ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Qualitative Study of Punica granatum, Daucus carota and Gymnema sylvestre Plants United States Pharmacopoeia (USP)
Archana Kumari1* and Sanjay Singh2
1Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, India
2Department of Pharmacy, Siddhartha Institute of Pharmacy, Hyderabad, India
- *Corresponding Author:
- Archana Kumari
Department of Pharmacognosy and Phytochemistry,
School of Pharmaceutical Education and Research, Jamia Hamdard,
India,
E-mail: aarchana1998@gmail.com
Received date: October 09, 2023, Manuscript No. IPAPCT-23-17983; Editor assigned date: October 12, 2023, PreQC No. IPAPCT-23-17983 (PQ); Reviewed date: October 26, 2023, QC No. IPAPCT-23-17983; Revised date: November 02, 2023, Manuscript No. IPAPCT-23-17983 (R); Published date: November 09, 2023, DOI: 10.36648/2321-2748.11.5.261
Citation: Kumari A, Singh S (2023) Qualitative Study of Punica granatum, Daucus carota and Gymnema sylvestre Plants United States Pharmacopoeia (USP). Am J Phytomed Clin Ther Vol.11 No.5: 261.
Abstract
The traditional medical system has been used for a very long period to treat numerous diseases. The study was carried to perform some qualitative dates includes plant extracts of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves. The study also contains the macroscopic (size, shape, surface view etc.), microscopic (xylem, phloem, etc.) and power microscopic characteristics of the mentioned plants along with physiochemical parameters determination such as foreign organic matter, ash value, acid insoluble ash value, extractive yield. The qualitative data includes the total phenolic, total flavonoid content, DPPH assay, and reducing power assay along with the TLC fingerprinting of each plant extracts to find out different metabolites (phytoconstituents) present in the given sample of plants also includes aflatoxin determination by using TLC method followed by TLC bio autography to find out antioxidant potential of the specific phytoconstituent and characterization of compounds via FTIR spectroscopy for identification for functional groups present in the plants.
Keywords
Phytoconstituents; FTIR; Plant extracts; Validation; Aflatoxin; TLC fingerprinting; Metabolites; Traditional medicine
Introduction
The status of a medication is established by its identity, purity, content and other chemical, physical, or biological features, as well as by the production process, which is referred to as the quality control of phytopharmaceuticals [1]. Standardisation comprises of the physicochemical assessment of crude pharmaceuticals, which covers topics like selection and treatment of crude material, according to the WHO chapter on standardization and quality control of herbal medications [2]. Due to their potential pharmacological properties, medicinal plants have been utilised to treat a variety of disorders. These properties include antineoplastic, antimicrobial, antioxidant, anti-inflammatory, analgesic, anti-diabetic, anti-hypertensive and other actions [3]. Medical plants have long been a significant source of bioactive substances with functions that influence health. Ayurveda, siddha, unani and Chinese traditional medicine are still effective and have been used for more than 1500 years [4]. Alkaloids, tannins, saponins, flavonoids, steroids, terpenoids, resins, cardiac glycosides, phenolic chemicals and coumarins were all found in the extracts after phytochemical screening [5]. The most popular approach is solvent extraction, in which the natural products go through a process in which the solvent permeates through the plant cell wall and the solute dissolves in the solvent before the extract is collected. According to reports, the extraction efficiency would depend on the size of the plant material, the solvent's characteristics, the solid-to-solvent ratio, the extraction temperature and the extraction duration [6]. This research paper contains quality control study of three plants which are; flower of Punica granatum, fruit of Daucus carota and leaves of Gymnema sylvestre on the basis of their scientific study.
Based on previous studies conducted on Punica granatum L., Daucus carota L. and Gymnema sylvestre R. Br., their phytochemical and pharmacological aspects were taken into consideration.
Phytochemical aspects
Punica granatum L: Traditional medicine has employed pomegranates to cure a range of ailments [7]. Pomegranate fruits are said to be used to get rid of parasites; their seeds, fruit peels, flowers, tree bark and roots, which are used to halt bleeding and heal ulcers; and their leaves, which are used to treat digestive problems and reduce inflammation [8].
The pomegranate has recently sparked a lot of consumer attention because of its alleged health benefits [9]. Due to the pomegranate's high phenolic content [10] and abundance of bioactive compounds, it has demonstrated a wide range of therapeutic effects. The peel of the pomegranate, in particular, contains significant amounts of hydrolyzed ellagitannins known as "pomegranate ellagitannins," which include punicalins, punicalagins, and pedunculagins. Ellagitannins are esters made of quinic acid or glucose-based polyols and hexahydroxydiphenic acid [11]. Apart from ellagitannins, other compounds found in pomegranate rind include anthocyanidins (such as cyanidin, pelargonidin and delphinidin) and flavonoids (such as kaempferol, luteolin, and quercetin). Anthocyanins, the pigment responsible for the red colour of the pomegranate, are abundant in the arils of this fruit. The most common anthocyanins are cyanidin-3-O-glucoside, cyanidin-3, 5-di-O-glucoside, delphinidin -3-O-glucoside, pelargonidin-3-O-glucoside [12]. Pomegranate arils also include phenolic acids, such as p-coumaric acid, chlorogenic acid, ellagic acid and gallic acid, in addition to anthocyanins [13].
Daucus carota L.: They have a significant impact on human nutrition due to their high food value and excellent preservation qualities [14]. Some of the phytochemicals found in carrots; including phenolic compounds (particularly chlorogenic acid), carotenoids, polyacetylenes and ascorbic acid (vitamin C), have biological activities that suggest they may have the potential to improve human health due to their anticancer, antioxidant, antiinflammatory, antibacterial, plasma lipid modification and serotonin-stimulating effects [15]. However, a number of variables, including carrot genotype (colour variations), climatic circumstances and the preparation and storage of carrot products, have an impact on the concentration and nature of phytochemicals [16].
Additionally, carrots can be processed to create anthocyaninsrich concentrate for the pigment industry, and the resulting pomace can be removed to create phenolic compounds with a high added value that can be used as functional food ingredients [17].
Gymnema sylvestre R. Br.: Gymnema sylvestre R. Br. (Asclepiadaceae), sometimes known as "gurmar" because of its unique ability to breakdown sugar is a well-known plant in the ayurvedic medical system. Gymnemasaponins, which are triterpene saponins also known as gymnemic acids, and gurmarin, a polypeptide are the phytoconstituents in plants that have the ability to reduce the appetite for sweets [18]. Plant extracts have alkaloids in them, which had been found. Acidic glycosides, anthraquinones and their derivatives are present in the leaves of Gymnema sylvestre R. Br. One of the nine closely related acidic glycosides that make up Gymnema's principal secondary metabolites is gymnemic acid [19].
Gurmarin was isolated from Gymnema sylvestre R. Br. and is a crucial 35 amino acid peptide with a 4209 molecular weight. Gymnemasins A, B, C and D as well as alkaloids are additional significant components that have been identified from leaves [20]. Gymnemic acid, deacyl gymnemic acid, gymnemagenin, 23- hydroxylnogispinogenin and gymnestrogenin are only a few of the saponins that have been isolated from Gymnema sylvestre R. Br. [21]. Gymnemic acids, gymnemosides, gymnemasaponins, gurmarin, gymnemanol, stigmasterol, d-quercitol, -amyrin related glycosides, lupeol, hydroxycinnamic acids and the coumarols group were revealed to be among the variety of various phytomolecules present in the plant [22].
Pharmacological aspects
Punica granatum L.: Punica granatum L. has strong antiproliferative, anti-inflammatory, anti-tumorigenic, pro-apoptotic, anti-estrogenic and antioxidant activities, according to the literature review [23].
Pomegranate trees produce blossoms in the summer that draw a variety of birds to the trees. The pale pink, piled oval petals that make up the pomegranate blooms are what give them their distinctive appearance [24]. Pomegranate flowers have long been used to treat diabetes, cardiovascular diseases, and obesity [25]. Pomegranate flowers have been utilized as a supplement in diet therapy and as an anti-diabetic drug in unani medicine for many years. With many potential methods, such as improved insulin receptor sensitivity, increased peripheral glucose utilization, and promotion of mRNA expression, the blossoms can considerably lower blood glucose levels in people with type II diabetes [26]. Although the exact processes causing these effects are uncertain, new studies indicate that pomegranate juice and blossoms may inhibit the progression of diabetes by binding to the peroxisome proliferator-activated receptor-gamma and releasing nitric oxide [27].
Daucus carota L.: The antioxidant, antibacterial, sedative, anti-inflammatory, antifungal, anthelmintic, anticancer, depressive, anti-cholesterol, gastric anti-ulcer and renal protective activities of Daucus carota L. has been noted. As a result, carrots can be utilized as a component of herbs; however, more investigation is required to identify carrots' therapeutic properties [28]. Carrots are a multi-nutrient food source because they include a high source of phytochemicals. Because of their anticancer, antioxidant, anti-inflammatory, antibacterial, plasma lipid modification and serotogenic properties, some phytochemicals found in carrots, including carotenoids, polyacetylenes, and ascorbic acid (vitamin C), have the potential to improve human health.
Gymnema sylvestre R. Br.: The herb Gymnema sylvestre R. Br. is frequently used to treat diabetes. Additionally, this plant contains anti-bacterial, anti-inflammatory and hypolipidemic properties. Additionally, it helps prevent cancer, asthma and cardiovascular illnesses [29]. Gymnema sylvestre R. Br. plant is frequently used in naturopathic medicine to cure diabetes. It also shows promise in the treatment of obesity, arthritis, hyperlipidemia, Parkinson's disease, and high cholesterol. The bioactive substances found in plants also have antibacterial, anti-inflammatory and anticancer activities [30]. Numerous bioactive substances that have been extracted from the plant and are either pure substances or crude extracts have been clinically studied in animal model systems for scientific validation [31].
Materials and Methods
Procurement of plant materials
All the three plant materials i.e., Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves were procured from different sources Khari Baoli, New Delhi.
Identification and authentication of plant materials
Identification of plant materials were carried out by performing macroscopic and microscopic analysis. Macroscopical feature involves odour, color, size shape and special feature of plants and microscopical features include stem bark, root, leaves, stem, flower and trichome content. The procured material was authenticated in house, in Bioactive Natural Product Laboratory (BNPL) [32].
Extraction of plant materials
In triplicate, plant materials were removed and washed with tap water and distilled water before being dried for 15 days in the sun. The ingredients were then ground into a coarse powder after drying. The hard narcotics were initially coarsely pulverized using a mortar and pestle before being put through the milling machine in the UG Lab at SPER, Jamia Hamdard. Using a grinder, soft medicines were reduced to a coarse powder [33].
Dried plant materials of each plant were subjected to reflux extraction process: Accurately weighed 300 g of powdered Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves from each vendor was taken in triplicate. Each drug was macerated for 24 hrs with continuous shaking on mechanical shaker for 8 hrs at 200 rpm with three solvents in triplicate i.e. 600 ml of 70% alcohol (methanol), 600 ml of distilled water and 600 ml of hydro-alcoholic solvent (50:50) (300 ml distilled water and 300 ml of 70% methanol). Further it was reflux for 2 to 3 hr. Each sample was filtered and pressed twice, firstly with the normal filter paper and then with muslin cloth. The filtrate was then poured in china dish after taking the empty weight of the china dish for calculation of extractive yield and evaporated to dryness on water bath under reduced pressure [34].
Extractive value:
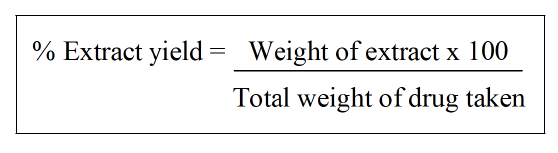
Macroscopy and microscopy
Macroscopy: The plant material was placed on a sheet of white paper for the macroscopic investigation, which involved the morphological description of the plant sections with just the naked eye. The shape, size, colour, odour and taste of leaves, flowers, and fruits were assessed as well as their organoleptic characteristics [35].
Microscopy: Plant samples were seen under the microscope for improved identification using various mounting media, stains, or other solutions. To soften the plant specimens, boiling water was used to soak the Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves for one to two hours. All three specimens were sliced into thin parts using a blade. Small plant tissue sections were transferred to clean slides in the centre, 1 or 2 drops of cotton blue dye and safranin dye (for each of the three specimens) were added, and then the slides were covered with a fresh cover slip. The cover slip was carefully positioned with its edge making initial contact with the slide in order to prevent the creation of air bubbles. It was then firmly pressed into place. Filter paper was used to wipe away extra fluid from the cover slip's margin. To stop the slide from drying out, glycerin was added to it. As a result, the produced slides were examined under a motic microscope (found in the Department of Food Technology at Jamia Hamdard) at 10x magnification. Images were taken and stored, which were then further examined for distinguishing characteristics and labeled appropriately [35].
Physio-chemical parameters
Foreign matter: The sample must be free of any unpleasant foreign matter, evident symptoms of mould growth, sliminess, bug contamination and other animal and animal product products, including animal excrement. Any organism, component, or byproduct of an organism that is not listed in the specification of the product is considered foreign matter, as are mineral admixtures such soils, stones, sand and dust. Beyond the bounds of the official parts of the organism, it must also comprise other parts. Unless otherwise stated, take 100 g of the sample and distribute it thinly on an appropriate platform. Separate the foreign material by examining in the light of day with your unaided eye or with a 6x or 10x magnifying glasses. The foreign stuff can also be separated using a suitable sieve. Sifting the sieve separates the dust that is thought to be a mineral admixture. Calculate the percentage of development of pharmacopoeia monograph according to United States Pharmacopoeia (USP) of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves. Foreign matter in the sample after it has been sorted through 250 different types of foreign material using the drug sample as a reference [31,36].
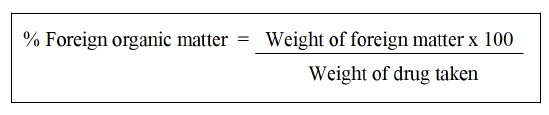
Determination of moisture content (loss on drying): Under the identical circumstances that will be used for the determination, dry the evaporating dish for 30 mins. In a tuned evaporating dish, add 5 g to 10 g of carefully weighed powder or medication. Prepare 10 g of the sample for the unpowered medication by cutting and shredding it into pieces that are about 3 mm thick. Crack any seeds and fruits that are less than 3 mm. Avoid using high-speed mills to prepare the samples and take care to ensure that the portion collected is an accurate representation of the official sample and that no considerable quantity of moisture is lost in the process. Distribute the test specimen as equally as you can by giving it a gentle shake to a depth of approximately 5 mm, or no more than 10 mm in the case of bulky materials. In the drying chamber, place the loaded bottle. Weigh the test specimen after 3 hrs of drying at 105°C. Continue drying and weighing every half an hour until the difference in weight between two subsequent measurements is no greater than 0.25% [37].
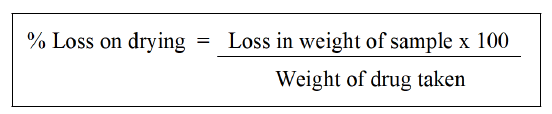
Determination of total ash: About 2 to 3 g of the pulverised drug, precisely weighed, should be incinerated in a tarte platinum or silica dish at a temperature no higher than 6000 until free from carbon. After cooling in a desiccator for 30 mins, weigh the substance right away. If carbon-free ash cannot be achieved in this manner, burn the charred mass with hot water, collect the residue on ashless filter paper, add the filtrate, burn the residue and filter paper until dry, and ignite at a temperature of no more than 600°C. Determine the amount of ash in relation to the air-dried medication [38].
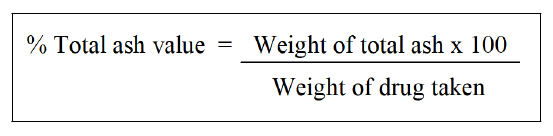
Determination of acid-insoluble ash: 25 ml of diluted hydrochloric acid should be added dropwise to the crucible containing complete ash. Collect the insoluble material using Whatman 41 ashless filter paper and then wash the filtrate in hot water until it is neutral. To ignite to a consistent weight, transfer the filter paper holding the insoluble material to the original crucible, dry on a hot plate and ignite. Wait 30 mins while the residue cools in suitable desiccators before weighing. Determine the amount of acid-insoluble ash in the drug's airdried form [39].
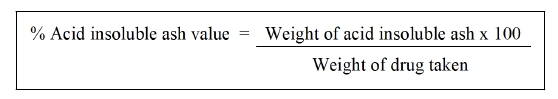
Determination of alcohol-soluble extractive: In a closed flask, macerate 5 g of the air dried medication, coarsely ground, with 100 ml of alcohol of the required strength for 24 hrs, stirring frequently for the first 6 hrs and allowing standing for the remaining 18. Filter quickly while taking steps to prevent solvent loss. Evaporate 25 ml of the filtrate until it is dry in a shallow dish with a flat bottom that has been tapered. Dry at 105°C, to constant weight, and weigh. Determine the amount of extractive that is soluble in alcohol using the air-dried medication as a reference [31,40].
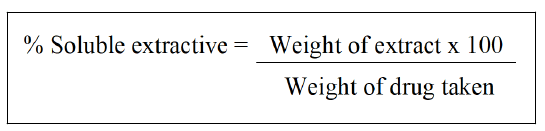
Quality control evaluation of extracts
Quality control evaluations of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves plant extracts were done by phyto-chemical screening and thin-layer chromatography.
Quality control evaluations of plant extracts by phytochemical screening: It includes TPC, TFC, spectrophotometer and determination of reducing power of extracts.
Total Phenolic Content (TPC): The Folin-Ciocalteu reagent was used to determine the total phenols. Folin-Ciocalteu reagent (5 mL, 1:10 diluted with distilled water) and aqueous Na2CO3 (4 mL, 1 M) were combined with a diluted plant extract (0.5 mL of 1:10 g/mL) or gallic acid (a common phenolic component). A ter allowing the combinations to remain for 15 mins, colorimetry at 765 nm was used to calculate the total phenols. Gallic acid solutions in methanol and water (50:50, v/v) solutions of 0, 50, 100, 150, 200, and 250 mg L-1 were used to create the standard curve. Gallic acid equivalent (mg of gallic acid/g of dry mass), which is a common reference compound, is used to express total phenol values [41].
Total Flavonoid Content (TFC):For lavonoid, the aluminum chloride colorimetric technique was employed. Each plant extract was combined separately with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. Each plant extract contained 0.5 mL of 1:10 g/mL in methanol. A single beam Systronics UV/ Visible spectrophotometer (India) was used to test the reaction mixture's absorbance at 415 nm a ter it had been le t at room temperature for 30 mins. Quercetin solutions in methanol were produced at concentrations ranging from 12.5 to 100 g/mL-1 to create the calibration curve [42].
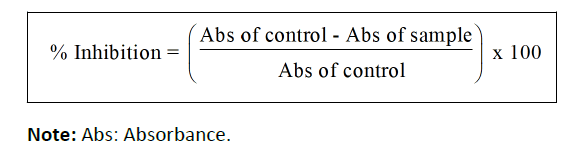
DPPH radical scavenging activity (Spectrophotometer): Using the DPPH assay, the ability of the methanol extracts to scavenge free radicals was evaluated. In methanol, a DPPH solution (0.004%, w/v) was created. Methanol was used to create a stock solution containing standard ascorbic acid (0.05 g/ml) and plant extract (1 mg/ml). Test tubes were illed with ascorbic acid and plant extract at varying quantities (10–500 g/ml), and 1 ml of freshly made DPPH solution was then added. The test tubes were then covered with aluminum foil to prevent light exposure. Each test tube's inal volume was increased to 2 ml with methanol before being incubated at room temperature for 30 mins in the dark. Using a spectrophotometer, the absorbance was measured at 517 nm following incubation [43]. The identical volume of methanol, DPPH, and reference ascorbic acid were used to prepare the control sample [44]. The blank was made of methanol. The following formula was used to determine the radical scavenging:
Determination of reducing power of extracts: By mixing 100 L of extract with 0.2 M phosphate buffer (5.0 mL, pH: 6.6) and 1% potassium ferricyanide (5.0 mL), the reducing power of the extracts was ascertained. 10% tricholoroacetic acid was added and centrifuged 12,000 g for 10 min at 50°C after 20 min of incubation at 50°C. The solution's top layer (5.0 mL) was read at 700 nm after being diluted with 5.0 mL of distilled water and 0.1% ferric chloride (1.0 mL). Three times the experiment was run [45].
Quality control evaluations of plant extracts by Thin-Layer Chromatography (TLC): Thin layer chromatography was used to determine the quantity of chemicals present in the prepared plant extract as well as to assess their quality. The steps were carried out as described below [46].
Preparation, drying and storage of the plates: Small HPTLC aluminum plates with 0.2 mm HPTLC silica gel 60 F254 layers were used. In a twin trough chamber, the plates were completely saturated in methanol for around 45 mins. The plates were then baked in an oven for 10 mins at 110°C while being piled on a drying rack. The active plates were stored in desiccators until they were needed for other applications [47].
Application of the sample: Glass capillaries were employed for applying test samples on TLC plates. A transparent template was used to aid apply the spots, and a minimum of 1 cm was left between each pair of neighboring spots. On the top of the plate, the sample spots were labelled with their respective names [48].
Solvent system: For the plant extract, a variety of developing solvent systems were tested, but the solvent system with the ratio of chromatographic chamber saturation to TLC plate development yielded the most desirable resolution.
The experiment made use of a chromatographic rectangular glass box (16.5 cm 29.5 cm). A smooth sheet of filter paper of 15 cm 40 cm in size was placed in the chromatographic chamber in a "U" shape and allowed to soak in the development solvent in order to prevent insufficient chamber saturation and the unwanted edge effect. The paper was moistened, and after being pressed against the chamber's walls, it clung to them. Before use, the chamber was given an hour to saturate. The studies were conducted in diffused daylight ta orom temperature [49].
Development and detection of spot: The TLC plates were dried for 5 min, and then the produced plates were examined in white light, 254 nm UV light, and 366 nm UV light. There were some places that could not be seen with the naked eye, thus detecting/visualizing chemicals like anisaldehyde solution were utilized [50].
Microbial toxins
Aflatoxins determination by Thin-Layer Chromatography (TLC): The aflatoxins in given drugs were analyzed by using TLC method [51].
Estimation of aflatoxin B–by TLC method: Methods of aflatoxin analysis are mainly based on their solubility characteristics in different solvents as well as on their characteristic fluorescent properties. The solubility of aflatoxins in organic solvents like chloroform, methanol, ethanol, acetone, acetonitrile, benzene etc., helps in their quantitative extraction from the various samples. Their insolubility in diethyl ether, petroleum ether and hexane helps in separating them from certain interfering pigments and fats. Their characteristic fluorescence and absorption in the long wavelength range have visible and UV light aids their detection and estimation [52].
Preparation of activated TLC plate: Prepare TLC plates of silica gel G (8-10 g with about 20 ml of water on a plate of 20 × 20 cm size) using an applicator (0.40 mm thickness). Air-dry the plates overnight. Activate the plates in oven at 110 ± 1°C for 1 h. Apply 5 or 10 μl of the diluted extract on the marked TLC plates (3 cm above the bottom edge of the plate) along with standard aflatoxin solution representing 2.5, 5.0, 7.5 and 10 μg concentrations. Run the plates to about 15 cm height in a solvent system (chloroform: Acetone, 90:10 v/v). Remove the plates and air dry. Observe the plates under UV light at 360 nm in a UV chromatography view cabinet. Compare the intensities of the fluorescent of the spots produced by the sample with those produced by the standard for quantifying the aflatoxin content in the sample [53].
TLC-bioautography for Reactive Oxygen Species (ROS)
In order to identify the antioxidant metabolites/reactive oxygen species in the best active extract, HPTLC bioautography technique was used [54].
Principle: The free radical scavenging activity of the extracts is evaluated by 1, 1-Diphenyl-2-Picryl-Hydrazyl (DPPH) reagent. DPPH is a free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. DPPH reacts with an antioxidant compound that can donate hydrogen and gets reduced. The change in colour (from deep violet to light yellow) was measured. The intensity of the yellow colour depends on the amount and nature of radical scavenger present in the sample or standard compounds [55].
Preparation of reagent: 0.1 mM methanolic DPPH solution was prepared by dissolving 1 mg of DPPH in 25 ml of methanol.
Selection of mobile phase: Two, pre coated aluminum sheet (10 × 10 cm) with silica gel 60 F254 of thickness 0.2 mm were used on which spots of best active extracts from both the plants in triplicate order with the band length of 5.0 mm were applied with the help of Linomat V5 applicator attached to HPTLC system which was programmed through WINCATS, the software which was installed with the system. After sample application, the plates were developed up to 80 mm in twin through glass chamber saturated with the respective solvent system. The plates were then dried at room temperature and dipped in with 0.1 mM methanolic DPPH solution and observed under visible light. Presence of reactive oxygen species were detected by yellowish spots against a purple background [56].
Fourier Transforms Infrared Spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy (FTIR) is a wellknown method for locating functional groups in a chemical molecule. The peaks in FTIR spectra assist identify the types of bonds and functional groups present in the chemical compound by illuminating the excitation energy of the molecules' vibration modes [57].
Principle: The fact that many gases absorb IR radiation at species-specific frequencies is the fundamental premise behind both NDIR analyzers and FTIR spectrometers. However, FTIR spectroscopy is a dispersing approach, which means measurements are carried out over a wide spectrum rather than a specific frequency range [58].
Using 2 ml cryotubes, the extract (2 g) was resuspended in 2 ml of the same solvent. The extract was dissolved using a vortex, and more emulsification was achieved by shaking the tube for nearly an hour. ATR-IR FTIR (Alpha 1; Bruker, Germany, Europe) was then used to examine the extract [59]. The extract was examined using the prescribed process in the scanning wave number range of 4000 to 500 cm-1 with a resolution of 4 cm-1 in order to produce IR spectra. By comparing the spectral data with references from the identification of functional groups present in the sample, interpretation of IR spectra acquired from the extract was accomplished [60].
Results
Procurement and identification of selected plant material
Procurement of plant materials: The selected plant materials i.e., Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. Leaves were procured from Khari Baoli, New Delhi.
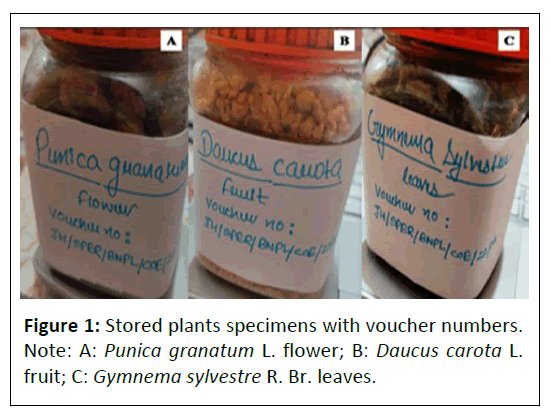
Identification and authentication of plant materials: The plant materials were identified and authenticated in-house using macroscopic and microscopic parameters. Macroscopical features include notable characteristics such as smell, colour, size and form. Microscopical characteristics include observational of specific microscopical characteristics. The authenticated sample specimens along with their voucher numbers have been deposited in the Bioactive Natural Product Laboratory (BNPL) for future reference which are enlisted in Table 1 and depicted in Figure 1.
| S. No | Plant species | Part used | Voucher no. |
|---|---|---|---|
| 1 | Punica granatum L. | Flower | JH/SPER/BNPL/COE/22/01 |
| 2 | Daucus carota L. | Fruit | JH/SPER/BNPL/COE/22/02 |
| 3 | Gymnema sylvestre R. Br. | Leaves | JH/SPER/BNPL/COE/22/03 |
Table 1: Authenticated plant specimens with their respective voucher numbers.
Macroscopy and microscopy
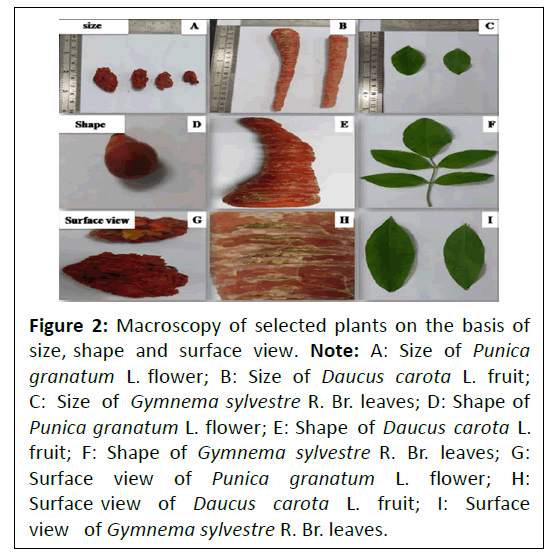
Macroscopic evaluation: Macroscopic characters of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves were evaluated on the basis of size, shape and surface view were shown in Figure 2.
Figure 2: Macroscopy of selected plants on the basis of size, shape and surface view. Note: A: Size of Punica granatum L. flower; B: Size of Daucus carota L. fruit; C: Size of Gymnema sylvestre R. Br. leaves; D: Shape of Punica granatum L. flower; E: Shape of Daucus carota L. fruit; F: Shape of Gymnema sylvestre R. Br. leaves; G: Surface view of Punica granatum L. flower; H: Surface view of Daucus carota L. fruit; I: Surface view of Gymnema sylvestre R. Br. leaves.
Punica granatum L. flowers were 3-7 cm long and 2 cm broad. Bright red in colour with 3-7 petals, rounded hexagonal in shape, orange to bright red surface view with red petals and numerous anthers attached to long red filament. Daucus carota L. fruits were found to be 5-50 cm in length with oblong-linear or narrow rhombic in shape and orange in colour. Gymnema sylvestre R. Br. leaves were 3-5 cm long and 3 cm broad, with elongated oval shape, brownish green in colour with no specific odour and bitter in taste.
Microscopy evaluation: The transverse sections of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves were evaluated for microscopic characters; which showed the presence of vascular bundles, vein inlets, epidermal cells, cuticle, xylem, phloem, cambium, palisade cells etc.
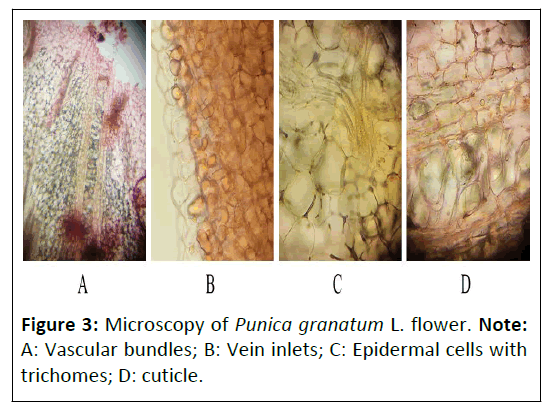
Punica granatum L. flower: The transverse section of Punica granatum L. showed the presence of epidermal cells with unicellular trichomes and were completely absent in the outer surface of epidermal cells, vein inlet was present in the outer region of epidermis. It also consisted of vascular bundles (xylem and phloem), the presence of rosette crystals and some parenchyma cells were also seen (Figure 3).
Figure 3: Microscopy of Punica granatum L. flower. Note: A: Vascular bundles; B: Vein inlets; C: Epidermal cells with trichomes; D: cuticle.
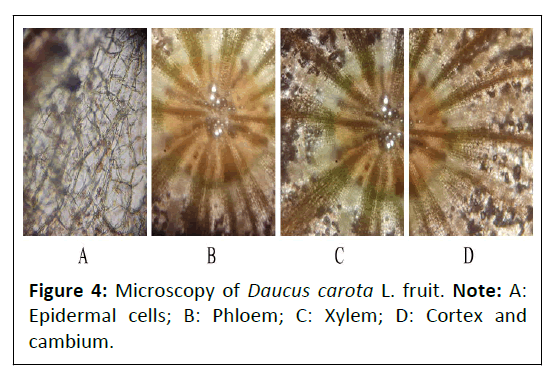
Daucus carota L. fruit: The transverse section of Daucus carota L. showed the presence of outer epidermis cells which consisted of different vascular bundles (xylem and phloem) in the upper epidermis layer, vein inlets were present at the margin of epidermal cells, multiple number of trichomes were present along with the epidermal cells and cuticle were present at the outer layer of epidermal cells (Figure 4).
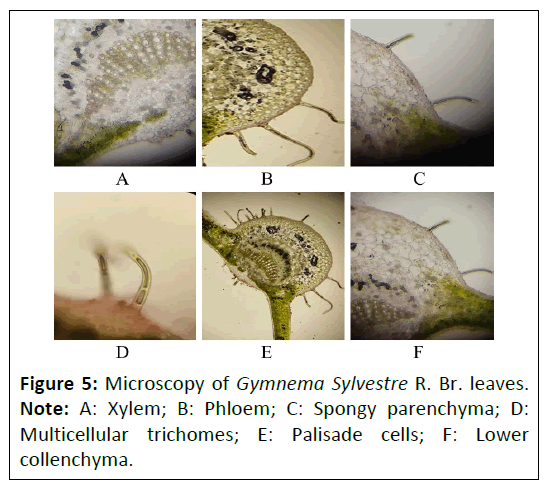
Gymnema sylvestre R. Br. leaves: The transverse section of leaf was found to be dorsiventral and showed the presence of midrib and lamina. The mid rib was broadly hemispherical with short lump on the adaxial side. Multicellular unbranched trichomes consist of single rows of cells were present on the both side of mid rib. Xylems were present in radial rows; multiple phloem cells were present on both upper and lower side of xylem stands. The epidermal cells of lamina were square shaped with outer convex and thin cuticle. Palisade tissue was found to be single layered, cylindrical. Four to five spongy parenchyma cells were loosely arranged. Anomocytic and paracytic stomata were also observed (Figure 5).
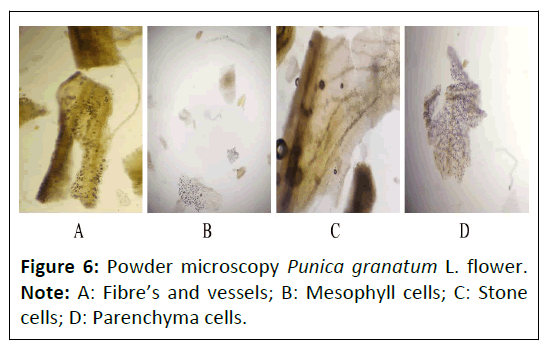
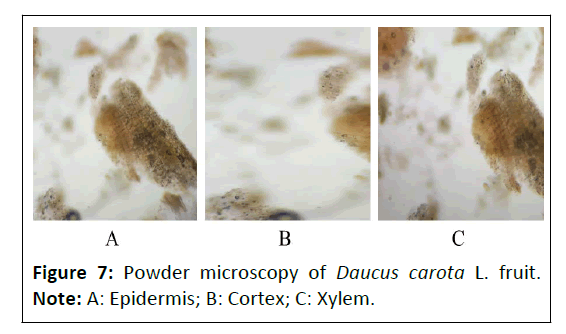
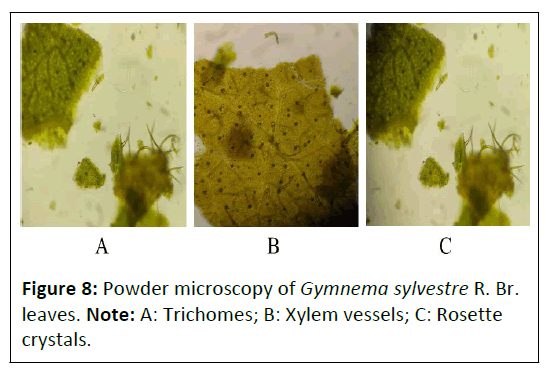
Powder microscopy: The powder characteristics of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves powder microscopy was shown in Figures 6-8, which showed the presence of many characteristics such as vascular bundles (xylem and phloem), epidermis layer, cortex, stomata, crystals, palisade, trichomes, fibers, stones cells mesophyll cells and parenchyma cells.
Powder microscopy of Punica granatum L. lower:The powder microscopy of Punica granatum L. flower showed the presence of different characteristics such as fibres and vessels, mesophyll cells, stone cells, parenchyma cells as depicted in Figure 6.
Powder microscopy of Daucus carota L. fruit: The powder microscopy of Daucus carota L. fruit showed the presence of different characteristics such as upper epidermis shows the presence of cortex layer and xylem vessels as depicted in Figure 7.
Powder microscopy of Gymnema sylvestre R. Br. Leaves: The powder microscopy of Gymnema sylvestre R. Br. leaves showed the presence of different characteristics such as upper epidermis shows the presence of glandular trichome, xylem vessels, crystal is present called rosette crystals as depicted in Figure 8.
Physico-chemical parameters: Physico-chemical parameters including foreign matter, moisture content total ash, acid insoluble ash, hexane soluble extractive yield and alcohol soluble extractive yield were evaluated. The results are tabulated in Table 2.
| Parameters | Punica granatum L. (Mean ± SD) | Daucus carota L. (Mean ± SD) | Gymnema sylvestre R. Br. (Mean ± SD) |
|---|---|---|---|
| Foreign matter (%) | 1 ± 0.251661 | 0.10 ± 0.036056 | 1 ± 0.655744 |
| Moisture content (%) | 0.6 ± 0.052042 | 1.34 ± 0.01 | 0.8 ± 0.025166 |
| Total ash (%) | 4.14 ± 0.360555 | 3.54 ± 0.01 | 12 ± 0.360555 |
| Acid insoluble ash (%) | 2.14 ± 0.075719 | 0.14 ± 0.015275 | 2 ± 0.251661 |
| Hexane soluble extractive yield (% w/w) | 7.4 ± 0.052915 | 0.8 ± 0.02 | 4.4 ± 0.064291 |
| Alcohol soluble extractive yield (% w/w) | 22 ± 0.360555 | 23 ± 0.5 | 6 ± 0.360555 |
Table 2: Physico-chemical parameters of Punica granatum, Daucus carota and Gymnema sylvestre plants.
The preparation of aqueous and hydro-alcoholic extracts was done using reflux condensation method (Figure 9).
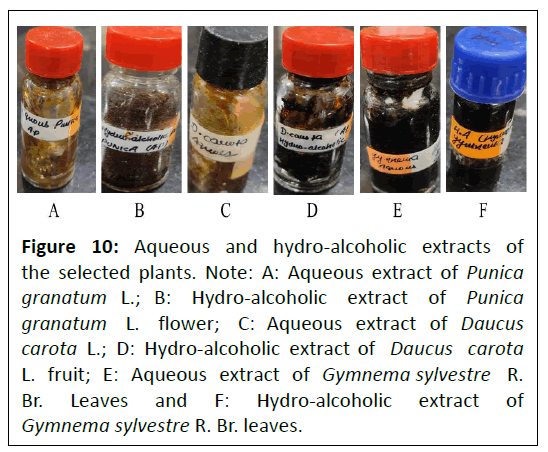
The prepared aqueous and hydro-alcoholic extracts of Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves are represented in Figure 10.
Figure 10: Aqueous and hydro-alcoholic extracts of the selected plants. Note: A: Aqueous extract of Punica granatum L.; B: Hydro-alcoholic extract of Punica granatum L. flower; C: Aqueous extract of Daucus carota L.; D: Hydro-alcoholic extract of Daucus carota L. fruit; E: Aqueous extract of Gymnema sylvestre R. Br. Leaves and F: Hydro-alcoholic extract of Gymnema sylvestre R. Br. leaves.
Extractive yields of selected plant materials were enlisted in Table 3.
| Plants name | Extractive yield (%) | |
|---|---|---|
| Aqueous (Mean ± SD) | Hydro-alcoholic (Mean ± SD) | |
| Punica granatum L. | 20 ± 0.51 | 16 ± 0.312 |
| Daucus carota L. | 20.04 ± 0.518 | 16.08 ± 0.555 |
| Gymnema sylvestre R. Br. | 33 ± 0.545 | 14 ± 0.513 |
Table 3: Extractive yields of the selected plants.
Quality control evaluations of extracts
Quality control evaluations of selected plant extracts were performed using spectrophotometric and chromatographic techniques.
Phyto-chemical screening: It includes TPC, TFC at 760 and 415, DPPH radical scavenging activity, Determination of reducing power.
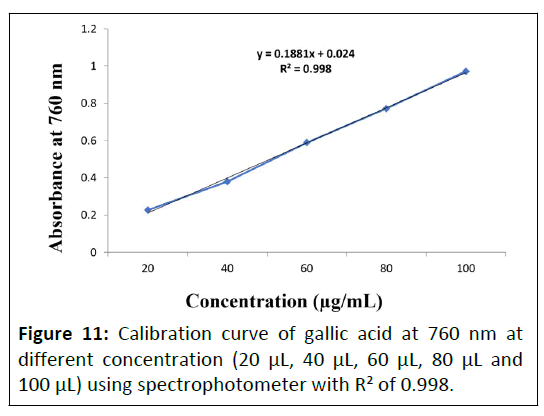
Total Phenolic Content (TPC) determination by spectrophotometer at 760 nm: Total phenolic determination of aqueous and hydro-alcoholic extracts of Punica granatum L. flower extract, Daucus carota L. fruit extract and Gymnema sylvestre R. Br. leaves were performed using spectrophotometer at 760 nm. Calibration curve of gallic acid standard is depicted in Figure 11 and obtained TPC of the prepared extracts were enlisted in Table 4.
| Plants | Extract | TPC (total phenolic content) (mg/g GAE) (Mean ± SD) |
|---|---|---|
| Punica granatumL. | Aqueous | 18.514 ± 0.298 |
| Hydro-alcoholic | 22.81 ± 0.464 | |
| Daucus carotaL. | Aqueous | 9.245 ± 0.0025 |
| Hydro-alcoholic | 11.75 ± 0.4202 | |
| Gymnema sylvestreR. Br. | Aqueous | 25.87 ± 0.4833 |
| Hydro-alcoholic | 18.53 ± 0.2926 |
Table 4: Total phenolic content of aqueous and hydroalcoholic extracts of Punica granatum L. lower extract, Daucus carota L. fruit extract and Gymnema sylvestre R. Br. leaves plants.
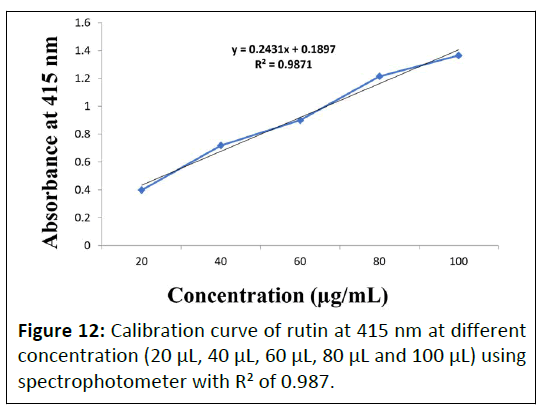
Total flavonoid determination by spectrophotometer at 415 nm: Total flavonoid determination of aqueous and hydroalcoholic extracts of Punica granatum L. flower extract, Daucus carota L. fruit extract and Gymnema sylvestre R. Br. leaves were performed using spectrophotometer at 415 nm. Calibration curve of rutin standard is depicted in Figure 12 and obtained TFC of the prepared extracts are enlisted in Table 5.
| Plants | Extract | TPC (total flavonoid content) (mg/g/RE) (Mean ± SD) |
|---|---|---|
| Punica granatum L. | Aqueous | 14.608 ± 0.335 |
| Hydro-alcoholic | 16.21 ± 0.1184 | |
| Daucus carota L. | Aqueous | 11.67 ± 0.3707 |
| Hydro-alcoholic | 13.17 ± 0.0953 | |
| Gymnema sylvestre R. Br. | Aqueous | 15.39 ± 0.2170 |
| Hydro-alcoholic | 15.87 ± 0.4808 |
Table 5: Total lavonoid content of aqueous and hydroalcoholic extracts of Punica granatum L. lower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves plants.
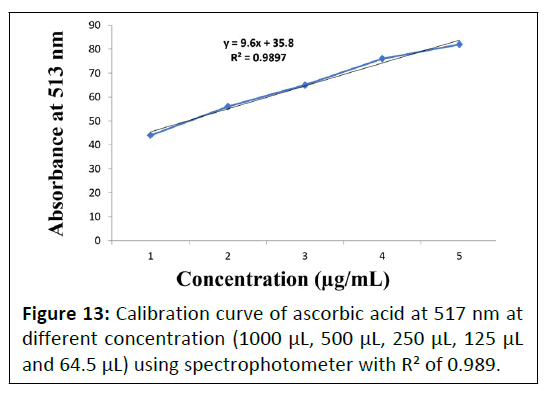
DPPH radical scavenging activity at 517 nm: DPPH radical scavenging activity of Punica granatum L. flower extract, Daucus carota L. fruit extract and Gymnema sylvestre R. Br. leaves were performed using spectrophotometer at 517 nm. Calibration curve of ascorbic acid standard is depicted in Figure 13 and obtained DPPH assay of the prepared extracts are enlisted in Table 6.
| Plants | Extract | DPPH radical scavenging activity Ic50 µg/mL (Mean ± SD) |
|---|---|---|
| Punica granatum L. | Aqueous | 37 ± 0.661 |
| Hydro-alcoholic | 68.6 0 ± 0.608 | |
| Daucus carota L. | Aqueous | 57.3 ± 0.404 |
| Hydro-alcoholic | 92 ± 0.53 | |
| Gymnema sylvestre R. Br. | Aqueous | 227 ± 1.527 |
| Hydro-alcoholic | 320 ± 2.516 |
Table 6: DPPH radical scavenging activity of aqueous and hydro-alcoholic extracts of Punica granatum L. lower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves plants.
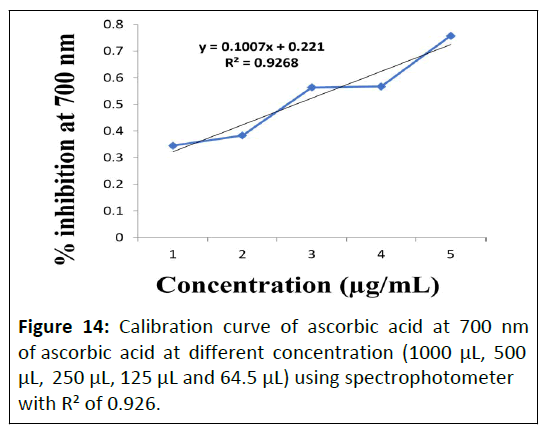
Determination of reducing power of extracts at 700 nm: Determination of reducing power assay of Punica granatum L. flower extract, Daucus carota L. fruit extract and Gymnema sylvestre R. Br. leaves were using spectrophotometer at 700 nm. Calibration curve of ascorbic acid standard is depicted in Figure 14 and obtained reducing power assay of the prepared extracts are enlisted in Table 7.
| Plants | Extract | Reducing power Assay Ic50 µg/mL (Mean ± SD) |
|---|---|---|
| Punica granatum L. | Aqueous | 21.49 ± 0.025 |
| Hydro-alcoholic | 68.6 ± 0.047 | |
| Daucus carota L. | Aqueous | 34.6 ± 0.052 |
| Hydro-alcoholic | 89.6 ± 0.025 | |
| Gymnema sylvestre R. Br. | Aqueous | 73.7 ± 0.084 |
| Hydro-alcoholic | 88.2 ± 0.011 |
Table 7: Determination of reducing power of aqueous and hydro-alcoholic extracts of Punica granatum L. lower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves plants.
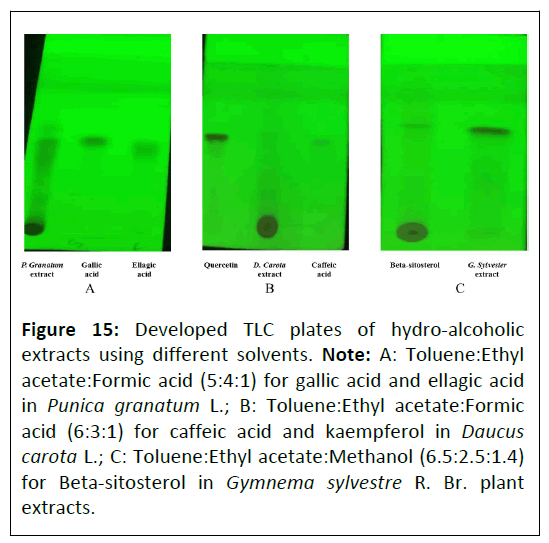
Thin layer chromatography: Thin layer chromatography of Punica granatum L., Daucus carota L. and Gymnema sylvestre R. Br. extracts was done by preparing sample solution in respective solvents by dissolving 30 mg of dried extracts per milliliter solvents. Then, prepared solutions were centrifuged separately to collect the supernatant. The collected supernatants were filtered, and samples were spotted on TLC plate. Developed TLC plates are illustrated in Figure 15.
Figure 15: Developed TLC plates of hydro-alcoholic extracts using different solvents. Note: A: Toluene:Ethyl acetate:Formic acid (5:4:1) for gallic acid and ellagic acid in Punica granatum L.; B: Toluene:Ethyl acetate:Formic acid (6:3:1) for caffeic acid and kaempferol in Daucus carota L.; C: Toluene:Ethyl acetate:Methanol (6.5:2.5:1.4) for Beta-sitosterol in Gymnema sylvestre R. Br. plant extracts.
High Performance Thin Layer Chromatography (HPTLC)
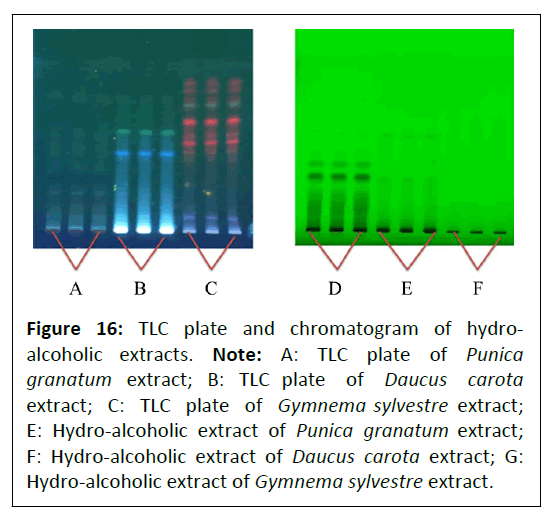
Qualitative estimation: Qualitative estimation of Punica granatum L., Daucus carota L. and Gymnema sylvestre R. Br. extracts was done using HPTLC. Prepared samples of hydro- alcoholic extracts were separately applied on the TLC plates as 5.0 mm bands with samples volume of 2.0 μL, 10 mm from the lower edge, with 15 mm distance from each side, and a minimum distance of 2.0 mm between each track. TLC plates were transferred in a development chamber (CAMAG) and developed using Toluene:Ethyl-acetate:Formic acid (5:4:1) as a solvent system. The developed plates were air-dried. Further, the developed plates were scanned at 254 and 366 nm for fingerprint analysis (Figure 16).
Figure 16: TLC plate and chromatogram of hydroalcoholic extracts. Note: A: TLC plate of Punica granatum extract; B: TLC plate of Daucus carota extract; C: TLC plate of Gymnema sylvestre extract; E: Hydro-alcoholic extract of Punica granatum extract; F: Hydro-alcoholic extract of Daucus carota extract; G: Hydro-alcoholic extract of Gymnema sylvestre extract.
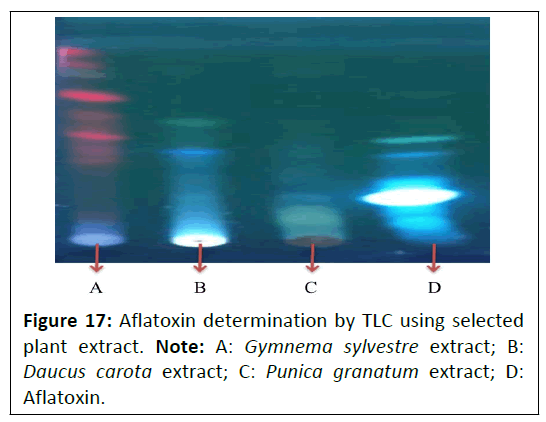
A latoxin determination by TLC: The determination of aflatoxins was done by TLC using Toluene:Ethyl-acetate:Formic acid (6:3:1) as solvent system. Aflatoxin determination of Punica granatum L., Daucus carota L. and Gymnema sylvestre R. Br. Extracts are depicted in Figure 17.
No aflatoxins were detected in the Punica granatum L., Daucus carota L., and Gymnema sylvestre R. Br. plant extracts.
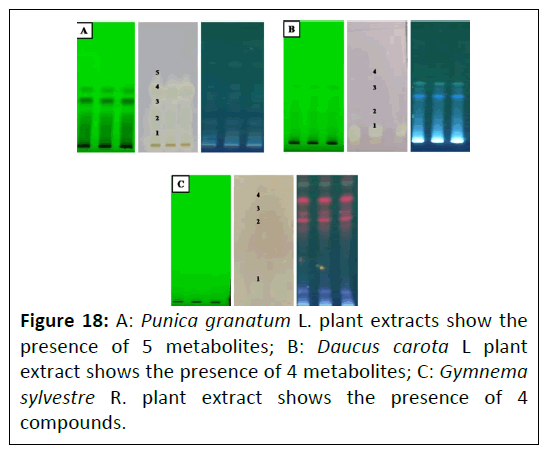
TLC-bioautography for Reactive Oxygen Species (ROS): The free radical scavenging activity of Punica granatum L., Daucus carota L., and Gymnema sylvestre R. Br. Plant extracts were confirmed using DPPH activity. After developing the samples on TLC plates, the plates were dipped in a solution of 5.0 mM DPPH. Bright yellowish- or cream-colored bands appeared on the purple background indicating the antioxidant metabolites, depicted in Figure 18; Punica granatum L. plant extracts show the presence of 5 metabolites, whereas Daucus carota L. plant extract shows the presence of 4 metabolites, while; Gymnema sylvestre R. Br.plant extract shows the presence of 4 compounds.
Fourier Transforms Infrared Spectroscopy (FTIR)
The FTIR spectra of different plant extracts of Punica granatum L., Daucus carota L., and Gymnema sylvestre R. Br. at different wavelengths along with their functional groups are depicted in Figures 19-21.
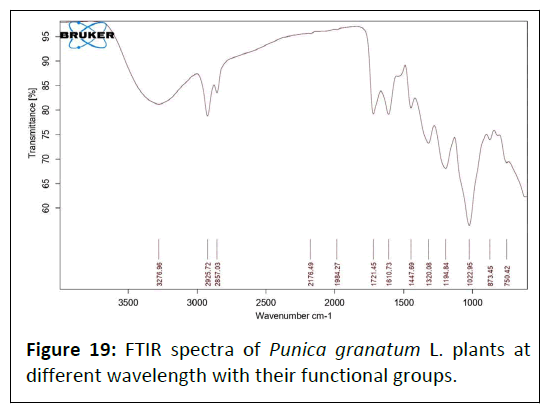
FTIR of Punica granatum L. plant: Fourier Transform Infrared (FTIR) spectrum of the Punica granatum L. extract is represented in Figure 19. The absorption peaks located mainly at 3276/cm are generally attributed to aromatic or aliphatic O–H stretching, 2325 and 2857 and 1984/cm are generally assigned to the alkyl C–H stretching, 2176/cm are represented to the alkenes C≡ C stretching band; whereas peak at 1721 assigned to the C=O group 1452, 1610/cm represented to the C=C, C=N groups, 1447, 1320 and 1194/cm depicted to the C-N (cyanide group) stretching, 1022/cm assigned to the N-N and C-H, 873 and 750/ cm assigned to the C-H wagging vibrations (Table 8).
| S. No | Wave number cm−1 | Functional group |
|---|---|---|
| 1 | 3276 | O-H |
| 2 | 2925 | C-H |
| 3 | 2857 | C-H |
| 4 | 2176 | C≡C |
| 5 | 1984 | C-H |
| 6 | 1721 | C=O |
| 7 | 1610 | C=C, C=N |
| 8 | 1447 | C-N |
| 9 | 1320 | C-N stretching + C-H |
| 10 | 1194 | C-N |
| 11 | 1022 | N-N + C-H |
| 12 | 873 | C-H wagging |
| 13 | 750 | C-H wagging |
Table 8: FTIR data of Punica granatum L. plant.
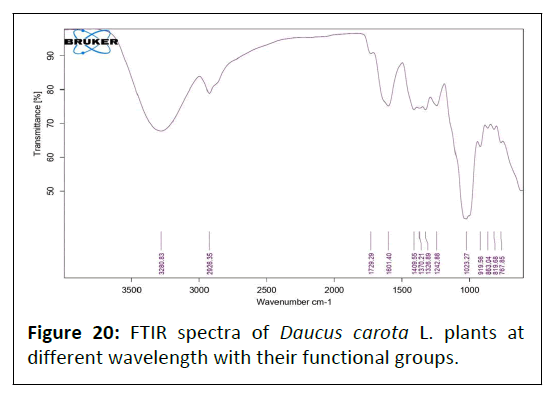
FTIR data of Daucus carota L. plant: Fourier Transform Infrared (FTIR) spectrum of the Daucus carota L. extract is represented in Figure 20. The absorption peaks located mainly at 3280/cm are generally attributed to aromatic or aliphatic O–H stretching, 2926/cm are generally assigned to the alkyl C–H stretching, 1729/cm are represented to the C=O (carboxyl) group, 1409/cm assigned to the C-N group; whereas peaks at 1370, 1326, 1242/cm assigned to the C-N stretching and C-H group, 1023/cm represented to the N-N stretching and C-H (alkyl) group, 919, 863, 819 and 767/cm assigned to the C-H wagging vibrations (Table 9).
| S. No | Wave number cm−1 | Functional group |
|---|---|---|
| 1 | 3280 | O-H |
| 2 | 2926 | C-H |
| 3 | 1729 | C=O |
| 4 | 1601 | C=N |
| 5 | 1409 | C-N |
| 6 | 1370 | C-N stretching, C-H |
| 7 | 1326 | C-N stretching, C-H |
| 8 | 1242 | C-N stretching |
| 9 | 1023 | N-N stretching + C-H |
| 10 | 919 | C-H wagging |
| 11 | 863 | C-H wagging |
| 12 | 819 | C-H wagging |
Table 9: FTIR data of Daucus carota L. plant.
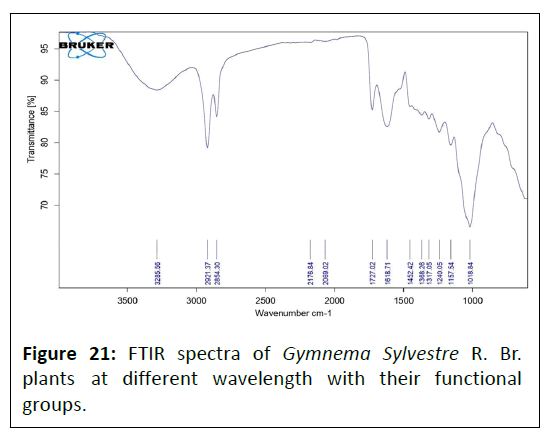
FTIR data of Gymnema sylvestre R. Br. plant: Fourier Transform Infrared (FTIR) spectrum of the Gymnema Sylvestre R. Br. extract is represented in Figure 21. The absorption peaks located mainly at 3285/cm are generally attributed to aromatic or aliphatic O–H stretching, 2921 and 2854/cm are generally assigned to the alkyl C–H stretching, 2176 and 2069/cm are represented to the C≡N (cyanide) group, 1727/cm assigned to the C=O stretching band, 1618/cm represented to the C=C, C=N groups, 1452/cm assigned to the C-N group; whereas peaks at 1368, 1317, 1240 and 1018/cm assigned to the C-N stretching and C-H group, 1157/cm represented to the N-N stretching and C-H (alkyl) groups (Table 10).
| S. No | Wave number cm−1 | Functional group |
|---|---|---|
| 1 | 3285 | O-H |
| 2 | 2921 | C-H |
| 3 | 2854 | C-H |
| 4 | 2176 | C≡N |
| 5 | 2069 | C≡N |
| 6 | 1727 | C=O |
| 7 | 1618 | C=C, C=N |
| 8 | 1452 | C-N |
| 9 | 1368 | C-N stretching + C-H |
| 10 | 1317 | C-N stretching + C-H |
| 11 | 1240 | C-N stretching |
| 12 | 1157 | N-N stretching + C-H |
| 13 | 1018 | C-N stretching + C-H |
Table 10: FTIR data of Gymnema sylvestre R. Br. plant.
Discussion
Traditional medicinal herbs have a long history and are still employed as first-line treatment alternatives. In developed and developing countries, traditional medicinal herbs are being used as alternatives to modern medicine to treat a variety of conditions.
In Ayurveda and Unani, a number of medicinal plants have been used individually or in combination with other herbs to treat a variety of ailments and health issues [4]. As mentioned in various pharmacopoeias, Punica granatum L. (pomegranate) can help in treatment of various disease risk factors including high blood pressure, high cholesterol, oxidative stress, hyperglycemia, and inflammatory activities (API, Part-I, Vol-II). Daucus carota L. (carrot) is a popular medicinal plant with various pharmacological activities including antioxidant, analgesic, anti-inflammatory, antimicrobial, antifungal, diuretic, lithontripic, intra ocular hypotensive, gastroprotective, hepatoprotective, aphrodistic, nephroprotective, antispasmodic, anticancer, antiestrogenic, cardioprotective and wound healing activities (UPI, Part-I, Vol-II) whereas, Gymnema sylvestre R. Br. (gurmar) has potent anti-diabetic properties. It is also used for controlling obesity in the form of Gymnema tea (API, Part-I, Vol- V). These plant materials were extracted in two different solvents to obtain aqueous and hydro-alcoholic extracts. Aqueous extract was found to exhibit a higher extractive yield as compared to hydro-alcoholic extract. The percentage yields for aqueous extracts were 20%, 20.04% and 33% and for hydroalcoholic 16.08%, 16% and 14% for Punica granatum L. flower, Daucus carota L. fruit and Gymnema sylvestre R. Br. leaves respectively. The obtained extractive values were in compliance with the reported values (API, Part-I, Vol -II), (UPI, Part-I, Vol-II) and (API, Part-I, Vol-V). that have long been in attention due to their health-promoting, disease-curing, and disease prevention properties. DPPH and reducing power assay is another method for the determination of antioxidant compounds (using spectrophotometer). Table 10 signifies the obtained values for the Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) of the aqueous and hydro-alcoholic extracts respectively using Folin-Ciocalteau and colorimetric assay. Many phenolic and flavonoid compounds have been reported to have antioxidants properties [60]. The obtained values for total phenolic content and total flavonoid content were in compliance with reported values [60]. The antioxidant capacity of the extracts of each plant material was expressed as equivalent of ascorbic acid and the obtained values for DPPH scavenging activity and reducing power assay. Hence the obtained values for DPPH and reducing power assay were in compliance with reported values.
The quality control analysis of herbal-based products is one of the major issues. To obtain metabolite patterns from any extract of plant materials, TLC profiling is frequently used. The TLC profiling of the plant materials can be used for quality control. TLC fingerprinting for identifying bioactive metabolites were shown in Figure 16 at 254 and 366 nm, respectively using and the selected solvent system Toluene:Ethyl acetate:Formic acid (5:4:1) [47].
To screen the antioxidant activity of different extract, TLCbioautography was performed [46]. After separation of prepared extracts on the TLC plates, the compounds with radical scavenging activity were determined using DPPH reagent. Maximum number of antioxidant metabolites were present in Punica granatum L. extract, whereas minimum number of metabolites were found in Daucus carota L. and Gymnema sylvestre R. Br. extract as represented in Figure 18, hence the obtained value were in compliance with reported values [46].
Fourier Transform Infrared Spectroscopy (FTIR) is a wellknown method for locating functional groups in a chemical molecule. The peaks in FTIR spectra assist identify the types of bonds and functional groups present in the chemical. The FTIR spectra of Punica granatum L. were obtained such as the absorption peak at 3280/cm showed the presence of O–H stretching, 2926/cm were generally assigned to the alkyl C–H stretching, 1729/cm were represented to the C=O (carboxyl) group, 1409/cm assigned to the C-N group; whereas peaks at 1370, 1326, 1242/cm assigned to the C-N stretching and C-H group, 1023/cm represented to the N-N stretching and C-H (alkyl) group, 919, 863, 819 and 767/cm assigned to the C-H wagging vibrations, hence the obtained FTIR spectra values of Punica granatum L. were in compliance with reported values [33]. The FTIR spectra of Daucus carota L. were obtained such as the peaks at 3280/cm showed the presence of O–H stretching, 2926/cm were generally assigned to the alkyl C–H stretching, 1729/cm were represented to the C=O (carboxyl) group, 1409/cm assigned to the C-N group; whereas peaks at 1370, 1326, 1242/cm assigned to the C-N stretching and C-H group, 1023/cm represented to the N-N stretching and C-H (alkyl) group, 919, 863, 819 and 767/cm assigned to the C-H wagging vibration, hence the obtained FTIR spectra values of Daucus carota L. were in compliance with reported values [1]. The FTIR spectra of Gymnema Sylvestre R. Br. were obtained such as the peaks at 3285/cm are generally showed the presence O–H stretching, 2921 and 2854/cm were generally assigned to the alkyl C–H stretching, 2176 and 2069/cm were represented to the C≡N (cyanide) group, 1727/cm assigned to the C=O stretching band, 1618/cm represented to the C=C, C=N groups, 1452/cm assigned to the C-N group; whereas peaks at 1368, 1317, 1240 and 1018/cm assigned to the C-N stretching and C-H group, 1157/cm represented to the N-N stretching and C-H (alkyl) groups, hence the obtained FTIR spectra values of Gymnema Sylvestre R. Br. were in compliance with reported values [57].
The developed article can serve as a reference standard for studying various phytochemical and pharmacognostical parameters and quality standards of the selected plants.
Conclusion
The quality control of natural products has become more challenging, demanding as well as stringent. With the remarkable worldwide increase in the use and demand of herbal drugs, the quality control has become imperative for consumers and health authorities to assure the use of standardized and authentic plant-derived therapeutic materials.
In the present study, the quality control studies of flower of Punica granatum L. fruit of Daucus carota L. and leaves of Gymnema sylvestre R. Br. plants were carried out based on physicochemical, phytochemical, and microbial tests. Simple, rapid, and selective HPTLC methods were developed for quantitative determination of major metabolites with versatile bio-functions. Furthermore, the TLC-bioautography of the plants led to the identification of major antioxidant compounds.
We have developed monographs for the plants Punica granatum L., Daucus carota L., and Gymnema sylvestre R. Br. Plants, respectively based on the quality control characteristics that would be included in pharmacopoeias.
Author Contributions
A.K., Conceptualization, literature review and draft writing, data curation, draft editing, and manuscript preparation, assistance in manuscript writing, draft editing, S.S., Investigation and manuscript preparation, visualization and critical evaluation of the manuscript.
Conflict of Interest
No potential conflict of interest to declare.
Data Availability Statement
All required data is mentioned in the manuscript and can be produced by the supervisor upon request.
Funding
No funding sources.
References
- Abbas FM, Shnawa AF, Hameed IH (2019) Daucus carota: In vitro antimicrobial activity and bioactive compounds of methanolic fruit extract using FTIR spectroscopic analysis. Indian J Public Health Res Dev 10: 948-953.
- Agnihotri AK, Khatoon S, Agarwal M, Rawat AKS, Mehrotra S, et al. (2004) Pharmacognostical evaluation of Gymnema sylvestre R. Br. Nat Prod Sci 10: 168-172.
- Agustin a JD (2018) Administration of moisture balance ointment from carrots (Daucus carota L.) and irrigation of boiled water from guava leaves (Psidium guajava Linn) are effective for accelerating the healing of acute contaminated wounds in mice (Mus musculus). STIKES Insan Medika Scholars Jombang, Indonesia.
- Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, et al. (2021) Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Front Pharmacol 11: 578970.
[Crossref] [Google Scholar] [Indexed]
- Akhtar S, Ismail T, Fraternale D, Sestili P (2015) Pomegranate peel and peel extracts: Chemistry and food features. Food Chem 174: 417-425.
[Crossref] [Google Scholar] [Indexed]
- Alam P, Kamal YT , Alqasoumi SI, Foudah AI, Alqarni MH, et al. (2019) HPTLC method for simultaneous determination of ascorbic acid and gallic acid biomarker from freeze dry pomegranate juice and herbal formulation. Saudi Pharm J 27: 975-980.
[Crossref] [Google Scholar] [Indexed]
- Anis M, Sharma MP, Iqbal M (2000) Herbal ethnomedicine of the Gwalior forest division in Madhya Pradesh, India. Pharm Biol 38: 241-253.
[Crossref] [Google Scholar] [Indexed]
- Arun N, Singh DP (2012) Punica granatum: A review on pharmacological and therapeutic properties. Int J Pharm Sci Res 3: 1240-1245.
- Ayeni EA, Abubakar A, Ibrahim G, Atinga V, Muhammad Z (2018) Phytochemical, nutraceutical and antioxidant studies of the aerial parts of Daucus carota L (Apiaceae). J Herbmed Pharmacol 7: 68-73.
- Bagri P, Ali M, Aeri V, Bhowmik M, Sultana S (2009) Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem Toxicol 47: 50-54.
[Crossref] [Google Scholar] [Indexed]
- Bakkiyaraj D, Nandhini JR, Malathy B, Pandian SK (2013). The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling 29: 929-937.
[Crossref] [Google Scholar] [Indexed]
- Ball DW (2001) The basics of spectroscopy 49: Spie press.
- Banihani S, Swedan S, Alguraan Z (2013) Pomegranate and type 2 diabetes. Nutr Res 33: 341-348.
[Crossref] [Google Scholar] [Indexed]
- Behera SK (2019) Phytochemical analysis and antioxidant activities of Gymnema sylvestre R. Br. Leaf Extracts. Free radic antioxid 9: 12-15.
- Bhowmik D, Gopinath H, Kumar BP, Kumar, KPS (2013) Medicinal uses of Punica granatum and its health benefits. J pharmacogn phytochem 1: 28-35.
- Bhujbal SS, Chawale BG, Kale MA (2022) Application based studies of HPTLC-bioautography in evaluation of botanicals: A review. J Anal Chemd 77: 473-483.
- Chaaib F, Queiroz EF, Ndjoko K, Diallo D, Hostettmann K (2003) Antifungal and antioxidant compounds from the root bark of Fagara zanthoxyloides. Planta Med 69: 316-320.
[Crossref] [Google Scholar] [Indexed]
- Choma IM, Grzelak EM (2011) Bioautography detection in thin-layer chromatography. J Chromatogr 1218: 2684-2691.
[Crossref] [Google Scholar] [Indexed]
- Clementz A, Torresi PA, Molli JS, Cardell D, Mammarella E, et al. (2019) Novel method for valorization of by-products from carrot discards. LWT 100: 374-380.
- Daisy ERAC, Rajendran NK, Houreld NN, Marraiki N, Elgorban AM, et al. (2020) Curcumin and Gymnema sylvestre extract loaded graphene oxide-polyhydroxybutyrate‑sodium alginate composite for diabetic wound regeneration. React Funct Polym 154: 104671.
- Dhawan BN, Patnaik GK, Rastogi RP, Singh KK, Tandon JS (1977) Screening of Indian plants for biological activity: Part VI. Indian J Exp Biol 15: 208-219
- Diem M, Chiriboga L, Lasch P, Pacifico A (2002) IR spectra and IR spectral maps of individual normal and cancerous cells. Biopolymers 67: 349-353.
[Crossref] [Google Scholar] [Indexed]
- Donald PL, Lampman GM, Kritz GS, Engel RG (2006) Introduction to organic laboratory techniques. (3rd edition) Thomson Brooks/Cole, Pacific Grove, California: 797–817.
- Engla K (2021) Phytochemical and pharmacological review of carrot (Daucus carota L). IJPSM 6: 75-82.
- Grdadolnik J (2002) ATR-FTIR spectroscopy: Its advantage and limitations. Acta Chimica Slovenica 49: 631-642.
- Griffiths PR (1983) Fourier transform infrared spectrometry. Science 222: 297-302.
[Crossref] [Google Scholar] [Indexed]
- Guerrero-Solano JA, Jaramillo-Morales OA, Jiménez-Cabrera T, Urrutia-Hernández TA, Chehue-Romero A, et al. (2020) Punica protopunica Balf., the forgotten sister of the common pomegranate (Punica granatum L.): features and medicinal properties-A review. Plants 9: 1214.
[Crossref] [Google Scholar] [Indexed]
- (2005) Validation of analytical procedures: Text and methodology Q2(R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Chicago, USA: 1-5.
- Gupta AK (2003) Anonymous: Quality standards of Indian medicinal plants. (1st edition) Indian Council of Medical Research, New Delhi, India, 1: 212–218.
- Haque N, Sofi G, Ali W, Rashid M, Itrat M (2015) A comprehensive review of phytochemical and pharmacological profile of Anar (Punica granatum Linn): A heaven’s fruit. J Ayu Herb Med 1: 22-26.
- (2016) Ayurvedic pharmacopeia of India. (1st edition) Pharmacopoeia Commission for Indian Medicine and Homoeopathy, Ghaziabad, India.
- Janssens M (2006) 2-Fundamental measurement techniques. Woodhead Publishing, United Kingdom: 22–62.
- Kaur P, Thakur R, Chaudhury A (2016) Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. GCLR 9: 33-38.
[Crossref] [Google Scholar] [Indexed]
- Kaur S, Gurumayum S, Rasane P (2015). effect of hot water blanching time and 660 drying temperature on the thin layer drying kinetics of and anthocyanin degradation in 661 black carrot (Daucus carota L.) Shreds. Food Technol Biotechnol 53: 324-330.
[Crossref] [Google Scholar] [Indexed]
- Khan F, Sarker MMR, Ming LC, Mohamed IN, Zhao C, et al. (2019) Comprehensive review on phytochemicals, pharmacological and clinical potentials of Gymnema sylvestre. Front Pharmacol 10: 1223.
[Crossref] [Google Scholar] [Indexed]
- Khramov VA, Spasov AA, Samokhina MP (2008) Chemical composition of dry extracts of Gymnema sylvestre leaves. Pharm Chem J 42: 29-31.
- Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4: 118-126.
[Crossref] [Google Scholar] [Indexed]
- Mabasa XE, Mathomu LM, Madala NE, Musie EM, Sigidi MT (2021) Molecular spectroscopic (FTIR and UV-Vis) and hyphenated chromatographic (UHPLC-qTOF-MS) analysis and in vitro bioactivities of the momordica balsamina leaf extract. Biochem Res Int 2021: 2854217.
[Crossref] [Google Scholar] [Indexed]
- Makita C, Chimuka L, Steenkamp P, Cukrowska E, Madala E (2016) Comparative analyses of flavonoid content in Moringa oleifera and moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. S Afr J Bot 105: 116-122.
- Marston A, Kissling J, Hostettmann K (2002) A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem Anal 13: 51-54.
[Crossref] [Google Scholar] [Indexed]
- Moga MA, Dimienescu OG, Bălan A, Dima L, Toma SI, et al. (2021) Pharmacological and therapeutic properties of Punica granatum phytochemicals: Possible roles in breast cancer. Molecules 26: 1054.
[Crossref] [Google Scholar] [Indexed]
- Bekbolatova EN, Kurbatova NV, SakipovaZB, Ibragimova LN, Alpysbayeva SI, et al. (2018) Macroscopic and microscopic diagnostic features of the potential herbal drug Crataegus Almaatensis Pojark endemic in Kazakhstan. Iran J Pharmaceut Sci 14: 39-50.
- Nadkarni KM (2006) Indian plants and drugs: (with their medicinal properties and uses). Asiatic Publishing House, Delhi, India.
- Newman RA, Lansky EP, Block ML (2007) Pomegranate: The most medicinal fruit. Basic Health Publications, California, USA .
- Nguyen HHV, Nguyen LT (2015) Carrot processing. (2nd edition) Handbook of Vegetable Preservation and Processing. CRC Press, Florida, USA: 449–466.
- Parveen R, Khan N, Zahiruddin S, Ibrahim M, Anjum V, et al. (2020) TLC-bioautographic evaluation for high-throughput screening and identification of free radical scavenging and antidiabetic compounds from traditional unani medicinal plant: Citrullus colocynthis Schrad. J AOAC Int 103: 669-677.
[Crossref] [Google Scholar] [Indexed]
- Parveen R, Zahiruddin S, Charegaonkar A, Khale A, Mallick S (2020) Chromatographic profiling of rose petals in unani formulations (Gulkand, Arq-e-Gulab, and Rose Sharbat) using HPTLC and GC–MS. J AOAC Int, 103: 684-691.
[Crossref] [Google Scholar] [Indexed]
- Patel MR (2017) Pharmacognostic and phytochemical evaluation of Gymnema sylvestre leaf. World J Pharm Pharm Sci 6: 1538.
- Potawale SE, Gabhe SY, Mahadik KR (2014) Quantification of Gymnemagenin and β-Sitosterol in marketed herbal formulation by validated normal phase HPTLC method. Chromatogr Res Int 2014: 92-97.
- Rajan S, Mahalakshmi S, Deepa VM, Sathya K, Shajitha S, et al.(2011) Antioxidant potentials of Punica granatum fruit rind extracts. Int J Pharm Pharm Sci, 3: 82-88.
- Roth L, Adler M, Jain T, Bempong D (2018) Monographs for medicines on WHO’s model list of essential medicines. Bull World Health Organ 96: 378-385.
[Crossref] [Google Scholar] [Indexed]
- Salisu B, Anua SM, Ishak WRW, Mazlan N (2021) Development and validation of quantitative thin layer chromatographic technique for determination of total aflatoxins in poultry feed and food grains without sample clean-up. J Adv Vet Anim Res 8: 656.
[Crossref] [Google Scholar] [Indexed]
- Schibli A, Reich E (2005) Stationary phases for planar separations-plates for modern TLC. LCGC North America 23: 458-469.
- Sheikh MK, Manjula N (2012) Effect of chemicals on control of fruit cracking in pomegranate (Punica granatum L.) var. Ganesh. II International Symposium on the Pomegranate 103: 133-135.
- Sherma J, Fried B (2003) Handbook of thin-layer chromatography. (3rd edn), CRC press, Boca Raton, United States: 1-1048.
- Smith BC (2011) Fundamentals of fourier transform infrared spectroscopy. CRC press, Boca Raton, USA.
- Subashini MS, Rajendran P, Ashok G, Kanthesh BM (2015) TLC, FTIR and GCMS analysis of leaves of Gymnema sylvestre R. Br from Kolli Hills, Tamil Nadu, India. Int J Curr Microbiol App Sci 4: 757-764.
- Sudberg S, Sudberg EM, Terrazas J, Sudberg S, Patel K, et al. (2010) Fingerprint analysis and the application of HPTLC to the determination of identity and quality of botanicals, from an industry perspective. J AOAC Int 93: 1367–1375.
- Tiwari P, Mishra BN, Sangwan NS (2014) Phytochemical and pharmacological properties of Gymnema sylvestre: An Important Medicinal Plant. Biomed Res Int. 2014: 830285
[Crossref] [Google Scholar] [Indexed]
- Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 5: 93.
[Crossref] [Google Scholar] [Indexed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences