5].
As a result of induced inversions of the hydrostatic pressure gradient (to −20 cm H2O), central injections of regulatory peptides (specifically, gastrin-17 and somatostatin-14) does not cause an elevation of their blood concentrations, even at high doses [38]. Under normal (positive) hydrostatic pressure gradient, central injections of regulatory peptides, as a rule, also does not lead to increases in their blood level concentrations. For example, this was shown in experiments with gastrin, calcitonin, corticotropinreleasing hormone and calcitonin gene-related peptide [39].
It has been hypothesized that some peptides injected into the brain are excreted to the blood so quickly that they have no time to exert the peripheral effects [40]. We consider this mechanism unlikely, asthe blood level of any regulatory peptide, independent of its concentration in cerebrospinal fluid, of which approximately 500 ml is produced per day, which is the same amount that is discharged to the blood. The half-life of regulatory peptides in the blood and other body fluids never exceeds 10 min [41].
Therefore, during 5 min (a rough half-life of most regulatory peptides) a little more than 1.5 ml of liquid dissolves in 3 L of circulating plasma, meaning a 2,000-fold dilution. Moreover, the liquid concentration of regulatory peptides is initially low. It is not without reason that there is a special vascular system providing the effect of releasing hormones on the adenohypophysis: its microscopic volume allows retaining their concentration at a quite high level. Active efflux of peptides. The efflux transport system has been described for brain ghrelin [42], while brain beta-endorphin gets into the blood via a more general-purpose system, involving P-glycoprotein [43]. It appears that the function of this mechanism consists of removing the excess regulatory peptides from the CNS. It is unlikely that the amount of a peptide, actively eliminated from the brain into the blood, would be sufficient for inducing any peripheral effects, although there is an opposite opinion that corticotropin-releasing hormone may be actively excreted into the blood and accumulated in the spleen, inducing its specific effects there [29]. Regulatory peptides are well known to penetrate, if at all, from the blood into the brain only in very small amounts. All the longstanding attempts to boost the passability of regulatory peptides across the BBB have not gained much success. Instead, it seems more productive to focus not so much on boosting the passability of regulatory peptides across the BBB as on increasing the efficacy of their binding to peptide receptors outside the brain — in the circumventricular organs and/or vagal afferents. The adoption of this paradigm prompts yet another practical approach—designing more stable, peptidase-resistant, forms of regulatory peptides.
Practical Consequences
Regulatory peptides are well known to penetrate, if at all, from the blood into the brain only in very small amounts. All the longstanding attempts to boost the passability of regulatory peptides across the BBB have not gained much success. Instead, it seems more productive to focus not so much on boosting the passability of regulatory peptides across the BBB as on increasing the efficacy of their binding to peptide receptors outside the brain — in the circumventricular organs and/or vagal afferents. The adoption of this paradigm prompts yet another practical approach —designing more stable, peptidase-resistant, forms of regulatory peptides.
Conclusion
In human, the morphological substrate of a BBB is a layer of brain microvessel endothelial cells interconnected by tight and adherens junctions. Permeability of the BBB to any substance including peptide is determined primarily by its lipophilicity.By separating the blood and brain parenchyma, the BBB maintains homeostasis of the brain and provides neurons with a unique extracellular milieu optimal for humoral transmission. Simultaneously, the BBB is an interface between peripheral tissues and brain.
Humoral communication between the periphery and the brain can be realized without any penetration of a substance into the brain parenchyma. Hydrophilic substances (including regulatory peptides) circulating in the blood can transmit information to the brain by binding to specific receptors on vagal afferents and/ or in the circumventricular organs (ie outside the BBB). In the circumventricular organs (small areas of brain tissue scattered along the perimeter of the ventricles), not capillary endothelium, but the ependyma of ventricles performs barrier function (the blood-cerebrospinal fluid barrier). The BBB selectively transports some regulatory peptides in the blood-to-brain or the brain-toblood direction. Efflux transporters protect the brain by clearing it from numerous potentially harmful exogenous and CNS-borne compounds. To understand the mechanism of central effect of any (endogenous or exogenous) substance, we have to find out wheter the substance penetrates through the BBB or affects the brain without penetrating into it.
References
- Witt KA, Davis TP (2006) CNS drug delivery: opioid peptides and the blood-brain barrier. AAPS J 8: 76-88.
- Jaspan JB, Banks WA, Kastin AJ (1994) Study of passage of peptides across the bloodbrain barrier: biological effects of cyclo(His-Pro) after intravenous and oral administration. Ann N Y.
- Begley DJ (1994) Peptides and the blood-brain barrier: The status of our understanding. Ann N Y Acad Sci 739: 89-100.
- Zlokovic BV, Begley DJ, Chain DG (1983) Blood-brain barrier permeability to dipeptides and their constituent amino acids. Brain Res 271: 65-71.
- Bradbury M (1979) The Concept of a Blood Brain Barrier. John Wiley & Sons Ltd.
- Habgood MD, Begley DJ, Abbott NJ (2000) Determinants of passive drug entry into the central nervous system. Cell Mol Neurobiol 20: 231-253.
- Kastin AJ, Pan W (2004) Brain influx of endogenous peptides affecting food intake. In: Sharma HS, Westman J, editors. Blood – Spinal Cord and Brain Barriers in the Health and Disease. Amsterdam.
- Zlokovic BV, Segal MB, Davson H, Lipovac MN, Hyman S, et al. (1990) Circulating neuroactive peptides and the blood-brain and blood-cerebrospinal fluid barriers. Endocrinol Exp 24: 9-17.
- Banks WA, Kastin AJ (1990) Peptide transport systems for opiates across the bloodbrain barrier. Am J Physiol 259(1 Pt 1): E1-10.
- Price TO, Samson WK, Niehoff ML, Banks WA (2007) Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptide 28: 2372-2381.
- Frey IM, Rubio-Aliaga I, Klempt M, Wolf E, Daniel H (2006) Phenotype analysis of mice deficient in the peptide transporter PEPT2 in response to alterations in dietary protein intake.
- Maresh GA, Kastin AJ, Brown TT, Zadina JE, Banks WA (19990 Peptide transport system-1 (PTS-1) for Tyr-MIF-1 and Met-enkephalin differs from the receptors for either. Brain Res 839: 336-340.
- Pan W, Tu H, Kastin AJ (2006) Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides 27: 911-916.
- Kastin AJ, Akerstrom V, Pan W (2000) Activation of urocortin transport into brain by leptin. Peptides 21: 1811-1817.
- Pan W, Kastin AJ (2008) Urocortin and the brain. Prog Neurobiol 84: 148-156.
- Kastin AJ, Akerstrom V (2002) Differential interactions of urocortin/corticotropin releasing hormone peptides with the blood-brain barrier. Neuroendocrinology 75: 367-374.
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM (1996) Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305-311.
- Banks WA, Uchida D, Arimura A, Somogyvári-Vigh A, Shioda S (1996) Transport of pituitary adenylate cyclase-activating polypeptide across the blood-brain barrier and the prevention of ischemia-induced death of hippocampal neurons. Ann N Y Acad Sci 805: 270-277.
- Dogrukol-Ak D, Tore F, Tuncel N (2004) Passage of VIP/PACAP/secretin family across the blood-brain barrier: therapeutic effects. Curr Pharm Des 10: 1325-1340.
- Urayama A, Yamada S, Kimura R, Zhang J, Watanabe Y (2002) Neuroprotective effect and brain receptor binding of taltirelin, a novel thyrotropin-releasing hormone (TRH) analogue, in transient forebrain ischemia of C57BL/6J mice. Life Sci 72: 601-607.
- Urayama A, Yamada S, Ohmori Y, Deguchi Y, Uchida S, et al. (2003) Blood-brain permeability of [3H] -(3-methyl-His2) thyrotropin-releasing hormone (MeTRH) in mice: effects of TRH and its analogues. Drug Metab Pharmacokinet 18(5): 310-318.
- Banks WA, Jaspan JB, Huang W, Kastin AJ (1997) Transport of insulin across the bloodbrain barrier: saturability at euglycemic doses of insulin. Peptides 18: 1423-1429.
- Ganapathy V, Miyauchi S (2005) Transport systems for opioid peptides in mammalian tissues. AAPS J 7:852-856.
- Fry M, Ferguson AV (2010) Ghrelin: central nervous system sites of action in regulation of energy balance. Int J Pept 2010: 1-8.
- Banks WA, Kastin AJ (1985) Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Res Bull 15: 287-292.
- Oldendorf WH (1970) Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res 24: 372-376.
- Pardridge WM, Oldendorf WH (1975) Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta 401: 128-136.
- Greenberg R, Whalley CE, Jourdikian F, Mendelson IS, Walter R, Nikolics K, et al. (1976) Peptides readily penetrate the blood-brain barrier: uptake of peptides by synaptosomes is passive. Pharmacol Biochem Behav 5: 151-158.
- Turner TD, Browning JL, Widmayer MA, Baskin DS (1998) Penetration of dynorphin 1-13 across the blood-brain barrier. Neuropeptides 32: 141-149.
- Cornford EM, Braun LD, Crane PD, Oldendorf WH (1978) Blood-brain barrier restriction of peptides and the low uptake of enkephalins. Endocrinology 103: 1297-1303.
- Nonaka N, Shioda S, Niehoff ML, Banks WA (2003) Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Ther 306: 948-953.
- Ermisch A, Rühle HJ, Kretzschmar R, Baethmann A (1991) On the blood-brain barrier to peptides: specific binding of atrial natriuretic peptide in vivo and in vitro. Brain Res 554: 209-216.
- Kastin AJ, Nissen C, Schally AV, Coy DH (1979) Additional evidence that small amounts of a peptide can cross the blood-brain barrier. Pharmacol Biochem Behav 11: 717-719.
- Kastin AJ, Nissen C, Coy DH (1981) Permeability of blood-brain barrier to DSIP peptides. Pharmacol Biochem Behav 15: 955-959.
- Banks WA (2001) Anorectic effects of circulating cytokines: role of the vascular bloodbrain barrier. Nutrition 17: 434-437.
- Christophe J (1993) Type I receptors for PACAP (a neuropeptide even more important than VIP?). Biochim Biophys Acta 1154: 183-199.
- Nussdorfer GG, Bahçelioglu M, Neri G, Malendowicz LK (2000) Secretin, glucagon, gastric inhibitory polypeptide, parathyroid hormone, and related peptides in the regulation of the hypothalamus-pituitary-adrenal axis. Peptides 21: 309-324.
- Chang TM, Passaro E Jr, Debas H, Yamada T, Oldendorf WH (1984) Influence of cisternal pressure on passage of neuropeptides from the cerebrospinal fluid into the peripheral circulation. Brain Res 300: 172-174.
- Lenz HJ, Klapdor R, Hester SE, Webb VJ, Galyean RF, Rivier JE, et al. (1986) Inhibition of gastric acid secretion by brain peptides in the dog. Role of the autonomic nervous system and gastrin. Gastroenterology 91: 905-912.
- Passaro E Jr, Debas H, Oldendorf W, Yamada T (1982) Rapid appearance ofintraventricularly administered neuropeptides in the peripheral circulation. Brain Res 241: 335-340.
- Guillemin R (1977)The endocrinology of the neuron and the neural origin of endocrine cells. Adv Exp Med Biol 87: 1-12.
- Banks WA, Tschop M, Robinson SM, Heiman ML (2002) Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302: 822-827.
- Martins JM, Banks WA, Kastin AJ (1997) Transport of CRH from mouse brain directly affects peripheral production of ββ-endorphin by the spleen. Am J Physiol 273: 1083-1089.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences