Radiological Imaging of Drug-Induced Pulmonary Lesions
D’souza M*, Rajiah P, Khan A and Irion K
Department of Radiology, Pennine Acute Trust Hospitals, England
- *Corresponding Author:
- Melosa D'souza
Department of Radiology
Pennine Acute Trust Hospitals, England
Tel: 07557091580
E-mail: melosadsouza@doctors.org.uk
Received date: January 31, 2018; Accepted date: February 10, 2018; Published date: February 18, 2018
Citation: D’souza M, Rajiah P, Khan A, Irion K (2018) Radiological Imaging of Drug-Induced Pulmonary Lesions. J Clin Radiol Case Rep Vol.2 No.1:02.
Copyright: © 2018 D’souza M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
There are many radiological manifestations of lung injury secondary to medication. Many of these pathologies have overlapping patterns on imaging, so classification can be a challenge. The most common presentations on high resolution CT are described in this pictorial essay. Also depicted are the common causative agents and their associated patterns of lung injury.
Keywords
Drug-induced; Lung lesions; Pulmonary lesions; Thorax; High resolution CT; Chest; Iatrogenic
Introduction
Many pharmacological agents have the potential to cause lung damage. This damage can either be acute or chronic. More than 100 cytotoxic and non-cytotoxic agents have been reported to cause pulmonary toxicity, and as more pharmaceuticals are developed, the number of causative agents is increasing. Other causes of lung injury must be ruled out before diagnosing drug-induced pulmonary toxicity, however, it should be considered in patients with new onset or progressive, respiratory complaints that correlate with the introduction of a new medication and improve on cessation of the drug.
There are different histopathological processes associated with different drugs and these relate to differing radiological manifestations. The patterns more commonly identified include diffuse alveolar damage (DAD), non-specific interstitial pneumonia (NSIP), hypersensitivity pneumonitis, eosinophilic pneumonia, bronchiolitis obliterans organizing pneumonia (BOOP), non-cardiogenic pulmonary oedema, pulmonary haemorrhage, acute respiratory distress syndrome (ARDS) and pulmonary veno-occlusive disease. This paper aims to characterize the different imaging patterns of pulmonary lesions by their underlying pathological processes. The paper also demonstrates lung injuries associated with common pharmaceuticals [1].
Patterns of Drug Induced Lung Damage
Diffuse Alveolar Damage (DAD)
This is a common manifestation of drug-induced lung injury. In the first week, there is an acute exudative-phase injury resulting in alveolar and interstitial oedema and hyaline membranes. After the first week, there is a reparative phase, characterized by proliferation of type II pneumocytes and interstitial fibrosis.
Those affected usually present with non-specific features of fever, cough and dyspnoea. Further lung function testing may reveal decreased carbon monoxide diffusing capacity. Chest radiographs demonstrate diffuse bilateral alveolar opacities in middle and lower zones. High resolution computed tomography (HRCT) shows diffuse or scattered areas of ground glass opacity, known as honeycomb lung. The resulting fibrosis can improve, stay stable, or worsen. Fibrosis lasting for as little as 1 week has the potential to progress to honeycomb lung.
Diffuse alveolar damage is commonly caused by cytotoxic chemotherapy agents such as: bleomycin, cyclophosphamide, carmustine, and mitomycin. Other iatrogenic causes may be found in Table 1.
| Lung Pathology | Causative Drugs |
|---|---|
| Diffuse alveolar damage | Bleomycin, Busulfan, Cyclophosphamide, Carmustine, Mitomycin, Melphalan, Gold salts |
| Non-specific interstitial pneumonia | Amiodarone, Methotrexate, Chlorambucil, Carmustine, Leflunomide |
| Hypersensitivity pneumonitis | Methotrexate, Sulfasalazine, Nitrofurantoin, Procarbazie, Gold salts |
| Eosinophilic pneumonia | NSAIDs, Nitrofurantoin, Para amino salicylic acid, Penicillamine, Sulfasalazine, ACE inhibitors |
| Bronchiolitis obliterans organizing pneumonia | Amiodarone, Bleomycin, Cyclophosphamide, Gold salts, Methotrexate, Nitrofurantoin, Pencillamine, Sulfasalazine |
| Non-cardiogenic Pulmonary edema | Acetylsalicylic acid, Amphotericin, Cyclophosphamide, Cyclosporin, Dexamethasone, Morphine, Methotrexate, Hydrochlorothiazide, Sulfasalazine |
| Pulmonary haemorrhage | Anticoagulants, Amphotericin B, Cyclophosphamide, Cytarabine, Pencillamine |
| Veno-occlusive disease | Bleomycin, Busulfan, Carmustine, Lomustine |
Table 1: Patterns of pulmonary lesions and their causative agents.
Non-specific Interstitial Pneumonia (NSIP)
Non-specific interstitial pneumonia, also known as chronic interstitial pneumonia, results from scattered interstitial inflammation, mild interstitial fibrosis, and hyperplasia of type II pneumocytes. Differential diagnoses include usual interstitial pneumonia and desquamative interstitial pneumonia; however, the interstitial inflammation is more homogenous and cellular than usual interstitial pneumonia.
Affected patients present similarly to those with DAD. Symptoms are typically those of fever, malaise, cough and dyspnoea. Carbon monoxide diffusion capacity is again reduced. Chest radiograph demonstrates diffuse, heterogeneous opacities. HRCT shows ground glass opacity in the early stages, with basal fibrosis (honeycombing and traction bronchiectasis) in the later stages.
The commonest drug causes are amiodarone and carmustine. Methotrexate, chlorambucil and leflunomide have also been implicated.
Hypersensitivity pneumonitis
Hypersensitivity reactions are mediated by the active immune system. The immune damage may be caused by drugspecific antibiotics, or drug-specific T cells. Deposition of antibody-drug complexes further perpetuates the reaction. Lymphocytes and plasma cell infiltrates lead to alveolar inflammation and subsequent damage to the lung parenchyma.
Presenting symptoms are generalized malaise, cough and dyspnoea. Chest radiograph may show alveolar infiltrates. HRCT is characterized by ground glass opacities, ill-defined centrilobular nodules, and extensive consolidation. Chronically, fibrosis, honeycombing and centrilobular nodules are seen. Non-caseating granulomas with giant cells may also be noted.
Nitrofurantoin and immunosuppressive drugs such as methotrexate, sulfasalazine and gold salts are known causes of hypersensitivity reactions.
Eosinophilic pneumonia
High circulating immunoglobulin type E (IgE) and eosinophilia cause infiltration of alveoli with eosinophils, lymphocytes and plasma cells, leading to thickening of alveolar septae.
Affected individuals demonstrate symptoms of dyspnoea, dry cough and fever. Blood tests show eosinophilia and high levels of IgE. Chest radiograph shows homogenous opacities typically in the peripheries and upper lobe, with a reverse pulmonary oedema pattern (bilateral peripheral airspace disease with sparing of the central lung parenchyma). HRCT shows peripheral pulmonary opacities.
Common drug causes are NSAIDs, para-amino salicylic acid, ACE inhibitors, nitrofurantoin, penicillamine and sulfasalazine. Cessation of the offending agent and treatment with steroids are the mainstay of management.
Bronchiolitis Obliterans Organizing Pneumonia (BOOP)
BOOP, also known as cryptogenic organizing pneumonia, is an inflammation of the bronchioles and surrounding tissue. It is characterized by proliferation of immature fibroblasts, called Masson bodies, causing polypoid plugs within respiratory bronchioles, alveolar ducts and alveolar spaces.
Symptoms are dyspnoea, dry cough and fever. Crackles may be heard on auscultation. Chest radiograph shows bilateral peripheral opacities in upper and lower lobes. These findings are similar to an extensive pneumonia. HRCT shows nodular consolidation, bronchial dilations, centrilobar nodules and branching linear opacities, known as “tree-in-bud”.
Common causative drugs are amiodarone, bleomycin, cyclophosphamide, gold salts, methotrexate, nitrofurantoin, penicillamine and sulfasalazine. Resolution of symptoms occurs on discontinuation of the drug and steroid therapy.
Non-cardiogenic pulmonary oedema
Symptoms include acute dyspnoea, cough and haemoptysis. Pathogenesis is likely to be endothelial injury and increased capillary permeability. Chest radiograph shows bilateral alveolar opacities and septal lines. The heart size is normal, as a pose to cardiomegaly seen in cardiogenic pulmonary oedema. HRCT shows alveolar opacification, interstitial lines, sub-pleural lines and effusions. Drugs known to cause noncardiogenic pulmonary oedema are listed in Table 1.
Pulmonary haemorrhage
This is an uncommon pulmonary complication. Patients often present with acute respiratory distress and, occasionally, haemoptysis. Chest radiograph shows bilaterally opacities occasionally with focal consolidation. HRCT demonstrates bilateral scattered or diffuse ground glass opacification [2,3].
Anticoagulants are common used drugs that can cause pulmonary haemorrhage. Other drugs are listed in Table 1.
Acute Respiratory Distress Syndrome (ARDS)
ARDS is characterized by diffuse injury to alveolar cells, leading to surfactant dysfunction, abnormal coagulation and activation of the innate immune response. This leads to non-cardiogenic pulmonary oedema and impaired gas exchange in the alveoli [4].
Patients present with worsening dyspnoea, tachypnoea and hypoxaemia. Chest radiograph shows diffuse bilateral opacities. HRCT shows dense consolidation in dependent areas, in the early stages. Later, a coarse reticular pattern is noted secondary with type II pneumocyte proliferation and fibrosis. Ground glass opacifications is seen in the nondependent part of the lungs.
Pulmonary veno-occlusive disease
This is a rare form of pulmonary hypertension, characterized by progressive intimal fibrosis and occlusion of pulmonary veins, leading to high pulmonary arterial pressures.
HRCT may show main pulmonary artery enlargement, ground-glass opacities in a centrilobar distribution, and mediastinal lymphadenopathy. However, lung biopsy may be required to make a definitive diagnosis [5].
The usual drugs responsible are cytotoxic agents such as bleomycin, mitomycin and carmustine.
Common Drugs Causing Lung Injury
Cardiovascular drugs
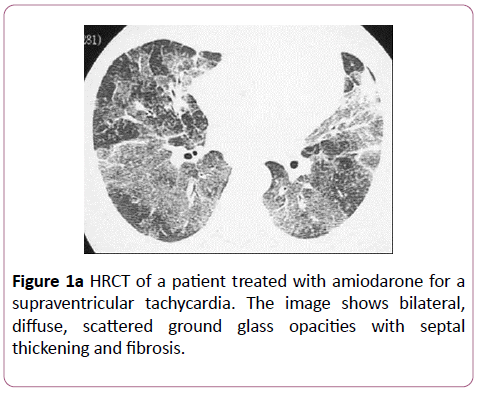
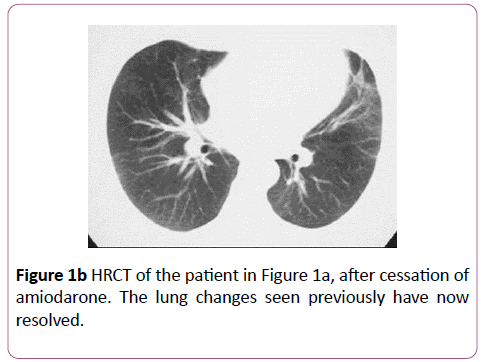
Amiodarone: Lung disease usually develops within months of initiating therapy in susceptible individuals. There is usually no correlation between dose or duration of treatment and development of pathology. Those most at risk are the elderly and patients administered a daily dose of greater than 400 milligrams. The pathology most often seen with Amiodarone is NSIP, pleural effusions and BOOP (Figure 1a). Usually patients respond well to cessation of amiodarone therapy (Figure 1b), although some may progress to death.
These features are consistent with a diagnosis of nonspecific interstitial pneumonitis.
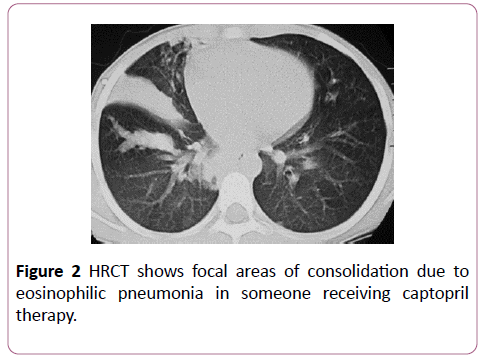
Captopril: Captopril is an angiotensin converting enzyme inhibitor used to treat hypertension. Eosinophilia is a common side effect of captopril therapy, which rarely manifests as an eosinophilic pneumonia (Figure 2). There is symptom resolution upon discontinuation of the drug.
Antibiotics
Nitrofurantoin
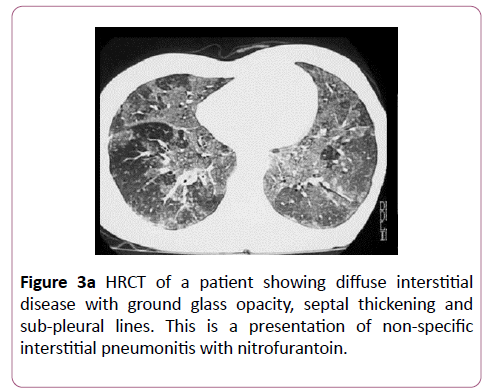
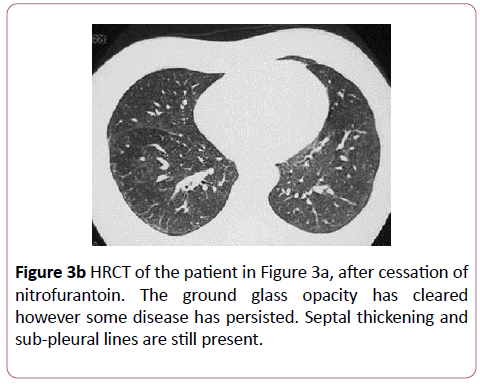
Nitrofurantoin is an uncommon cause of drug-induced lung disease. An acute reaction mimics hypersensitivity pneumonitis, and occurs within 2 weeks with an eosinophilia, fever, dyspnoea and cough. Imaging shows bilateral, diffuse, basal heterogeneous opacities. Chronic toxicity occurs months to years after therapy. The most common presentation is nonspecific interstitial pneumonitis (Figure 3a), although it can rarely present as BOOP. Nitrofurantoin induced lung disease has a good prognosis and responds to cessation (Figure 3b).
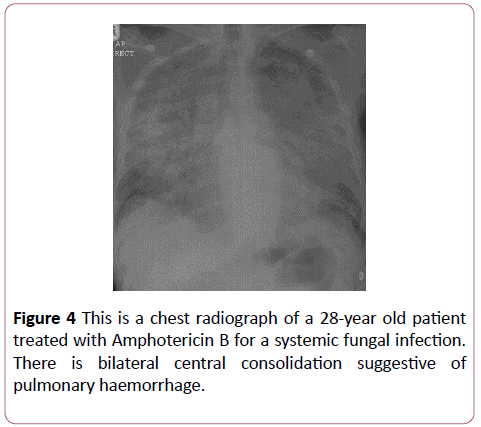
Amphotericin B
Amphotericin is typically used to treat fungal infections (Figure 4).
Anti-Inflammatory Drugs
Methotrexate
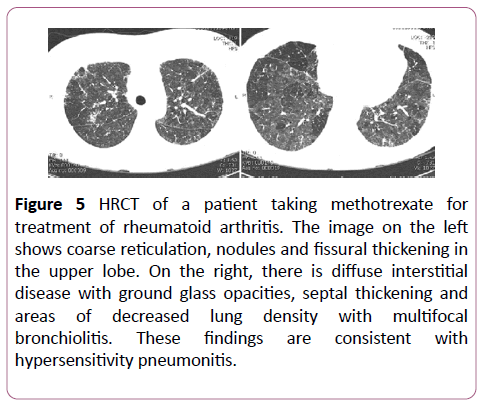
Methotrexate is commonly used to treat rheumatological conditions. 5-10% of those treated develop some lung disease. This occurs within months of therapy, with no relation to dose or duration of treatment. NSIP and hypersensitivity pneumonitis (Figure 5) are most commonly seen with methotrexate treatment, and these usually improve on stopping the drug.
Figure 5: HRCT of a patient taking methotrexate for treatment of rheumatoid arthritis. The image on the left shows coarse reticulation, nodules and fissural thickening in the upper lobe. On the right, there is diffuse interstitial disease with ground glass opacities, septal thickening and areas of decreased lung density with multifocal bronchiolitis. These findings are consistent with hypersensitivity pneumonitis.
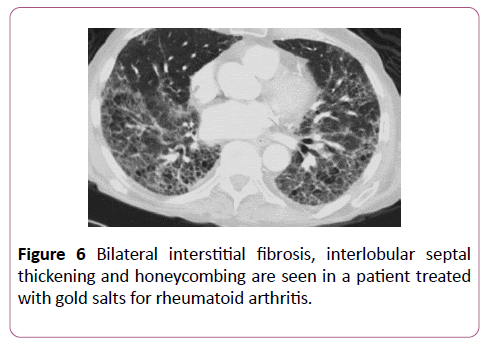
Gold salts
Gold salts are also used to treat rheumatological conditions. 1% of these patients go on to develop lung disease within 2-6 months of initiating therapy. DAD, NSIP and BOOP are the usual pathologies found with gold salts (Figure 6) and cessation has a good response.
Sulfasalazine
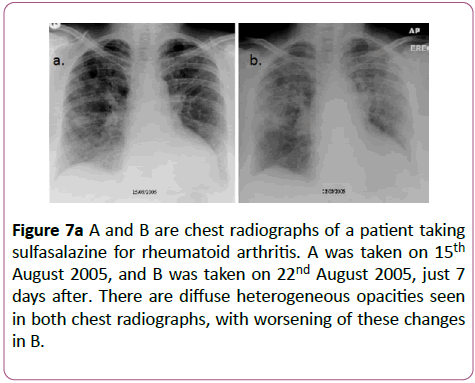
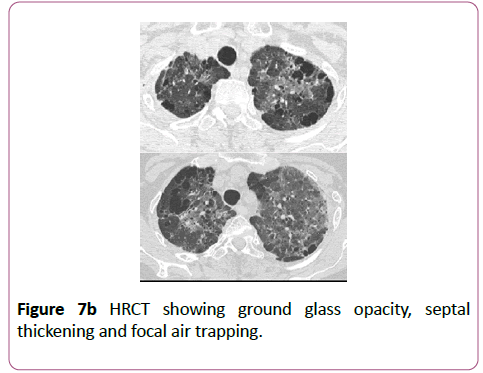
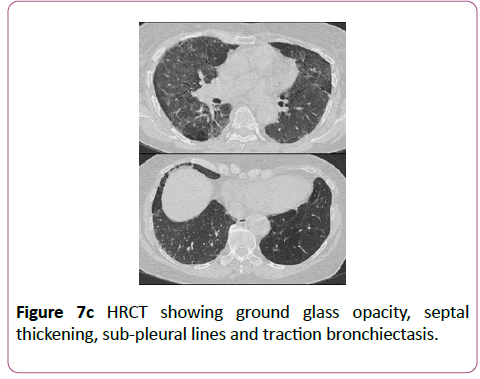
Sulfasalazine is used to treat inflammatory bowel disease and inflammatory arthritis, although its use has decreased in preference for the 5-aminosalicylic acid derivatives which have a more acceptable side effect profile. The lung lesions caused by this drug can be variable (Figures 7a-7c).
Figure 7a: A and B are chest radiographs of a patient taking sulfasalazine for rheumatoid arthritis. A was taken on 15th August 2005, and B was taken on 22nd August 2005, just 7 days after. There are diffuse heterogeneous opacities seen in both chest radiographs, with worsening of these changes in B.
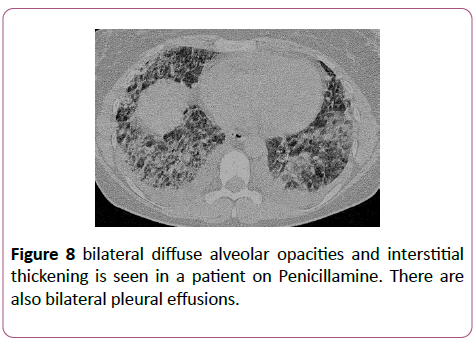
Penicillamine
Penicillamine is primarily used as a chelating agent in the treatment of Wilson’s disease. It can also be used as a disease modifying agent in patients with severe rheumatoid arthritis that have failed to respond to conventional therapy. Penicillamine has been reported to cause BOOP (Figure 8).
Cyclosporine
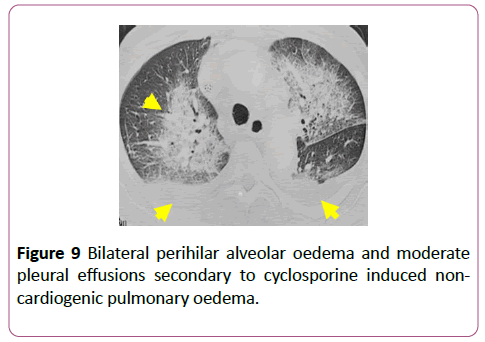
Cyclosporine is an immunosuppressive agent used as posttransplant prophylaxis against graft rejection. It can also be used to treat rheumatological conditions. Non-cardiogenic pulmonary oedema is a complication of cyclosporine treatment (Figure 9).
Chemotherapeutic Agents
Bleomycin
Bleomycin is one of the most commonly implemented drug. The risk of developing lung disease is increased with a cumulative dose of >450 units. Additionally, being elderly, or having a history of oxygen therapy or irradiation are risk factors. Reinstitution of bleomycin treatment within 6 months of cessation also increases risk.
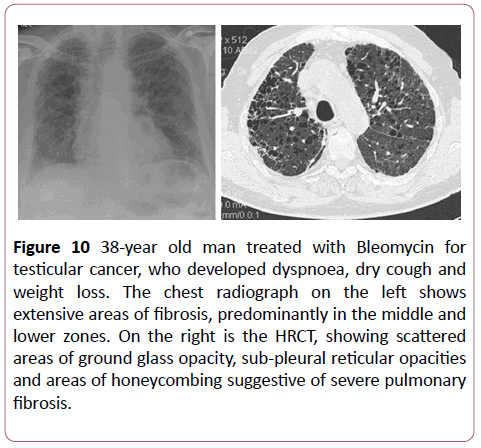
Bleomycin can cause lots of lung pathologies but the most commonly seen is diffuse alveolar damage, then non-specific interstitial pneumonia and BOOP (Figure 10). Bleomycin related lung damage is associated with a poor prognosis, with respiratory failure in 3 months [6].
Figure 10: 38-year old man treated with Bleomycin for testicular cancer, who developed dyspnoea, dry cough and weight loss. The chest radiograph on the left shows extensive areas of fibrosis, predominantly in the middle and lower zones. On the right is the HRCT, showing scattered areas of ground glass opacity, sub-pleural reticular opacities and areas of honeycombing suggestive of severe pulmonary fibrosis.
Carmustine
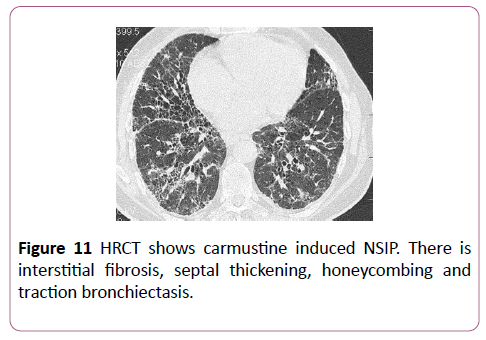
20-30% of patients treated with carmustine develop druginduced lung disease. There is a clear relationship between cumulative dose and lung injury; if the cumulative dose is greater than 1.5 gm/m2, more than 50% of the lung is affected. A history of radiation increases the risk of developing lung disease. The pathologies most associated with carmustine are DAD and NSIP (Figure 11).
Cyclophosphamide
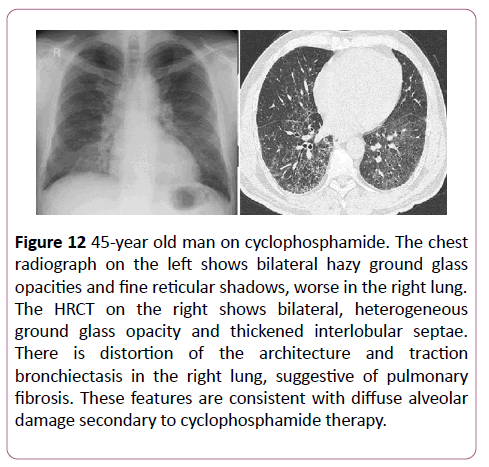
Unlike carmustine, there is no relationship between the dose of cyclophosphamide and the severity of lung injury. The onset of symptoms can be variable with some developing symptoms 2 weeks after treatment, some 13 years after. DAD is the most common lung pathology seen, however HSIP or BOOP can also occur (Figure 12). The prognosis is usually improved with discontinuation of cyclophosphamide.
Figure 12: 45-year old man on cyclophosphamide. The chest radiograph on the left shows bilateral hazy ground glass opacities and fine reticular shadows, worse in the right lung. The HRCT on the right shows bilateral, heterogeneous ground glass opacity and thickened interlobular septae. There is distortion of the architecture and traction bronchiectasis in the right lung, suggestive of pulmonary fibrosis. These features are consistent with diffuse alveolar damage secondary to cyclophosphamide therapy.
Conclusion
Drug induced damage should be suspected in patients presenting with new or progressive respiratory symptoms, and receiving medications known to cause pulmonary toxicity, particularly chemotherapeutic agents. The clinical and radiological findings in drug-induced lung disease can be nonspecific and may imitate non-drug related disease processes, such as infection.
It can be difficult to determine the aetiology of lung lesions without histological confirmation of the underlying pathological process. Thorough drug histories are essential to identify iatrogenic causes for the lung injury, as different medications are associated with different patterns of radiological lesions. As more pharmaceuticals are developed, the list of causative agents will undoubtedly increase. Identifying the pulmonary reaction and underlying cause can improve the management of iatrogenic lung disease.
References
- Ellis SJ, Cleverley JR, Mu¨ller NL (2000) Drug-induced lung disease: high-resolution CT findings. American Journal of Roentgenology 175: 1019-1024.
- Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC (2000) Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics 20: 1245-1259.
- Camus PH, Foucher P, Bonniaud PH, Ask K (2001) Drug-induced infiltrative lung disease. European Respiratory Journal 18: 93-100.
- Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM (1999) Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology 210: 29-35.
- Frazier AA, Franks TJ, Mohammed TLH, Ozbudak IH, Galvin JR (2007) Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. Radiographics 27: 867-882.
- Schwaiblmair M, Behr W, Haeckel T, Märkl B, Foerg W, et al. (2012) Drug induced interstitial lung disease. The Open Respiratory Medicine Journal 6: 63.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences