Quercetin Hydrogel for Antioxidant Activity
Abstract
Hydrogels have received considerable attention in the present years. They possess also a degree of flexibility very similar to natural tissue due to their large water content. Their ability to absorb moisture and their compatibility with different molecule have led to the development of quite a few hydrogel formulations. One of such formulation the Quercetin hydrogel which can be used topically for its antioxidant effect. The additional advantage of hydrogel is that they possess further wound healing property. Thus Quercetin hydrogel formulation would be helpful in several dermal conditions where its antioxidant mechanism is necessary.
INTRODUCTION
Hydrogels have received considerable attention in the present years. They possess also a degree of flexibility very similar to natural tissue due to their large water content. Their ability to absorb moisture and their compatibility with different molecule have led to the development of quite a few hydrogel formulations. One of such formulation the Quercetin hydrogel which can be used topically for its antioxidant effect. The additional advantage of hydrogel is that they possess further wound healing property. Thus Quercetin hydrogel formulation would be helpful in several dermal conditions where its antioxidant mechanism is necessary.
Source of Quercetin
Quercetin is a plant pigment and chemically a flavonoid. It is found abundantly in many plants and food material, such as red wine, onions, green tea, apples, berries, Ginkgo biloba, St. John's wort, American elder, and others. The name has been in use since 1857, and is derived from quercetum (oak forest), after Quercus. In red onions, higher concentrations of quercetin occur in the outermost rings (or in the peels) and in the part closest to the root, the latter part of the plant with the highest concentration. Studies show that organically grown tomatoes had 79% more quercetin than non-organically grown fruit. Quercetin is present in various kinds of honey from different plant sources.
Pharmacological importance of Quercetin
- Quercetin is a flavonoid and is mainly used for the following,
- Anti-inflammatory- Quercetin has showed significant reduction in the plasma levels of cyclooxygenase (COX) and lipooxygenase (LOX). Further it has shown reduction in the inflammatory mediators like the NO synthase, COX-2, C- reactive protein (CRP). It also inhibits xanthine oxidase and thus reducing the levels of uric acid.
- Cardiovascular disease prevention- quercetin is found to provide protection against coronary heart disease (CHD) and also reduces risk of mortality in higher levels of low density lipoproteins (LDL) in Quercetin inhibits fat accumulation in the maturing fat cells and causes destruction of the existing fat cells.
- Neurodegenerative diseases- quercetin along with ascorbic acid is found to protect against the oxidative damage in the lymphocytes and neurovascular structures in the skin and in the neurons of the brain. It protects the brain cells against oxidative stress leading to Alzheimer’s disease and other neurological conditions.
- Cancer and apoptosis- quercetin has potent anti-cancer properties and is an apoptosis It decreases the growth of tumour in brain, colon, liver and other tissues. It is an anti- proliferative, growth factor suppressor and an antioxidant.
- Ulcer and gastritis- quercetin can inhibit the gastric acid secretion and lipid peroxidation of the gastric cells to some It can also inhibit Helicobacter pylori infection and thus preventing formation of gastric ulcers.
CHEMISTRY AND STRUCTURE
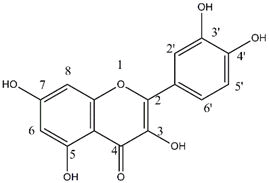
The basic flavonoid structure consists of two phenyl groups joined by a three carbon bridge. Flavonoids are of two main classes, those which have the three-carbon bridge "open" and those which have the three-carbon bridge involved with a heterocyclic ring, referred to as ring C. Variations in ring C and the various substitution patterns available for rings A and B allow for a variety of flavonoid structures with different mechanism of actions and different pharmacological properties.
The subclasses of flavonoids mostly vary by the functional group placed on ring C. Flavanol and anthocyanidins are the only two subclasses that lacks a 4-oxo group and they do contain a 3- hydroxyl group along with the flavonol basic group. The flavones, isoflavones, and flavonols contains a 2-3 double bond on ring C, and anthocyanidins have two double bonds at 1-2 and 3-4 positions. The C ring on the subclasses is joined to ring B on carbon 2 for all classes of flavonoids, except for isoflavones which are joined at carbon 3. These various substitution patterns define the subclasses and affect their pharmacokinetics and their mechanism as an antioxidant.
The main mechanism of action of quercetin is that it reacts with a free radical, it donates a proton to become a radical itself, but the resulting unpaired electron is delocalized by resonance, decreasing the energy of quercetin radical to be reactive. Three structural groups help in quercetin'stoabmiliatyintain its
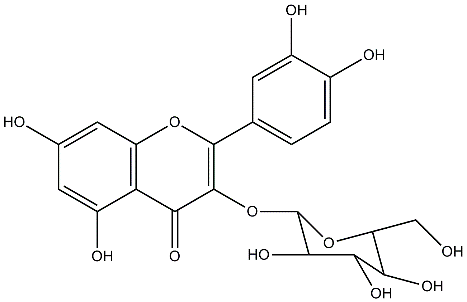
stability and act as an antioxidant when reacting with free radicals: the B ring o-dihydroxyl groups, the 4-oxo group in conjugation with the 2, 3-alkene, and the 3- and the 5-hydroxyl groups. The functional groups can also contribute electrons to the rings, which increases the number of resonance forms. Many flavonoids are attached to sugars in their natural state, the O-glycoside form, where glycosylation occurs at any hydroxyl group to yield a sugar moiety. The most common quercetin glycosides have a sugar group at the 3-poistion, one of them is quercetin-3-O-β-glucoside. These glycosylated structures are most common in nature, not the aglycone, or parent compound.
The biosynthesis of phytochemicals, like flavonoids by the plants is a kind of defensive response to the environmental stress factors. Flavonoids can also function as protection from ultraviolet sunlight and lipid peroxidation for the plants itself.
The target of most of the studies regarding quercetin is its aglycone part. However, plasma analysis after quercetin consumption indicates that quercetin metabolites, like glucuronide (quercetin-3-O-βon-Did-gel)u, caure the primary compounds that circulate in the blood. The metabolites are also found primarily in plants. Due to scarcity of quercetin metabolites commercially, the aglycone is mostly under study. The chemical synthesis of the metabolites, however, is possible but is mostly difficult.
MECHANISM OF ACTION AS AN ANTIOXIDANT
Materials and method
Fresh dry outer scales of onion (which contains the highest amount of quercetin) were collected from different brown cultivars of onion (mainly Sochaczewska and Blonska). The scales were pulverized in laboratory mill and sieved. Particles smaller than 0.2 mm was used to experiments with extraction of quercetin.
Extraction methods:
- Extraction is done by shaking the pulverised dry scales of onion for 4 h of material in laboratory shaker using different organic media with different conditions.
- a) Extraction by 2 hours heating under reflux condenser with ethyl acetate
- b) Extraction by 2 hours heating under reflux condenser with 60% ethanol (can also be done with 80% ethanol and 96% ethanol)
- Proportion of solvent to material was: 50 ml of solvent per 1 g of powder.
- c) Extraction by shaking of material in laboratory shaker with cold ethyl acetate during 4 hours [proportion volumes of solvent (ml) to weight of powdered onion scales (g) that can be used are 10 : 1, 20 : 1, 30 : 1, 50 : 1, 100 : 1].
- Extracts were filtered under vacuum, and 20 microliters of clear supernatants were taken to analysis. Solvent was evaporated in rotary evaporator under vacuum in 40°C. Weight of residue after evaporation of solvent was used for calculation of extraction efficiency (in %). Extracts were filtered under vacuum, and 20 microliters of clear supernatants were taken to Solvent was evaporated in rotary evaporator under vacuum in 40°C. Weight of residue after evaporation of solvent was used for calculation of extraction efficiency (in %). Analysis of the residue is done by LKB (Sweden) HPLC apparatus. Equipped with Rheodyne 7125 injection system (loop 20 µl), UV detector (2151 Variable Wavelenght Monitor) set at 370 nm, and Shimadzu C-R6A Chromatopac integrator was used.
- The quercetin was isocratically separated on Lichrosorb RP18 (4 x 250 mm, 10 µm) column, and a mobile phase was methanol: water mixture (55:45, v/v) contained 2% ortho- phosphoric acid. The flow rate was 0.8 ml/min. In such conditions retention time of quercetin was 13.1 min. The standard curve was prepared for concentration range 0.2 to 10.0 µg/ml. Analyses were done in three replicates, and results were statistically calculated.
Results
Highest yield of extraction of quercetin from powdered dry scales of onion was obtained by hot extraction with 60% of ethanol, although content of quercetin in crude extract was only 21%. Obtained through such method product was liquid and oily. In all cases when ethanol solutions were used crude quercetin was always oily. Such crude quercetin was contaminated up to
78% with soluble carbohydrates, phenolic acids and non-volatile organic acids. Addition of water to ethanol increased efficiency of extraction process, but decreased clearly its purity. When quercetin was extracted from powdered onion scales by shaking with cold ethyl acetate the yield of crude product was ranged from 3.57% to 4.56%, and purity reached level 70%. Half hour, and 1 hour of extraction process is too short time to get high yield of crude quercetin. The process should be done for 4 hours, and but longer time did not increase the extraction efficiency. When hot ethyl acetate was used the yield of crude quercetin was higher - 4.66%, but its purity declined to value 60%. The results indicated that for proper extraction process 50 volumes of solvent on one weight of powdered onion scales have to be used. The conclusions drawn from the above extraction method is that,
- Cold extraction with ethyl acetate is effective, fast and simple method of isolation of crude quercetin from powdered dry scales of onion. Using such procedure yellow, powdered quercetin can be obtained with 70%
- Hot extraction with ethyl acetate improved non-significantly yield of crude quercetin, but decreased its purity.
- Yield of crude quercetin obtained by its extraction with ethanol-water solutions was much higher, but product was oily, and contained up to 78% of contaminants. It needs further purification
CHEMICAL TESTS OF QUERCETIN
The quercetin is classified under flavonoids. So the chemical identification tests of quercetin are same as the tests of flavonoids:
- Shinoda test to dry powder or extract, add 5 ml of 95% ethanol, few drops of conc. HCl and 0.5 g magnesium turnings. Pink colour observed.
- To small quantity of residue, add lead acetate Yellow coloured precipitate is formed. Addition of increasing amount of sodium hydroxide to the residue show yellow coloration, which decolouration after addition of acid.
QUERCETIN HYDROGEL FORMULATION
A brief introduction to Hydrogel
The major focus this brief review is primarily hydrogel formulation, which are actually polymer networks which gets extensively swollen by absorbing water. Hydrophilic gels that are usually referred to as hydrogels are networks of polymer chains that can be found as colloidal gels in which water is the dispersion medium. The most common definition of hydrogel is that hydrogel is a water-swollen, and cross-linked polymeric network produced by the simple reaction between one or more monomers. Another definition is that it is a polymeric material that exhibits the ability to swell and retain a significant fraction of water within its structure without dissolving in water. Hydrogels have received considerable attention in the past 50 years, due to their exceptional promise in wide range of applications and their compatibility with wide range of drugs. They possess also a degree of flexibility which resembles the
natural tissue due to their large moisture content. The ability of hydrogels to absorb water is due to their hydrophilic functional groups attached to the polymeric backbone, while their resistance to dissolution arises from cross-links between network chains. Many materials, both naturally occurring and synthetic, are fit to be formulated as hydrogel. During last two decades, natural Hydrogels were gradually replaced by synthetic hydrogels due to their long service life, high capacity of water absorption, and high gel strength. Fortunately, synthetic polymers usually have well-defined structures that can be physically or chemically modified to yield more stable forms of hydrogel. Hydrogels can also be synthesized from purely synthetic components. Also, it is highly stable in the conditions of sharp and strong fluctuations of temperatures. Recently, hydrogels have been defined as two- or multi- component systems consisting of a three-dimensional network of polymer chains with water that filling in the space between macromolecules. Depending on the properties of the polymer (or polymers) used, as well as on the nature and density of the polymeric network joints, such structures in an equilibrium can contain varying amounts of water; typically in the swollen state, the mass fraction of water in a hydrogel is much higher than the mass fraction of polymer. In practice, to achieve high degrees of swelling, it is preferred to use synthetic polymers that are water- soluble when in non-cross-linked form.
Desired features of Quercetin Hydrogel formulation
- The functional features of an ideal formulation can be listed as follows,
- Must have highest absorption capacity (maximum equilibrium swelling) in saline.
- Must show desired rate of absorption (preferred particle size and porosity) depending on the application requirement.
- Must exhibit the highest absorbency under load (AUL).
- Should show lowest soluble content and residual monomer.
- Must have highest durability and stability in the swelling environment and during the storage.
- Must have highest biodegradability without formation of toxic species following the degradation.
- The polymeric material and quercetin should not be incompatible to each other.
- PH-neutrality after swelling in water.
- Colourless, odourless, and absolutely non-toxic.
- Must have good photo stability.
- Re-wetting capability (if required) the hydrogel has to be able to give back the imbibed solution or to maintain it; depending on the application requirement (e.g., in agricultural orhygienic applications)
- Have lowest price.
Advantages of the formulation
- Psesses high degree of flexibility similar to natural tissues
- Bio compatible, bio degradable (hydrogels can also be injected)
- Applied locally so by passing first pass metabolism
- Sustained and prolonged action in comparison to conventional drug delivery systems
- Decreased dose of administration
- Decreased side-effects
- Improved drug utilization
- Improved patient compliance
- Drug loss is prevented by extensive first pass
- Lower daily cost to patient due to fewer dosage units are required by the patient in therapy.
- Easy to modify
- Timed release of growth factors and other nutrients to ensure proper tissue growth
- Entrapment of microbial cells within polyurethane hydrogel beads with the advantage of low toxicity
- It is environmentally sensitive that means it have the ability to sense changes of pH, temperature or the concentration of metabolite and release their load as result of such a change.
- It also possess good transport properties and easy to modify.
- It is more elastic and stronger than available hydrogels of similar softness. Poly (methyl acrylate- cohydroxyethylacrylate) hydrogel implant material of strength and softness.
Disadvantages of the formulation
- They can cause a sensation like the movement of maggots.
- Its disadvantage includes thrombosis at anastomosis sites and then surgical risk associated with the device implantation and retrieval.
- The main disadvantage of the formulation is that they are nonadherent and may need to be secured by a secondary. Hydrogels have low mechanical strength and difficult to handle and are expensive.
Polymers that can be used to formulate the quercetin hydrogel
The polymers that are available for the formulation are categorised into synthetic polymers and natural polymers. They are as follows,
Synthetic polymers- poly(vinylpyrrolidone) (Benamer et al., 2006; Rosiak et al., 1995); starch, poly(vinylpyrrolidone), poly(acrylic acid) (Kumar et al., 2008; Spinelli et al., 2008); carboxymethyl cellulose, hydroxypropyl methyl cellulose (Barbucci et al., 2004; Porsch & Wittgren, 2005); polyvinyl alcohol, acrylic acid, methacrylic acid (Nho et al., 2005); chitosan, αβ-glycerophosphate (Zhou et al., 2008); κ- carrageenan, acrylic acid, 2-acrylamido-2- methylpropanesulfonic acid (Campo et al., 2009; Pourjavadi & Zohuriaan-Mehr, 2002); acrylic acid, carboxymethyl cellulose (El-Naggar et al., 2006; Said et al., 2004).
Natural polymers- Starch (Trksak & Ford, 2008); gum arabic (Al-Assaf et al., 2006b; Al-Assaf et al., 2007b; 2006; Katayama et al., 2008); xanthan, pectin, carrageenan, gellan, welan, guar gum, locust bean gum, alginate, starch, heparin, chitin and chitosan (Phillips et al., 2003; Phillips et al., 2005).
Technologies adopted to hydrogel preparation
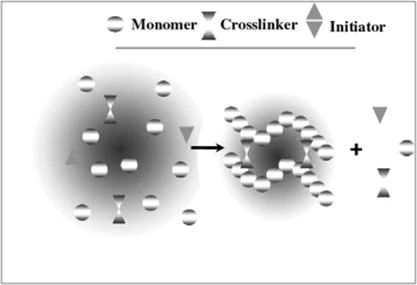
By definition, hydrogels are polymeric networks having hydrophilic properties. While hydrogels are prepared with hydrophilic monomers, hydrophobic monomers are sometimes used in hydrogel preparation to regulate the properties for
gels, including bulk, solution, and suspension polymerization. In general, the three integral parts of the hydrogels preparation are monomer, initiator, and cross-linker. For controlling the heat of polymerization and the final hydrogels properties, diluents can be used, such as water or other aqueous solutions. Then, the hydrogel mass needs to be washed for removing impurities left from the preparation process such as non- reacted monomer, initiators, cross- linkers, and unwanted products produced via side reactions.
Preparation of hydrogel based on acrylamide, acrylic acid and its salts by inverse-suspension polymerization and diluted solution polymerization have also been investigated. Fewer studies have been done on highly concentrated solution polymerization of acrylic monomers, which are mostly patented. Hydrogels are usually prepared from polar monomers. According to their starting materials, they can be divided into natural polymer hydrogels, synthetic polymer hydrogels or can be combinations of the two classes. From a preparative point of
view, they can be obtained by graft polymerization, cross-linking polymerization, networks formation of water-soluble polymer, and radiation cross-linking, etc. There are many types of hydrogels; mostly, they are lightly cross-linked copolymers of acrylate and acrylic acid, and grafted starch-acrylic acid polymers prepared by inverse- suspension, emulsion polymerization, and solution polymerization. The polymerization techniques have been described below,
Bulk polymerization- There are many vinyl monomers which can be used for the productions of Bulk hydrogels can be formulated using one or more types of monomers. The wide variety of monomers enables preparation of hydrogels with desired physical properties for a specific application. Usually, a small amount of cross-linking agent is added in any hydrogel formulation. The polymerization reaction is normally initiated with radiation, ultraviolet rays, or chemical catalysts. The choice of a suitable initiator depends upon the type of monomers and solvents being used. The polymerized hydrogel can be produced in a wide variety of forms including films and membranes, rods, particles, and emulsions. Bulk polymerization is the simplest technique in which only monomer and monomer-soluble initiators are used. High rate of polymerization and degree of polymerization occur because of the high concentration of monomer. However, the viscosity of reaction increases markedly with the conversion which produces the heat during polymerization which can be avoided by controlling the reaction at low conversions. The bulk polymerization produces a glassy, transparent polymer matrix of a homogenous hydrogel which is very hard which when immersed in water, the glassy matrix swells to become soft and flexible.
- Solution polymerization/cross linking- In solution copolymerization/cross-linking reactions, the ionic or neutral monomers are mixed with the multifunctional cross-linking The polymerization is initiated thermally by UV- irradiation or by a redox initiator system. The solvent serves as a heat sink which is the major advantage of the solution polymerization over the bulk polymerization. The prepared hydrogel needs to be washed with distilled water to remove the monomers, oligomers, cross-linking agent, the initiator, the soluble and extractable polymer, and other impurities. Phase separation may occur which results in the formation of heterogeneous hydrogel when the amount of water during polymerization is more than the water content corresponding to the equilibrium swelling. The typical solvents which are employed for solution polymerization of hydrogels include water, ethanol, water–ethanol mixtures, and benzyl alcohol. The synthesised solvent may then be removed after formation of the gel by swelling the hydrogel in water.
- Suspension polymerization or Inverse suspension polymerization- Dispersion polymerization is one of the most advantageous methods because the products are obtained as powder or microspheres (beads), which do not require The use of water-in-oil (W/O) process instead of the more common oil-in-water (O/W), makes it ‘‘inverse-suspension’’. In this technique, the monomers and initiator are dispersed in the hydrocarbon phase as a homogenous mixture. The viscosity of the monomer solution, agitation speed, rotor design, and dispersant type are the factors governing resin particle size and shape. The dispersion is thermodynamically unstable and requires both continuous agitation and addition of a low hydrophilic–lipophilic-balance (HLB) suspending agent.
- Grafting to a support- Generally, bulk polymerization creates hydrogels with inherent weak structure. To improve the mechanical properties of a hydrogel, it can be grafted on surface coated onto a stronger This technique that involves the production of free radicals onto a stronger support surface and then polymerizing monomers directly onto it as a result a chain of monomers are covalently bonded to the support. A variety of polymeric supports have been used for the synthesis of hydrogel by grafting techniques.
- Polymerization by irradiation- Ionizing with high energy radiation, like gamma rays and electron beams has been used as an initiator to prepare the hydrogels in the previously discussed The irradiation of aqueous polymer solution produces radicals on the polymer chains. Also, radiolysis of water molecules results in the formation of hydroxyl radicals, which also attack the polymer chains, resulting in the formation of macro-radicals. Recombination of the macro-radicals on different chains results in the formation of covalent bonds which finally produces a cross-linked structure. Examples of polymers cross- linked by the radiation method are poly (vinyl alcohol), poly (ethylene glycol), and poly (acrylic acid). The major advantage of the radiation initiation over the chemical initiation is that it causes the production of relatively pure and initiator- free hydrogels.
EVALUATION OF THE FORMULATED QUERCETIN HYDROGEL
Physical appearance
The physical appearance and homogeneity of the prepared gels were tested by visual observations. The marketed formulation was considered as reference for the mentioned test.
Spread ability test
Spread ability can be determined by applying the gel over an even surface and observed for the emollient property of hydrogel or any gritty nature of the hydrogel if present.
PH determination
The pH of the gel formulations was determined by using a pH meter. For pH determination, 1% of hydrogel formulation in deionized water was prepared and pH was determined.
Drug content
For assay of the drug (quercetin) in hydrogel, quercetin was extracted from 1 g of each gel formulations with 20 mL of suitable buffer system and the assay for estimation of drug content was carried out as per protocol.
Determination of viscosity
The viscosity of the gel formulations was determined using Brookfield viscometer with spindle no. 7 at 100 rpm at the temperature of 250C. Ex vivo drug permeation studies: The abdominal hair of Wistar male albino rats, weighing 150- 200 g, was shaved using a razor after sacrificing by spinal dislocation. The abdominal skin was surgically removed and adhering subcutaneous fat was carefully cleaned. The epidermis was then separated from dermis by soaking the full thickness skin in 2 M sodium bromide solution in water for 6-8 h. The epidermis was thoroughly washed with water and stored in freezer for further use. For ex vivo permeation studies, skins were allowed to hydrate for 1 h before being mounted on the Franz diffusion cell with the stratum corneum (SC) facing the donor compartment. The receptor compartment was filled phosphate buffer pH7.4 and receptor phase was maintained at 32 ± 0.5oC. 1 g of the gel was placed on the SC side in the donor compartment. The amount of drug permeated was determined spectrophotometrically at 276 nm by removing 1 mL aliquot through a hypodermic syringe fitted with a 0.22 mm membrane filter, at designated time intervals for 30 min. The volume was replenished with the same volume of pre-warmed receiver solution to maintain sink conditions.
Accelerated stability studies
Stability studies were carried out on optimized formulation according to International Conference on Harmonization (ICH) guidelines. The formulation packed in aluminium tube was subjected to accelerated stability testing for 3 months as per ICH norms at a temperature (40 ± 2oC) and relative humidity 75 ± 5%. Samples were taken at regular time intervals of 1month for over a period of 3months and analysed for the change in pH, spreadability, drug content and in-vitro drug release by procedure stated earlier. Any changes in evaluation parameters, if observed were noted. Tests were carried out in triplicate and mean value of the observed values was noted along with standard deviation.
DISCUSSIONS
Thus it can be concluded that the formulation will show excellent skin permeability. We can also suggest that the hydrogels can be very useful for improving the skin permeability of water soluble flavonoid antioxidants. The quercetin hydrogel formulation will actually play a very important role as an antioxidant topical formulation for conditions like psoriasis and other conditions caused due to reactive oxygen species generated from environmental pollutants and will help to provide prolonged skin protection as a cosmetic product.
REFERENCES
- Jaganathan, K.; Mani, M.P.; Khudzari, A.Z.M.; Ismail, A.F.; Ayyar,M.; Rathanasamy, R. Enriched
- physicochemical and blood-compatible properties of nanofibrous polyurethane patch engrafted with
- Juniper oil and titanium dioxide for cardiac tissue Int. Polym. Anal. Character 2019, 24, 696â??708.
- Vignesh, ; Gopalakrishnan, A.; Poorna, M.R.; Shantikumar, V.N.;Jayakumar, R.; Mony, U. Fabrication of micropatterned alginate- gelatin and k-carrageenan hydrogels of defined shapes using simple wax mould method as a platform for stem cell/induced Pluripotent Stem Cells (iPSC) culture. Int. J. Biol. Macromol. 2018, 112, 737â??744.
- Bahrami, S.B.; Kordestani, S.S.; Mirzadeh, H.; Mansouri, Poly(vinyl alcohol)-chitosan blends: Preparation, mechanical and physical properties. Iran. Polym. J. 2003, 12, 139 :146.
- Iqbal, N.; Tariq, M.; Khan, S.M.; Gull, N.; Iqbal, S.S.; Aziz, A.;Nazir, A.; Iqbal, M. Synthesis and
- Characterization of chitosan and guar gum based ternary blendswith polyvinyl Int. J. Biol. Macromol.2020, 143, 546â??554.
- Harrison, P.; Spada, F. Hydrogels for Atopic Dermatitis andWound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71.
- Ghasemiyeh, ; Mohammadi-Samani, S. Hydrogels as DrugDelivery Systems; Pros and Cons. TiPS 2019, 5, 7â??24.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences