Recent Trends in Pharmaceutical Biotechnology
Karna Venkata Ramana*, Janifer Raj Xavier and Rakesh Kumar Sharma
Defence Food Research Laboratory, Defence Research and Development Organization, Mysore, Karnataka, India
- *Corresponding Author:
- Karna Venkata Ramana
Food Biotechnology Discipline, Defence Food Research Laboratory, Siddarthanagar, Mysore, Karnataka, India.
Tel: +918212473686
Fax: +9182122473468
E-mail: karnavr1@gmail.com
Received date: December 12, 2016; Accepted date: January 24, 2017; Published date: January 27, 2017
Citation: Ramana KV, Xavier JR, Sharma RK. Recent Trends in Pharmaceutical Biotechnology. Pharm Biotechnol Curr Res. 2017, 1:1.
Abstract
The foundations of pharmaceutical biotechnology mainly lie in the capability of plants, microorganism and animals to produce low and high molecular weight compounds useful as therapeutics. Although molecules from plants and microorganisms are preferred extraction from plant biomass needs tedious downstream processing while in case of microorganisms it is easy with fewer amounts of impurities. Pharmaceutical biotechnology is poised to flourish for the last 4-6 decades with the advent of recombinant DNA technology and metabolic engineering supported by the well-developed bioprocess technology. Large scale production and cost effectiveness and affordability could be achieved by way of synergising all these technologies. In the current review importance of microorganism in conjunction with recombinant DNA technology is discussed for the production of biopharmaceuticals and development of therapeutic applications using recently developed molecular methods and mechanisms of disease progression. Bacterial enzymes are being used in applications from drug modification to therapeutic uses.
Keywords
Pharmaceutical biotechnology; DNA technology; Bioprocess technology; Biopharmaceuticals
Introduction
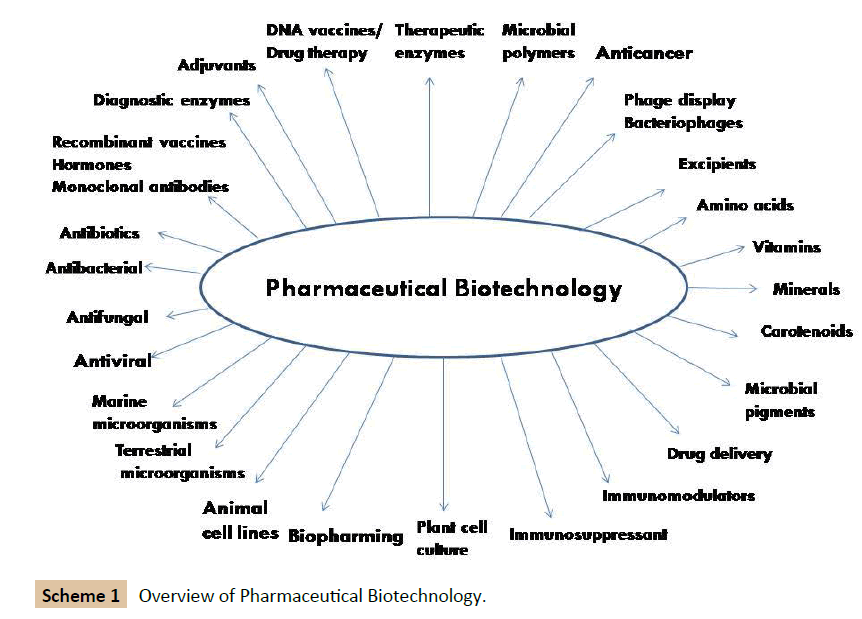
Plant and microbial products, in form of natural preservatives have been known since centuries for preservation of fruits, vegetables and milk in the form of bread, yoghurt, beer, wine, vinegar, cheese, pickles and other fermented materials. After the accidental discovery of the antibiotic molecule penicillin in 1929 by Alexander Fleming, the economic importance of microorganisms gained importance and bioprocesses such as fermentation, bioconversion and enzymatic biotransformation for production of various valuable products from microorganisms were explored [1]. These products included antibiotics, amino acids, enzymes, enzyme inhibitors, nucleotides, vitamins, organic acids, vaccines and polysaccharides with applications in the field of medicine to improve human health. Plants and microorganisms, particularly bacteria, fungi, yeast and microalgae are a vast resource of biopharmaceuticals and could be produced using fermentation process or direct extraction from plant biomass. Secondary metabolites from microorganisms are reported to be important for pharmaceutical applications. Secondary metabolism consists of synthesis of special metabolites possessing unusual chemical structures and is not critical for the growth and development of producing organisms. Secondary metabolites initiation and biosynthesis are possibly due to the deficiency of nutritional growth limiting components. Actinomycetes, mycobacteria, eubacteria, algae, fungi are reported to produce a large repertoire of bioactive showing therapeutic properties. In the last 4-5 decades much attention has been paid for isolation and characterisation of pharmaceuticals from endosymbiotic microorganisms that thrive in plant tissues, sponges and animals. Marine ecosystem has been credited as a source of microorganisms that produce inexhaustible and valuable molecules for application as pharmaceuticals. The areas are even expanding using the knowledge of microbiology, recombinant DNA technology, metabolic engineering combined with synthetic chemical technology (Scheme 1). Even, some complex biomolecules could be synthesised using chemical methods; however, they suffer with inherent drawbacks such as purity, containing isomeric forms and product purification.
Microorganisms have unique characteristics which make them irreplaceable, industry friendly hosts, as they are the real workhorses for large scale production of an array of useful metabolites. Microorganisms have a high ratio of surface area to volume, which facilitates the rapid uptake of nutrients required to support high rates of metabolism and biosynthesis suitable for variety of reactions (Table 1). The microorganisms can be isolated from various ecological niches allowing them to be transplanted from nature to the laboratory flask and ultimately to the production scale fermenter, where it is capable of growing on inexpensive carbon and nitrogen sources and producing valuable compounds. With the development of recombinant DNA technology, microorganisms could be manipulated genetically with ease, to increase production of the products. Although microbes are awfully superior in presenting us with an astonishing array of valuable products, they usually produce them only in small amounts. To overcome this bottleneck, in early 1970s traditional industrial microbiology in combination with molecular biology, tailor made microbial strains have been developed for large scale production. The modern biotechnology industry has made a major impact in the business world; the biopharmaceuticals (recombinant protein drugs, vaccines and monoclonal antibodies) have a market of around 15 billion dollars. As a result of technological improvements in screening programs, separation and product purification techniques, the number of natural compounds discovered so far is estimated to be more than one million. From the 22500 biologically active compounds that have been obtained so far from microbes, 45% are produced by Actinomycetes, 38% by fungi and 17% by unicellular bacteria, it is obvious that most microbial products are made by fermentation technology. Despite the efficiency of the chemical route to riboflavin synthesis, much of the production of this compound is carried out currently by fermentation. Most natural products are so complex and contain so many centres of asymmetry that they probably will never be made commercially by chemical synthesis [2,3]. Several biopharmaceuticals are produced using recombinant DNA technology as they are safer and are effective than conventionally produced molecules and also to make them cost-effective. For instance insulin was proved effective and safer in comparison to the insulin obtained from animal sources. The biopharmaceuticals obtained through recombinant DNA technology include recombinant insulin, interferon’s, hepatitis B vaccine, somototrophin, etc., A large number of biopharmaceuticals are produced and marketed by biotechnology companies which come under one of the below mentioned categories.
| S. No. | |
|---|---|
| 1 | Cytokines |
|
|
|
|
|
|
|
|
| 2 | Enzymes |
|
|
|
|
|
|
| 3 | Hormones |
|
|
|
|
|
|
| 4 | Clotting factors |
|
|
|
|
| 5 | Vaccines |
|
|
|
|
|
|
| 6 | Monoclonal antibodies |
| MoAb muromonab-CD3 (treatment of immune system rejection) | |
| MoAb Infliximab cA2 (treatment of Crohn’s Disease) | |
| 7 | Enzyme inhibitors |
| Clavulinic acid | |
| Ancovenin | |
| Streptovaricin | |
| Streptonigrin | |
| Lovastatin | |
| Monacolin K | |
| 8 | Immunosuppressors |
| Cyclosporin | |
| Rapamycin | |
| 9 | Polyamino acids |
| Epsilon poly-L-lsine | |
| Polyglutamic acid | |
| cyanophycin |
Table 1 Some of the Biopharmaceutical categories.
Bacterial polysaccharides as pharmaceuticals
Microbial exopolysaccharides (EPS) are biopolymers synthesized by several microorganisms which include several genera of bacteria, molds and yeasts. These are gum like polymers synthesized by these organisms and released into the surrounding environment. EPS mainly protects the microorganisms from the surrounding environment and also acts as a reserve food material, and the producing organisms can use this as a main carbon source. Based on the sugar composition, EPS are classified as homopolymers (with a single type of sugar, glucose, or xylose,) and heteropolymers (with more than one type of sugar moieties, glucose, rhamnose, mannose, etc.,). Based on the presence or absence of uronic acid, EPS are categorized as acidic or neutral EPS, respectively. Some of these EPS can form a film, some form gels, some more can increase the viscosity of solutions. Due to their varied functional properties, microbial EPS found applications in various fields such as agriculture, cosmetics, food, oil recovery, packaging, textile, wastewater treatment, pharmaceuticals, medicine, and in the form of membranes. Several health benefits such as antitumor activity, anti-atherosclerotic effect, immunomodulation activity, and prebiotic effect of lactic acid bacteria and other microbial EPS are reviewed recently [4,5]. Antarctic bacterium Pseudoalteromonas sp. S-5 producing an EPS, showing anticancer activity, was recently reported [6]. Gellan finds a variety of applications as an ingredient of oral, ophthalmic, and nasal formulations, tissue engineering, and dressing material [7]. Applications of xanthan gum has been reviewed in drug delivery, due to its potential in retarding the drug release, in the form of liposomes, hydrogel, niosomes, nanoparticles, matrix system, or microspheres [8]. Recently, phosphorylated curdlan micro gels were prepared for in vitro drug release, and these micro gels were found to show excellent biocompatibility [9]. The production and applications of microbial celluloses in medical and pharmaceutical fields was reviewed [10]. Bacterial cellulose is considered to be a best alternative to wound dressing material due to its water-holding capacity, porosity, and efficient barrier properties as well as nanofiber material. Several investigations have been reported on application of bacterial cellulose as artificial skin or membrane or as wound dressing material over burn injuries. Some studies were carried out to use bacterial cellulose as excipient and as to use it as slow drug release material in the field of pharmaceutical biotechnology. The authors have carried out extensive research on the production of bacterial cellulose produced by Gluconacetobacter xylinus for various applications (Figure 1). Derivatization increased functionality of levan in terms of increased reducing power, scavenging activity, antioxidant, and anticancer activity [11]. At 0.1 to 1.0% concentrations, levan is an excellent immunostimulant in fishes [12]. Fructooligosaccharides derived from acid hydrolysis of levan are considered as prebiotic agents [13]. Levan has several medical applications such as an anticlotting factor in heart surgery, healing wounds, after angioplasty anti-AIDS agent, and in the subcutaneous dental filling [14].
Algal pharmaceuticals and therapeutics
Microalgae possess unique characteristics compared to conventional microorganisms, such as photosynthetic ability and massive species and bio product diversity. As such, algae are highly attractive candidates for application as cell factories. Macroalgal pigments also have demonstrated their lack of toxicity and biological activity in a wide range of biological applications, including prevention of acute and chronic coronary syndromes, atherosclerosis, rheumatoid arthritis, muscular dystrophy, cataract and neurological disorders. They are also recommended to protect the skin and eyes against UV radiation [15]. Lutein is one of the major xanthophylls found in green microalgae. It is accumulated in the macula of the human retina and elicits protection for the eyes from oxidative stress, and acts as a filter of the blue light preventing macular degeneration and age-related cataract [16-18]. Because of their antioxidant and anti-inflammatory activity, most microalga pigments have neuroprotective effects in cultured rat cerebellar neurons, and hepatoprotective effects in hepatocytes grown in vitro (e.g., phycocyanin, phycoerythrin). Antiviral and antifungal activities were noticed with allophycocyanin and phycocyanin [19]. In addition to the utilization of microalgal biomass rich in proteins and minerals for food and feed purposes, there are beneficial special compounds produced by these organisms, such as pigments, enzymes, sugars, lipids with valued fatty acids, sterols, and vitamins i.e., β-carotene, thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folic acid. Additionally, the generation of other scarce bioactive compounds, displaying immune mediation, anticancer, anti-inflammatory and antibiotic activity, is reported [20-22]. Algae can act as chemical platforms for cosmetic purposes (e.g., coloring pigments and especially anti-aging skin supplements. Extracts from Chlorella vulgaris are reported to support collagen repair in addition to pharmaceutical and therapeutic applications [23]. The exploitation of microalgae for special metabolites is highly attractive because these frequently display exceptionally high market values. Sulphated polysaccharides extracted from marine algae were found to be anti-oxidant, anti-coagulant, anti-inflammatory, anti-viral, antibacterial, anti-tumour, immunomodulatory and radio-protective.
Microalgae produce a variety of compounds for adaptation and survival at different environmental conditions. Many marine microalgal strains produce a high percentage of total lipids (up to 30-70% of dry weight) [24]. The accumulation of fatty acids is closely linked to microalgal growth stages, functioning as an energy stockpile during unfavourable conditions or cell division. Omega-3 fatty acids are accumulated due to its high energy content, as well as the good flow properties crucial for cellular functions [25,26]. To date, the ω-3 fatty acid content of numerous microalgae strains have been studied. Strains from the genera Phaeodactylum, Nannochloropsis, Thraustochytrium and Schizochytrium have demonstrated high accumulation of Eicosapentaenoic acid (EPA) and/or Decosahexaenoic acid (DHA). Phaeodactylum tricornutum and Nannochloropsis sp. demonstrated an EPA content of up to 39% of total fatty acids, whereas the strains such as Thraustochytrium and Schizochytrium limacinum contained a DHA percentage of 30–40% of total fatty acids. Omega-3 fatty acids represent an important structural component of human cell membranes, particularly neuronal cells [27-31]. The consumption of EPA and DHA supplements has been shown to prevent cardiovascular, nervous system and inflammatory diseases. Regular consumption of ω-3 fatty acids reduce the risk of hypertension, thrombosis, myocardial infarction and cardiac arrhythmias due to the increase in the high-density lipoprotein/low-density lipoprotein (HDL/LDL) ratio and decrease the total cholesterol/ HDL ratio [32]. In addition to cardiovascular benefits, omega-3 fatty acids have also demonstrated positive effects on brain function and the nervous system (Table 2). In pregnant women, the adequate intake of EPA and DHA is crucial for healthy development of the fetal brain [33,34]. In infants, arachidonic acid (ARA), an omega-6 fatty acid, and DHA were found to be essential for normal growth and functional development [35]. The increased consumption of DHA may also diminish the severity of depression [36]. Immunomodulatory effects have been observed when ω-3 fatty acids were used in the treatment of inflammatory conditions such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, asthma, lupus and cystic fibrosis [37-39]. There is currently a large demand for microalgae in the nutraceutical and pharmaceutical industry due to their health-promoting effects. Macroalgalderived PUFA, such as ARA and DHA are added as fortifications to infant formulae an industry that is worth $10 billion per year. To date, microalgal extracts can be found in many face and skin care products, e.g., anti-aging cream, refreshing or regenerative care products, sun cream, emollient and anti-irritant in peelers [40]. Dermochlorella is extracted from Chlorella vulgaris, which is known to stimulate collagen synthesis in skin supporting tissue regeneration and wrinkle reduction [41]. Protulines is a proteinrich extract from Arthrospira (Spirulina), which helps combat early skin aging, exerting a tightening effect and preventing wrinkle formation [42].
| Bioactive Compound | Source | Pharmaceutical application |
|---|---|---|
| Polyunsaturated fatty acids (PUFA) | ||
| Eicosa pentenoic acid (EPA) | Isochrysis galbana, Nannochloropsis oculata, Nitzschia laevis, Phaeodactylum cornutum, Porphyridium cruentum, Porphyridium purpureum, Phaeodactylum tricornutum |
Nutritional supplement, heart diseases, lowers blood pressure, lowering plasma levels of cholesterol and other lipids, anti-thrombosis and anti-arthrosclerosis. |
| Docosa hexaenoic acid (DHA) | Crypthecodinium cohnii, Pavlova lutheri, Schizochytrium limacinum, Ulkania sp. |
Important for brain and eye development during fetus stage and in children, adult dietary supplement to improve cardiovascular health and cancer prevention. |
| Eicosatetraenoic acid (arachidonic acid, ARA) | Porphyridiumsp. | Nutritional supplement, Anti-inflammatory, muscle anabolic formulations. |
| Octadecatrienoic acid (γ-linolenic acid, GLA) | Spiriulinasp. | Nutritional supplement, Anti-inflammatory useful to suppress tumor growth and metastasis. |
| Pigments | ||
| Marrenine | Haslea ostrearia | Anti-proliferative effect on lung cancer model, antiviral and anticoagulant properties. |
| Pyropheophytin a fucoxanthin (Fuco) |
Brown macroalgae such as Eicenia bicyclis, Hijikia fusiformis |
Hepatoprotective |
| Phycoerythrin | Brown macroalgae such as Eicenia bicyclis, Hijikia fusiformis |
Hepatoprotective |
| β-carotene | Dunaliella salina, Dunaliella bardawil, Botryococcus braunii |
Pro-vitamin A, antioxidant, food additive. |
| Microcolins A and B | Cyanaobacter | Immuno suppresive activity |
| Vitamin E | Euglena gracilis | Vitamin supplement. |
| Lutein | Green microalgae Chlorella protothecoides, Chlorella zofingiensis, Botryococcus braunii, Chlorococcum citriforme, Dunaliella salina, Muriellopsis sp., Neospongiococcum gelatinosum |
Treatment of age-related cataract, anti-macular degeneration, anti-colon cancer, cosmaceutical. |
| Phycoerythrin | Cyanobacteria, Porphyridium | Immunofluorescent techniques, labelled antibodies, receptors and other biological molecules. |
| Phycocyanin | Spirulina(Cyanobacteria) | Cosmetics, immunofluorescent techniques, antibody label. |
| Zeaxanthin | Botryococcus braunii, Dunaliella salina, Nannochloropsis oculata, Nannochloropsis gladitana |
Anti-colon cancer, eye health. |
| Astaxanthin | Haematococcus pluvialis | Anti-colon cancer, eye health. |
| Amino acids | ||
| Amino acids | Diatom | Dermatological applications and as cosmaceuticals. |
| Mycosporine-like amino acids (MAAs) | Microalgae | Sunscreens to reduce UV-induced damage and as ROS scavengers. |
| Vitamins | ||
| α-Tocopherol | Chlorella sp., Nannochloropsis oculata, Stichococcus bacillaris, Euglena gracili. |
Vitamin E, food additive, antioxidant and cosmaceuticals. |
| Polysaccharides | ||
| Polysaccharides | Red microalgae such as Porphyridium sp., |
Antiviral activity. |
| ß-1,3-glucan | Chlorella | Immune-stimulator, antioxidant and reduction of blood cholesterol |
| Alginates, cellulose, or carrageenan | Rhodophyta group | Several pharmaceutical applications. |
| Sulfated exopolysaccharide | Cytotoxic effect towards human cancer cell lines | |
Table 2 Algal pharmaceuticals and therapeutics.
For deployment as oral vaccines, some algae are permitted and they have been certified as generally recognized as safe (GRAS) for human consumption by the Food and Drug Administration. In addition, algal vaccines offer a number of unique advantages over conventional plant therapeutics, such as rapid growth (relative to terrestrial plants), minimal environmental impact from lateral gene transfer, and minimal processing requirements [43]. Algal plastid production of fully active, monoclonal human antibodies was first reported in C. reinhardtii chloroplasts [44]. The RuBisCO large subunit (rbcL) and plastidial ATP synthase (atpA) promoters were utilized in conjunction with rbcL 50 and 30 UTR sequences for plasmid construction, generating a single-chain antibody targeting glycoprotein D of the herpes simplex virus. In another development, production of a chimeric protein composed of a mucosal adjuvant (CtxB) and a malaria transmission-blocking vaccine candidate (Pfs25) was demonstrated in C. reinhardtii [45]. Oral vaccination in mice was found to elicit both IgG and IgA antibodies’ production. Tran and colleagues recently built upon this work to express single chain antibodies targeting the B cell surface antigen CD22 fused to an immunotoxin. Fusion protein produced dimeric immunotoxins capable of binding and reducing B-cell lymphoma viability [46,47].
Microbes and their metabolites as drug and enzyme delivery systems
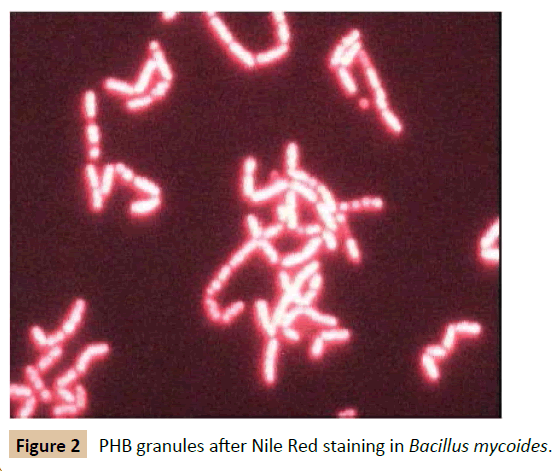
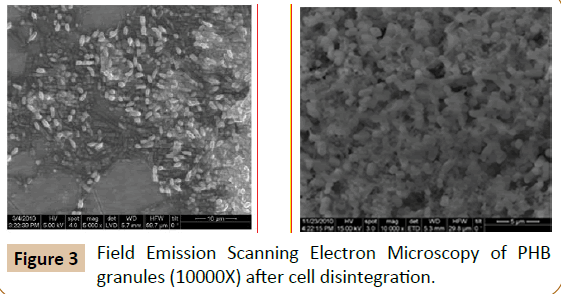
To achieve efficient and biocompatible targeted drug delivery, various technologies have been developed to deliver chemotherapeutic agents, antibiotics, therapeutic proteins, and biomolecules. One novel aspect could be the application of biofilm formation by drug-carrying bacteria to deliver drugs/ antibiotics to the site of infection. Biofilm formation in the human body via various commensals is very common. These commensal biofilms can be productively used to deliver various compounds such as drugs, enzymes, etc. [48]. A non-pathogenic commensal surviving in the human system as a biofilm can be further augmented with the population enriched with specific enzyme-secreting engineered bacteria. The same principle can be applied to eliminate biofilm via targeted delivery of alginate lyase or mucinase-overproducing bacterial strains (Table 3). A chimeric E. coli strain expressing the glucan digesting enzymes mutanase and dextranase, which decompose the Streptococcus mutans biofilm under in vitro conditions was recently designed by Otsuka [49]. Previous reports claim the treatment of murine colitis by implanting the engineered Lactococcus lactis -secreting interleukin-10 in mice colon [50]. Other E. coli engineered systems were used for gut inflammation [51]. In an identical study, the attenuated strains of Salmonella typhimurium are genetically modified to trigger the production of the tumour necrosis factor related apoptosis- inducing ligand (TRAIL) under a hypoxiainduced targeted promoter system nirB [52]. When administered, this strain specifically targeted the malignant melanoma of mice. Various bacteria invade tumours and have been engineered to destruct them by releasing a chemotherapeutic prodrug, via secretion of TNFα and cytokines [53-55]. However, a synthetic biological approach would allow designing a bacterium rationally with desired capabilities and safety requirements. The Voigt lab constructed an E. coli strain that senses a low-oxygen microenvironment such as found in tumour tissues. Hypoxia is the cue in this particular E. coli strain to upregulate a Yersinia pseudotuberculosis adhesin protein (invasin) sufficient for E. coli to invade mammalian cells. Because invasion is only efficient from a certain cell density, the Voigt lab further equipped this E. coli with a second sensor including another genetic circuit that senses cell density (quorum sensing circuit from Vibrio fischeri) [56]. Thus, this E. coli strain should specifically invade tumour cells in a population density-dependent manner. The authors have carried out research on the production of polyhydroxybutyrate (PHB) by B. mycoides which is useful for biomedical applications such as surgical threads, slow release of antibiotics, medical disposables and as scaffolds with slow drug release functions (Figures 2-4).
| Recombinant therapeutic proteins | Source | Pharmaceutical Application |
|---|---|---|
| Artemisinin | Recombinant E. coli | Anti-malarial drug. |
| Paclitaxel (Taxol) | Recombinant E. coli | Anti-cancer agent anti-neoplastic drug. |
| Eleutherobin | Recombinant E. coli | Anti-cancer agent. |
| Erythromycin and Epothilone C and D |
Recombinant E. coli | Anti-cancer drugs. |
| Aromatic bacterial polyketides |
Recombinant E. coli | Precursor to several antitumor polyketides. |
| Humulin | Recombinant E. coli | Diabetes. |
| Protropin | Recombinant E. coli | hGH deficiency. |
| Roferon A | Recombinant E. coli | Hairy cell leukemia Cancer, genital warts and hepatitis. |
| Interferon alpha | Human lymphoblastoid cells |
AIDS-related Kaposi’s sarcoma, multiple myeloma, non-Hodgkin lymphoma. |
| Interferon alpha-2a | Recombinant E. coli | Treatment of Kaposi’s sarcoma, follicular lymphoma, cutaneous T-cell lymphoma, melanoma, chronic myelocytic leukemia, kidney cancer. |
| Interferon alpha-2b | Recombinant E. coli | Treatment for cancers, pancreatic melanoma, non-Hodgkin lymphoma, leukemia, hairy cell leukaemia, renal cell carcinoma, multiple myeloma, follicular lymphoma. |
| Interferon alpha-1b | Recombinant E. coli | Renal cell carcinoma, hairy cell leukemia. |
| Interferon gamma-1a | Recombinant E. coli | Kidney cancer. |
| Tasonermin | Recombinant E. coli Natural Cytokine | Soft tissue sarcoma |
| Molgramostim | Recombinant E. coli | Myelo dysplastic syndrome |
| Nartograstim | Recombinant E. coli | Solid tumour |
| Filgrastin | Recombinant E. coli | Stimulates hematopoiesis and treatment for neuatropenia. |
| Humatrope | E. coli | hGH deficiency |
| Recombivax | Recombinant Saccharomyces cerevisiae | Hepatitis B |
| Intron A Orthoclone OKT3 | Hybridoma cell line | Reversal of acute kidney and transplant rejection |
| Denileukin diftitox | E. coliFusion protein | Cutaneous T-cell lymphoma |
| Activase | CHO cells | Acute myocardial infarction |
| Epogen | CHO cells | Anaemia |
| Trastuzumab biosimilar | CHO cells | Breast and gastric cancer. |
| Thyrotropin alpha | CHO cells | Thyroid cancer |
| Darbepoetin α (Aranesp) | CHO cells | Red blood cell production. |
| Epoetin α (Binocrit) | CHO cells | Stimulates production of RBCs. |
| Hyaluronic acid/ Hyaluronan | Bacillus subtilis | Cell therapy, tissue engineering and regenerative medicine. |
Table 3 Recombinant therapeutic proteins from microorganisms.
Recombinant therapeutic proteins from microorganisms
Since the production of recombinant insulin in the late 70s, the emergence of molecular biology and biotechnology has enabled the biological fabrication of a long list of active therapeutic proteins. Today, recombinant DNA and hybridoma cell technologies are mainstream platforms to obtain most of the currently marketed protein drugs such as monoclonal antibodies, hormones, cytokines and growth factors [57,58]. Over 200 protein drugs are expected to be available over the next few years to treat expanding human disorders such as diabetes, cancer, respiratory, cardiovascular and inflammationrelated diseases, as well as other rare diseases. In fact, the global market for protein drugs already exceeds US $50 billion, with an average annual growth rate of almost 4%, according to BCC Research. Unfortunately, the costs of protein drugs are often extremely high. As a representative example, recombinant human Erythropoietin (EPO), which is used as treatment for anaemia due to kidney failure or anticancer treatments costs over 2 billion US $ per kg, probably being the most expensive existing substance today. Enzyme replacement therapies such as for lysosomal storage diseases (LSD) representing excess of 0.15 million $ per year per patient. Such high costs are partially explained not only by the investment in product development but also by the expenses associated to quality analysis and control [59,60]. In addition, the immature state of the current production methods raises manufacturing costs to a level often unaffordable from an industrial point of view. With the exception of short peptides, suitable to be produced by chemical synthesis, protein pharmaceuticals have to be produced in living cells or transgenic organisms, poses important challenge to industriallyscaled production [61]. Manufacturing a recombinant protein or an antibody might represent up to 25% of the global sale figures, imposing a strong need to find cost-effective alternatives to the current recombinant fabrication systems [62,63]. Among the different steps of recombinant protein production, downstream processing might impose a load of up to 80% of the total process cost, a figure that can be slightly reduced by adapting the equipment, specially moving from steel bioreactors to disposable tanks [64,65]. Interestingly, the major factor influencing the process cost is the biological platform used as cell factory [66]. On the other hand, many protein drugs are often unstable during production and/or purification and tend to aggregate [67]. Systemic administration of protein drugs is especially sensitive to aggregation, which can also occur during storage or in a form of unnoticed soluble aggregates. Many side effects and undesired immunoreactions have been found to be linked to protein instability and aggregation in the body [68-70]. Improving protein solubility represents a continuous challenge in the development of protein drugs [71]. As a result, biopharmaceuticals still suffer from batch-to-batch conformational heterogeneity, a currently unavoidable disadvantage inherent to recombinant biological production. These drawbacks complicate the consideration of proteins as clinical drugs from the regulatory point of view. Moreover, proteins with therapeutic value often undergo posttranslational modifications necessary for the natural biological function.
Conclusion
Plants, animals and microorganisms have become dispensal source for the production of pharmaceuticals. Current, microbial sources, either wild strains are developed bu recombinant DNA technology, conventional mutagenesis or metabolic engineering, are mostly preferred for the production of biopharmaceuticals under various categories (Table 1). For the production of monoclonal antibodies or large number high value biopharmaceuticals and vaccines animal cell lines are being used in production scale fermenters to achieve costeffective and to address safety concerns. The biopharmaceutical technology is mammoth field including biopharmaceuticals to cure and prevent diseases such as cancer, cardiovascular problems and growth retardation in children, to treat viral, bacterial and mycotic infections. The repertoire of therapeutic molecules is further burgeoning using microorganisms, plants and animals from marine and fresh water sources. Further the endosymbiotic microorganisms from plants, sponges and animals have enhanced the prospects of finding a plethora of bioactive molecules. The overall, biopharmaceutical production platform includes microorganisms, animal cell lines, plants and animals. In the near future metabolic engineering and synthetic biology would further augment drug discovery and biopharmaceutical production to treat diseases effectively.
References
- Fleming A (1929) On the antibacterial action of cultures of Penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol 10: 226-236.
- Lenz J (2004) Biotechnological advantages of laboratory-scale solidstate fermentation with fungi. Appl Microbiol Biotechnol 64: 175-186.
- Berdy J (2005) Bioactive microbial metabolites. A personal view. J Antibiot 58: 1-26.
- Cragg GM, Newman DJ, Snader KM (1997) Natural products in drug discovery and development. J Nat Prod 60: 52-60.
- Madhuri KV, Vidya Prabhakar K (2014) Microbial exopolysaccharides: biosynthesis and potential applications. Oriental J Chem 30: 1401-1410.
- Patten DA, Laws AP (2014) Lactobacillus -produced exopolysaccharides and their health benefi ts: a review. Benefi cial Microbes (in-press).
- Chen G, Qiana W, Lia J, Xua Y, Chena K (2015) Exopolysaccharide of Antarctic bacterium Pseudoaltermonas sp. S-5 induces apoptosis in K562 cells. Carbohydr Polym 121: 107-114
- Osmałek T, Froelich A, Tasarek S (2014) Application of gellan gum in pharmacy and medicine. Int J Pharm 466: 328-340.
- Benny IS, Gunasekar V, Ponnusami V (2014) Review on application of xanthan gum in drug delivery. Int J PharmTech Res 6: 1322-1326.
- Popescua I, Pelina IM, Butnarub M, Fundueanua G, Sufl DM, et al. (2013) Phosphorylated curdlan microgels. Preparation, characterization, and in vitro drug release studies. Carbohydr Polym 94: 889-898.
- Chawla PR, Bajaj IB, Survase SA, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 47: 107-124.
- Liu J, Luo J, Ye H, Zeng X (2012) Preparation, antioxidant and antitumor activities in vitro of different derivatives of Levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem Toxicol 50: 767-772.
- Gupta SK, Das P, Singh SK, Akhtar MS, Meena DK, et al. (2011) Microbial levan, an ideal prebiotic and immunonutrient in aquaculture. World Aquacult 42: 63-66.
- Huang MY, Lee CF, Ho ST, Lin KJ, Pan CL (2013) Highyield levan produced by Bacillus licheniformis FRI MY-55 in high-sucrose medium and its prebiotic effect. J Pure Appl Microbiol 7: 1585-1599.
- Srikanth R, Reddy CHSSS, Siddartha G, Ramaiah MJ, Uppuluri KB (2015) Review on production, characterization and applications of microbial levan. Carbohydr Polym 120: 102-114.
- Sies H, Stahl W (2004) Nutritional protection against skin damage from sunlight. Annu Rev Nutr 24: 173-200.
- Hashimoto T, Ozaki Y, Mizuno M, Yoshida M, Nishitani Y, et al. (2011) Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. The British journal of nutrition 107: 1-4.
- Alves-Rodrigues A, Shao A (2004) The science behind lutein. Toxicol Lett 150: 57-83.
- Granado F, Olmedilla B, Blanco I (2003) Nutritional and clinical relevance of lutein in human health. Brit J Nutr 90: 487-502.
- Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J Appl Phycol 20: 113-136.
- Abed RMM, Dobretsov S, Sudesh K (2009) Applications of cyanobacteria in biotechnology. J Appl Microbiol 106: 1-12.
- Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12: 499-506.
- Pasquet V, Cherouvrier JR, Farhat F, Thiery V, Piot JM, et al. (2011) Study on the microalgal pigments extraction process: performance of microwave assisted extraction. Process Biochem 46: 59-67.
- Bumbak F, Cook S, Zachleder V, Hauser S, Kovar K (2011) Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl Microbiol Biotechnol 91: 31-46.
- Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of production. Process Biochem 40: 3627-3652.
- Tiez L, Zeiger E (2010) Plant physiology. 5th edn. Sunderland. Sinauer Associates Inc Publishers.
- Cohen Z, Khozin-Goldberg I, Adlerstein D, Bigogno C (2000) The role of triacylglycerol as a reservoir of polyunsaturated fatty acids for the rapid production of chloroplastic lipids in certain microalgae. Biochem Soc Trans 28: 740-744.
- Derrien A, Coiffard LJM, Coiffard C (1998) De Roeck-Holtzhauer, Y. Free amino acid analysis of five microalgae. J Appl Phycol 10: 131-134.
- Sukenik A (1991) Ecophysiological considerations in the optimization of eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour Technol 35: 263-269.
- Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72: 1161-1169.
- Zhu L, Zhang X, Ji L, Song X, Kuang C (2007) Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochem 42: 210-214.
- Brunner E (2006) Oily fish and omega 3 fat supplements. BMJ 332: 739-740.
- Sijtsma L, Swaaf ME (2004) Biotechnological production and applications of the n-3 polyunsaturated fatty acid docosahexaenoic acid. Appl Microbiol Biotechnol 64: 146-153.
- Horrocks LA, Yeo YK (1999) Health benefits of docosahexaenoic acid (DHA). Pharmacol Res 40: 211-225.
- Simopoulos AP, Bazan NG, Karger S (2009) Omega-3 fatty acids, the brain and retina. Karger.
- Damude HG, Kinney AJ (2008) Enhancing plant seed oils for human nutrition. Plant Physiol 147: 962-968.
- Dyerberg J, Leaf A (1995) GA CISSFAL board statement: recommendations for the essential fatty acid requirement for infant formulas. J Am Coll Nutr 14: 2.
- Hibbeln JR, Salem N Jr (1995) Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr 62: 1-9.
- Oren A, Gunde-Cimerman N (2007) Mycosporines and mycrosporine-like amino acids: UV protectants or multipurpose secondary metabolism? FEMS Microbiol Lett 269: 1-10.
- Simopoulos AP (1991) Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 54: 438-463.
- Calder PC (1996) Sir david cuthbertson medal lecture. Immunomodulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Proc Nutr Soc 55: 737-774.
- Braud JP (1997) Simultaneous culture in pilot tanks of the macroalga Chondrus crispus (Gigartinaceae) and the microalga Odontella aurita (Eupodiscaceae) producing EPA. Marine Microorganisms for Industry; Le Gal Y, Muller-Feuga A (Eds) Ifremer: Plouzane pp: 39-47.
- Exymol SAM: Protulines: Spirulina extract (2012) In Exymol SAM. Edited by. Monaco.
- Specht E, Miyake-Stoner S, Mayfield S (2010) Micro-algae comes of age as a platform for recombinant protein production. Biotechnol Lett 32: 1373.
- Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci USA 100: 438.
- Gregory JA, Topol AB, Doerner DZ, Mayfield S (2013) Alga produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl Environ Microbiol 79: 3917.
- Tran M (2013) Production of anti-cancer immunotoxins in algae: ribosome inactivating proteins as fusion partners. Biotechnol Bioeng 110: 2826.
- Tran M (2013) Production of unique immunotoxin cancer therapeutics in algal chloroplasts. Proc Natl Acad Sci USA 110: E15.
- Clasen J, Michael AF (2014) Synbiotic microbes as drug delivery systems. ACS Synthetic bio 4: 358-364.
- Carpentar J, Randolph TW. Journal of pharmaceutical sciences 98: 3247-3268.
- Steidler L, Wolfing H, Lieven S, Sabeine N, Florian O, et al. (2000) Treatment of Murine Colitis by Lactococcus lactis Secreting Interleukin-10. Science 289: 1352-1355.
- Archer EJ, Robinson, Suel GM (2012) Engineered e.coli that detect and respond to gut inflammation through nitric oxide sensing. ACS Synthetic biology 19: 451-457.
- Chen J, Yang Cheng X, Qiau Y, Tang Chen G, Wei J, et al. (2011) Samonella mediated tumor targeting TRAIL gene theraphy significantly suppresses melanoma growyh in mouse model. Cancer Science 103: 325-333.
- Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, et al. (2003) Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther 10: 737-744.
- Nuyts S, Van Mellaert L, Theys J, Landuyt W, Bosmans E, Anne J, Lambin P (2001) Radio-responsive recA promoter significantly increases TNF alpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther 8: 1197-1201.
- Murray PJ, Aldovini A, Young RA (1996) Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette–Guerin strains that secrete cytokines. Proc Natl Acad Sci USA 93: 934-939.
- Anderson JC, Clarke EJ, Arkin AP, Voigt CA (2006) Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol 355: 619-627.
- Ferrer-Miralles N, Domingo-Espin J, Corchero JL, Vazquez E, Villaverde A (2009) Microbial factories for recombinant pharmaceuticals. Microb Cell Fact 8: 17.
- Walsh G (2010) Biopharmaceutical benchmarks 2010. Nat Biotechnol 28: 917-924.
- Eon-Duval A, Broly H, Gleixner R (2012) Quality attributes of recombinant therapeutic proteins: an assessment of impact on safety and efficacy as part of a quality by design development approach. Biotechnol Prog 28: 608-622.
- Medrano G, Dolan MC, Condori J, Radin DN, Cramer CL (2012) Quality assessment of recombinantproteins produced in plants. Methods Mol Biol 824: 535-564.
- Kemsley JN (2009) Analyzing Protein Drugs. Chem Eng News 87: 20-23.
- Chadd HE, Chamow SM (2001) Therapeutic antibody expression technology. Curr Opin Biotechnol 12: 188-194.
- Farid SS, Washbrook J, Titchener-Hooker NJ (2005) Decision-support tool for assessing biomanufacturing strategies under uncertainty: stainless steel versus disposable equipment for clinical trial material preparation. Biotechnol Prog 21: 486-497.
- Roque AC, Lowe CR, Taipa MA (2004) Antibodies and genetically engineered related molecules: production and purification. Biotechnol Prog 20: 639-654.
- Rouf SA, Moo-Young M, Scharer JM, Douglas PL (2000) Single versus multiple bioreactor scale-up: economy for high-value products. Biochem Eng J 6: 25-31.
- Vazquez (2011) Post production protein stability: Trouble beyond cell factories. Microbial cell factories 10: 60.
- Antosova Z, Mackova M, Kral V, Macek T (2009) Therapeutic application of peptides and proteins: parenteral forever. Trends Biotechnol 27: 628-635.
- Antonelli G (2008) Reflections on the immunogenicity of therapeutic proteins. Clin Microbiol Infect 14: 731-733.
- De Groot AS, Scott DW (2007) Immunogenicity of protein therapeutics. Trends Immunol 28: 482-490.
- Garcia-Fruitos E, Vazquez E, Gonzalez-Montalban N, Ferrer-Miralles N, Villaverde A (2011) Analytical approaches for assessing aggregation of protein biopharmaceuticals. Curr Pharm Biotechnol 10: 60.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences