ISSN : 0976-8505
Der Chemica Sinica
Preparation and Application of Zeolitic Adsorbents for Removal of Fuoride from Aqueous Solution; Equilibrium, Kinetic and Thermodynamic Studies
Z. Abaei, H Faghihian* and N. Esmaeeli
Department of Chemistry, Shahreza Branch, Islamic Azad University, Shahreza, Iran. Shahreza, Isfahan, Iran
Abstract
The presence of fluoride in drinking water has profound effect on teeth and bones strengthening. But at concentrations higher than 1.5 mgL-1, fluoride becomes a serious contaminant of water. In this study, removal of fluoride from aqueous solution was studied by adsorbent prepared by modification of Y-zeolite. The parent and modiï¬ÂÂed zeolites were characterized by Fourier Transform Infrared Spectrometry (FTIR), scanning electron microscopy (SEM) and X-ray diffraction (XRD. (The effect of different parameters including pH, fluoride concentration, contact time and temperature on the adsorption capacity of the adsorbents was investigated. At the optimized conditions, the adsorption capacity of 200 mgg-1 was obtained which was higher than the previous reported values. The fluoride adsorption on modified zeolite (Fe-Y) was well described by the Freundlich adsorption isotherm model. The kinetic of adsorption followed pseudo second-order model and the process was endothermic.
Keywords
Fluoride, Y-zeolite, Modified zeolite, Ion exchange, Adsorption
Introduction
When fluoride concentrations are above the permissible level, it is considered as a health hazardous compound. World Health Organization classified fluoride as one of the contaminants of water for human consumption [1,2]. Pollution of water by fluoride occurs through natural resources and human activities [3]. Various minerals such as fluorite, biotites, topaz, and their corresponding host rocks such as granite, basalt, and syenite, contain fluoride that is gradually released into groundwater [4-6]. Besides the natural geological sources, various industries intensively contribute to fluoride pollution. The industries which discharge wastewater containing high fluoride concentrations include nuclear installments, glass and ceramic production, semiconductor manufacturing, electroplating, coal ï¬ÂÂred power stations, beryllium extraction plants, brick and iron works, and aluminum smelters [7,8]. Fluoride as a strong electronegative anion is combined with positively charged calcium of teeth and bones resulting dental, skeletal, and non-skeletal forms of fluorosis and associated health complaints [9]. For removal of excessive amount of fluoride from drinking water, and waste streams several methods including adsorption [10,11] and precipitation [12] have been investigated. Among them, adsorption method due to its simplicity, low cost, and environmental friendly is frequently used. Different adsorbents have been examined for fluoride removal, including activated alumina [13], carbon nanotubes [14], calcite [15], red mud [16] and zeolite [17].
Zeolites are three dimensional aluminosilicate materials with porous structure that have valuable properties such as cation exchange, molecular sieving, catalysis and adsorption [18]. The fundamental building structure of zeolites consisted of tetrahedron composed of four oxygen atoms surrounding silicon or aluminum atom. Since aluminum has lower positive charge than silicon, the framework usually has a net negative charge which is balanced by exchangeable cation such as K+, Na+, Mg2+ and Ca2+ [19]. Modification of zeolite can be conducted by different methods including ion exchange in which the exchangeable cation of the parent zeolite is replaced by appropriate ingoing cations. Modified zeolites have been used for removal of some anions from aqueous solutions and showed high adsorption capacity and selectivity [20,21].
In this research, the Fe-exchanged form of Y-zeolite was prepared by ion exchange process. The modified zeolite (Fe- Y) was used for removal of fluoride from aqueous solutions under different experimental conditions.
Materials and Methods
NaF (99.9%), ferric chloride (FeCl3.6H2O), sodium hydroxide (NaOH), hydrochloric acid (HCl), sodium chloride (NaCl) were supplied from Merck company (Germany) and Y-zeolite we purchased from Johnson Matthey Company (Germany).
Modification of Y-zeolite
The Fe-exchanged zeolite was prepared through ion exchange process. 5.0 g the synthesized Na-Y zeolite was shaken with 100 mL of 0.15M FeCl3.6H2O solution for 48 h at 45ºC. The zeolite exchanged form was separated and washed thoroughly with deionized water. The modified zeolite (Fe-Y) was dried at 110ºC and stored in a desiccator containing saturated sodium chloride solution.
Adsorption studies
Stock solution (1000 mgL-1) of fluoride was prepared by dissolving 0.221 g of anhydrous sodium fluoride into 1000 mL of deionized water. The standards and fluoride solutions were prepared by the stock solution by dilution. The adsorption performance of the Na-Y and Fe-Y adsorbents was studied by contacting accurately weighed amount of the adsorbent with 25 mL of fluoride solution in a plastic bottle. The mixture was continuously shaken until the equilibrium was established. The sample was separated by filtration and the fluoride concentration in the remaining solution was measured by fluoride ion selective electrode (Sartoius PY-I01). Before measurement, the sample and the standard solutions were diluted with TISAB adjusting buffer (TISAB) solution (1:1V/V) in order to eliminate the interfering effect of ions and to maintain the ionic strength and the pH of the solution constant. To realize the influence of the surface charge of the adsorbent on removal of fluoride, the pH (pzc) of the adsorbents was measured as follows:
A series of solutions was prepared by transferring 25 mL of 0.01 M NaCl solution into polyethylene vessels. The pH of the solutions was accurately adjusted between 1 and 9 by addition of HCl or NaOH (0.1M solutions). N2 was continuously bubbled into the solution to prevent the dissolving of CO2. 0.1 g of the adsorbent was then added to the solution and after 3 hours, the final pH was measured. The measured pH was plotted versus the initial pH of the solution. The straight line of “pH initial=pH final” was also drawn. The pH at which the curve crossed the straight line was taken as the point of zero charge (pHpzc).
The uptake of fluoride was calculated by the following equation:
q=((Ci-Cf).V)/m (1)
Where (q), expressed as the amount of ions removed per unit mass of the adsorbent, Ci and Cf are initial and final concentrations (mgL-1) respectively, m is the mass of the adsorbent (g) and V is the volume of the solution (L).
Results and Discussion
Characterization of the adsorbents
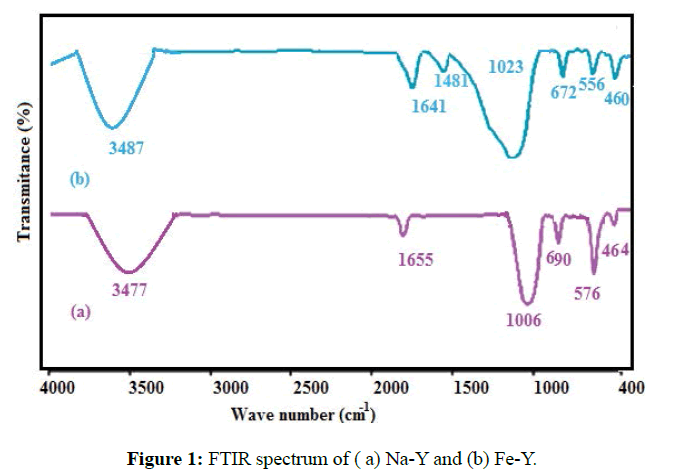
FTIR spectra of the Na-Y and Fe-Y samples were taken by Perkin Elmer Spectrum 65 Model infrared spectrophotometer (Figure 1). For Na-Y sample, the absorption bands appeared at 464, 576, 690, 1006, 1655, and 3477 cm-1 agreed well with the infrared spectral data reported by Flanigen et al. (Figure 1a) [22]. The similarity in the FTIR spectra of Na-Y and Fe-Y zeolites implied that the original zeolite structure was retained after replacement of Fe with Na (Figure 1b). The adsorption bands appeared at 1480 cm-1 indicated the presence of the Fe on the structure of the modified zeolite. Moreover the band ate 464 cm-1 was enhanced by the presence of iron.
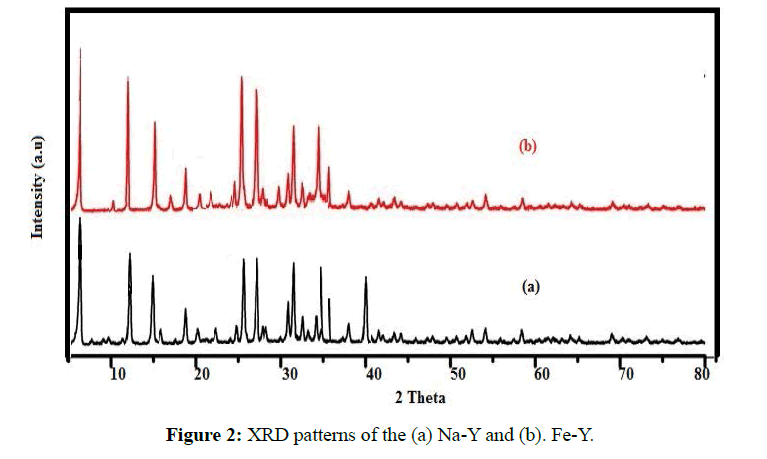
The XRD pattern of the adsorbents was taken by use of Xpertpro diffractometre instrument (Netherland). CuKα was used as radiation source and Ni as filter. The range of scanning angle (2θ) was between 0-80º (Figure 2). Comparison of the of Na-Y(a) and Fe-Y(b) XRD patterns indicated that the diffraction lines basically remained intact after replacement of Na by Fe indicating that the no crystalline transformation occurred during treatment of the sample.
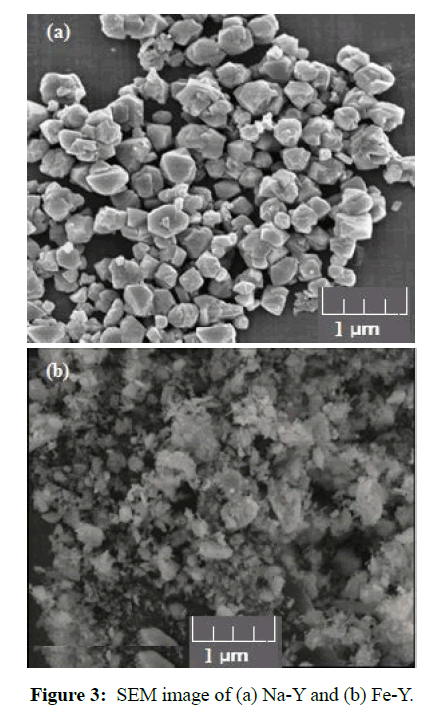
The SEM images of the adsorbents were prepared by scanning electron microscope, MIRA3LMU model, TESCAN Company. The surface morphology of Na-Y sample was changed after ion exchange with Fe (Figure 3). In the SEM image of Na-Y sample well defined separated crystals with average diameter of 1μm were observed while in Fe-Y sample the particles became powder in form with smaller particle size.
Optimized adsorption capacity
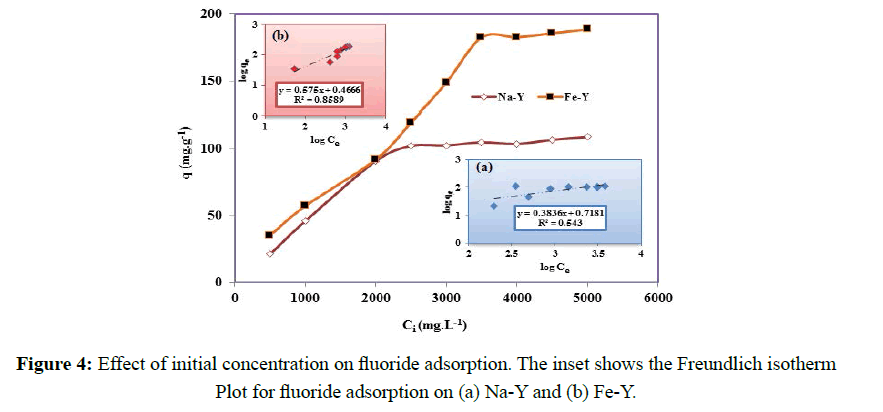
The primary experiments showed that the adsorption capacity of Fe-Y was significantly higher that Na-Y zeolite indicating the enhancing effect of Fe on the adsorption of fluoride. However to optimize the adsorption capacity of the modified adsorbent, the effect of different parameters on the adsorption capacity was investigated. The effect of initial concentrations of fluoride on the adsorption capacity was examined in concentration range of 500-5000 mgL- 1 (Figure 4). The maximal adsorption capacity of 100 and 180 mgg-1 was obtained at initial fluoride concentration of 2500 and 3500 mgL-1 respectively for Na-Y and Fe-Y samples. This indicated that replacement of Na by Fe significantly increased the adsorption capacity of the adsorbent. The adsorption capacity obtained for the modified zeolite was higher than the previous reported values; in fly ash [23], polypyrrole/Fe3O4 magnetic nano-composite [24], and hydrous ferric oxide [25]. The higher adsorption capacity obtained for Fe-Y adsorbent can be justified by the following reactions [26,27]:
Zeo-FeOH+H+ ⇌ Zeo-FeOH2+ (2)
Zeo-FeOH ⇌ H++Zeo-FeO- (3)
The fluoride ions (F-) subsequently adsorbed through ion exchange process according to Equations (4) and (5).
Zeo-FeOH+F- ⇌ Zeo-FeF+OH- (4)
Zeo-FeOH+2+F- ⇌ Zeo-FeF+H2O (5)
Or undergo non-speciï¬ÂÂc columbic interaction through reaction (6):
Zeo-FeOH+2+F- ⇌ Zeo-FeOH+2…..F - (6)
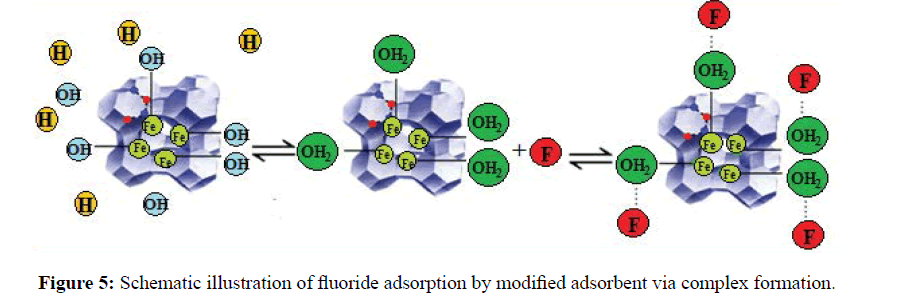
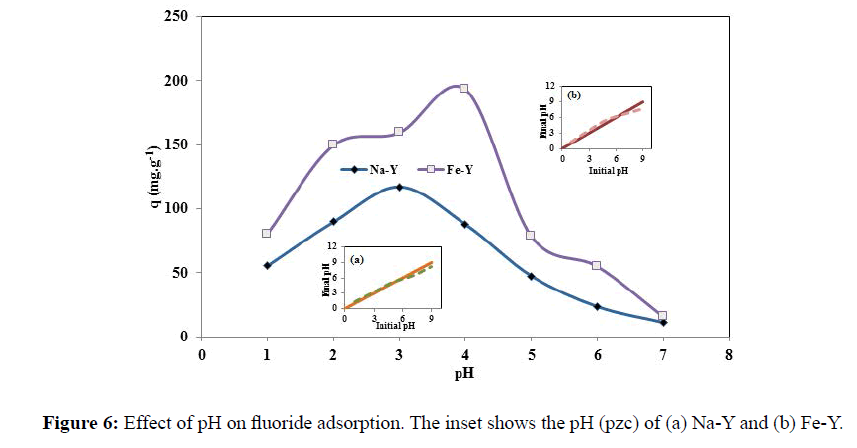
The schematic representation of fluoride adsorption by iron atoms is illustrated in Figure 5. The influence of pH on the adsorption of fluoride was studied in pH range of 1.0-7.0 (Figure 6). The adsorption performance of zeolite towards F- strongly was governed by the pH of the solution. As the solution pH increased, the adsorption capacity increased and the maximum adsorption capacity of 193.2 and 117.1 mgg-1 was obtained respectively for Fe-Y and Na-Y at pH=4 and at pH=3.
The pH(pzc) of the adsorbent was close to 5.0 and 6.0 respectively for Na-Y and Fe-Y adsorbents. Basically when the pH of the solution is below the pH(pzc), the adsorbent surface is positively charged and the fluoride anions are attracted to the surface of the adsorbents. In the meantime, at very low pHs, the adsorption was not favored because fluoride was converted to weakly ionized hydrofluoric acid. Thus fluoride adsorption was maximized at pHs lower than pH (pzc) and when the F- concentration was high. This was occurred at pH =3 and pH=4 respectively for Na-Y and Fe-Y zeolites. At high pHs, fluoride adsorption was sharply decreased because of competition of hydroxide ions for the adsorption sites. However the adsorption capacity obtained in this work was significantly higher that the results reported by other workers. Sun et al. modified natural zeolite stilbite with Fe3+ for removal of fluoride and obtained the maximal adsorption capacity of 2.31 mgg-1 at pH=6.94. Karthikeyan et al. used polyaniline/alumina adsorbent for removal of fluoride from aqueous solutions and reported the maximal adsorption value of 6.6 mgg-1 at pH value ranging from 3 to 9 [28].
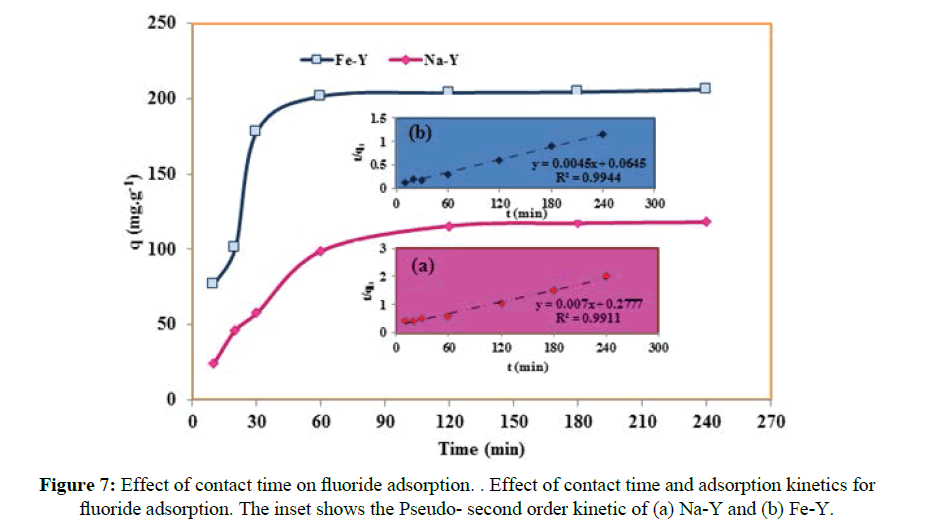
The effect of contact time on the adsorption of fluoride indicated that the uptake was at equilibration after 60 minutes (Figure 7) and the equilibrium adsorption capacity of 201.6 mgg-1 and 115.6 mgg-1 was obtained respectively for Fe-Y and Na-Y adsorbents. The required time for equilibration in this work was significantly shorter than the reported valued for adsorbents such as activated alumina [29], nano-hydroxyapatite [30], and carbon nanotubes [31]. The fast kinetic of this work encourages the application of the prepared adsorbent for removal of fluoride from aqueous solutions by column operation.
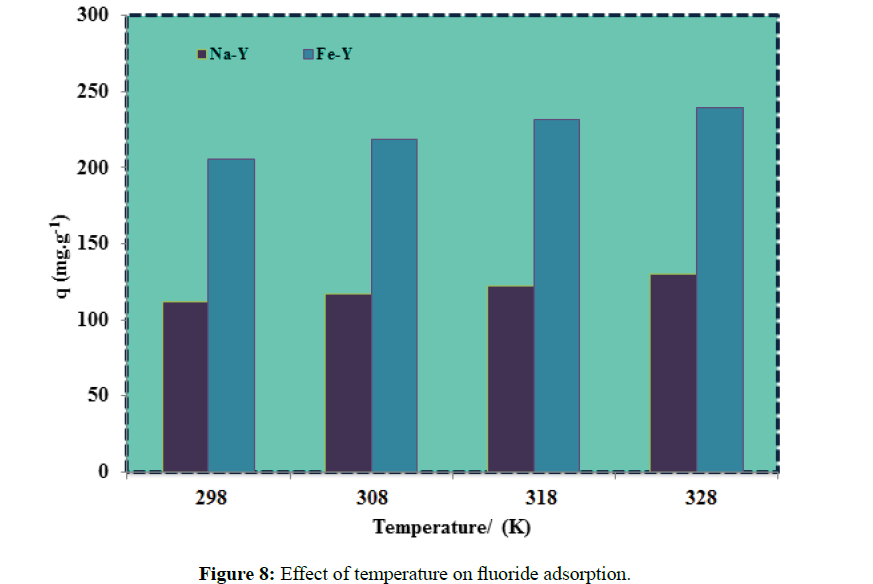
The influence of temperature on the fluoride adsorption was studied at 298K to 328K (Figure 8). At higher temperature the mobility of highly electronegative fluoride ions was increased, diffusion of fluoride ions into the adsorption sites was accelerated and consequently the formation of fluoride complex was enhanced. The effect of temperature on the adsorption capacity of the adsorbent of this work was not as significant as in the previous studied adsorbents such as aluminium complexed amino phosphonic type resins [32]. This was attributed to the wider opening Y-zeolite channels.
Adsorption isotherms
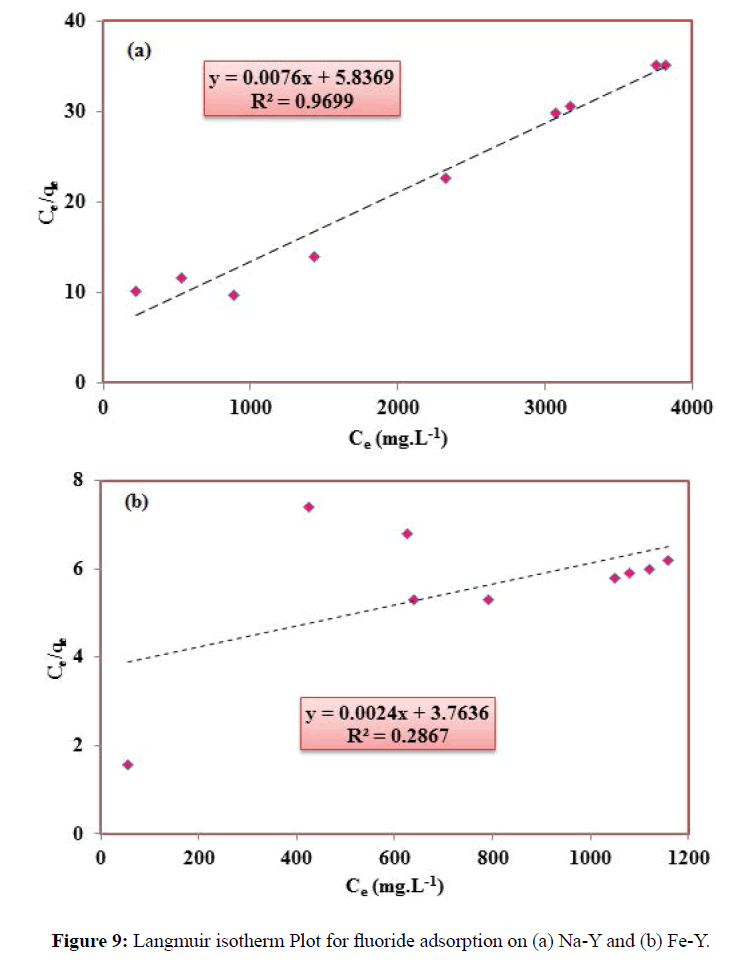
The adsorption data were analyzed by Langmuir and Freundlich isotherm models. The Langmuir isotherm equation assumes monolayer coverage of the adsorbate over a homogeneous adsorbent surface. The linear form of Langmuir isotherm equation is given by Equation (7).
Ce/qe=1/qmb+Ce (1/qm) (7)
Where Ce denotes the equilibrium concentration, qe is the adsorption capacity, b (Lmol-1) relates to the heat of adsorption, and qm refers to the maximum adsorption capacity, which is the amount of ions at complete monolayer coverage. The Langmuir constants qe and b were determined from the slope and intercept of the plot. This model implies that the adsorption occurs on a homogenous surface and all adsorption sites energetically equal to each other. The plot of specific adsorption (Ce/q) versus equilibrium concentration (Ce) is shown in Figure 9 and the related parameters are summarized in Table 1. The essential characteristics of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor RL that is given by Equation (8):
| Isotherm models | ||||||
|---|---|---|---|---|---|---|
| Adsorbents | Langmuir Isotherm | Freundlich Isotherm | ||||
| qm | RL | R2 | n | Kf | R2 | |
| Na-Y | 131.6 | 0.24 | 0.9699 | 2.6 | 5.2 | 0.5430 |
| Fe-Y | 417 | 0.32 | 0.2867 | 1.7 | 2.9 | 0.8589 |
Table 1: Langmuir and Freundlich adsorption isotherms of the Na-Y and Fe-Y.
RL=1/1+bCo (8)
Where Co is the highest initial concentration of the adsorbate (mgL-1), and b (Lmg-1) is Langmuir constant. The value of RL indicates the shape of the isotherm to be either unfavorable (RL>1), linear (RL=1), favorable (0<RL<1), or irreversible (RL=0).
The Freundlich isotherm is an empirical equation, assuming that the adsorption process takes place on heterogeneous surfaces. The linear form of Freundlich isotherm equations is given as:
Log qe=log kF+(1/n)log Ce (9)
Where kF is the Freundlich constant representing the adsorption capacity, qe is the extent of fluoride anion adsorbed per unit mass of adsorbent (mgg-1), 1/n is a constant indicative of the adsorption intensity or surface heterogeneity, showing the favorable adsorption if 1/n˃1, and becoming more heterogeneous as its value get closer to zero. The values of Freundlich constants with the correlation coefficients are shown in Table 1 and Figure 4.
KF and n are empirical constants, indicating the adsorption capacity and adsorption intensity respectively. The Freundlich model contents KF and n are calculated from the slope and intercept of the plot of logqe versus log Ce (Figure 4). According to the analysis of the experimental data by Langmuir and Freundlich models, it was found that fluoride adsorption by Fe-Y followed Freundlich adsorption model and by Na-Y follows the Langmuir model. The RL values between 0 and 1 indicate favorable adsorption. Although the value of RL of the Langmuir isotherm for Na-Y and Fe-Y was respectively 0.24, 0.32 indicating that the adsorption of fluoride by the adsorbents was favored by this model.
The fitness of the data related to Na-Y adsorbent to the Freundlich isotherm implied that the adsorbent surface of heterogeneous while Fe-Y adsorbent provides homogeneous adsorption sites. In fact, in Na-Y sample the exchange sites are uniquely occupied by Na cations resulting a homogenous surface and monolayer adsorption of fluoride, but in Fe-Y, sodium cations of the parent zeolite were partially exchanged with Fe resulting a heterogeneous surface at which the adsorption sites were not energetically equivalent. Similar results were reported for removal of fluoride onto polypyrrole/Fe3O4 magnetic nano-composite [24].
Kinetic study
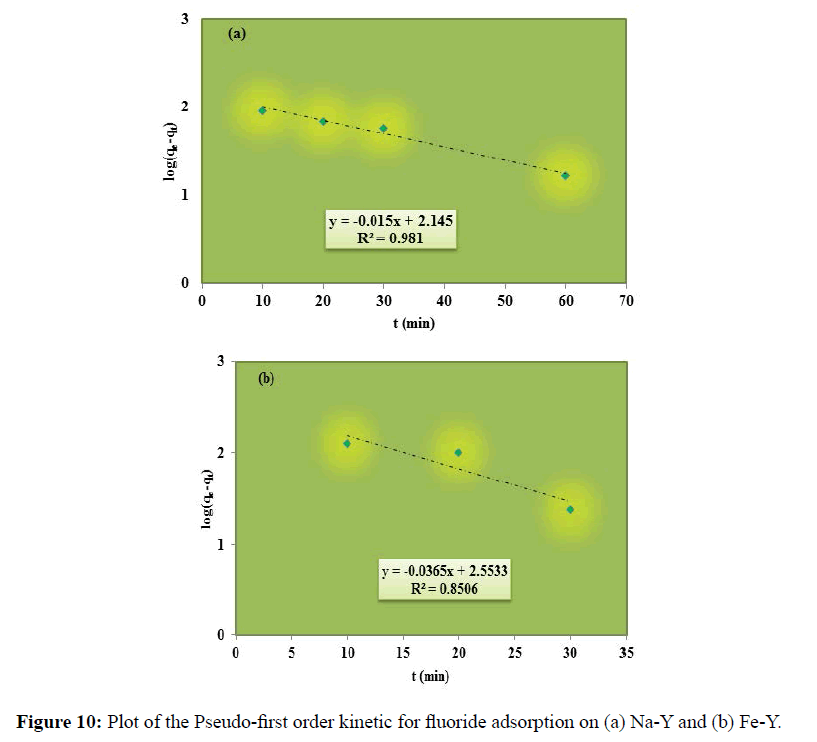
Two commonly used kinetic models; pseudo-first order and pseudo-second order kinetic models were employed to analyze the adsorption data. The linearized form of Lagergren pseudo-ï¬ÂÂrst-order and the pseudo-second order rate equations are given respectively in the following Equations (10) and (11).
log(qe-qt)=log qe-(K1/2.303)t (10)
t/qt=(1/k2qe2)+(1/qe)t (11)
Where, qe and qt are the amount of solute on the surface of the sorbent at equilibrium, and at t time (mg.g-1) respectively. k1 and k2 are the rate constant of pseudo-ï¬ÂÂrst order and pseudo-second order respectively. Figure 10 demonstrates the plot of pseudo-first order model; log (qe-qt) versus time for the adsorption of fluoride by the adsorbents.
The plot of t/qt versus t for the linear pseudo-second-order model has been depicted in Figure 7. The calculated results of the kinetic constants are given in Table 2. The higher correlation coefficient (R2) values obtained for pseudosecond- order model indicated the applicability of the pseudo-second-order model for the fluoride sorption onto the studied adsorbents. The pseudo-second order model is based on chemical adsorption and confirms that the adsorption of fluoride on the adsorbents is chemical in the nature as indicated in Equations 2-6. (Section 3.2). Similar results were reported for the removal of fluoride onto Brushite [33], alumina of alkoxide nature [34] and charcoal contained dispersed aluminum oxide [35].
| Kinetic models | ||||
|---|---|---|---|---|
| Adsorbents | Pseudo-first-order | Pseudo-second-order | ||
| K1 | R2 | K2 | R2 | |
| Na-Y | 0.035 | 0.9810 | 0.00018 | 0.9907 |
| Fe-Y | 0.084 | 0.8506 | 0.00031 | 0.9944 |
Table 2: Kinetic parameters for pseudo-first-order and pseudo-second-order.
Adsorption thermodynamics
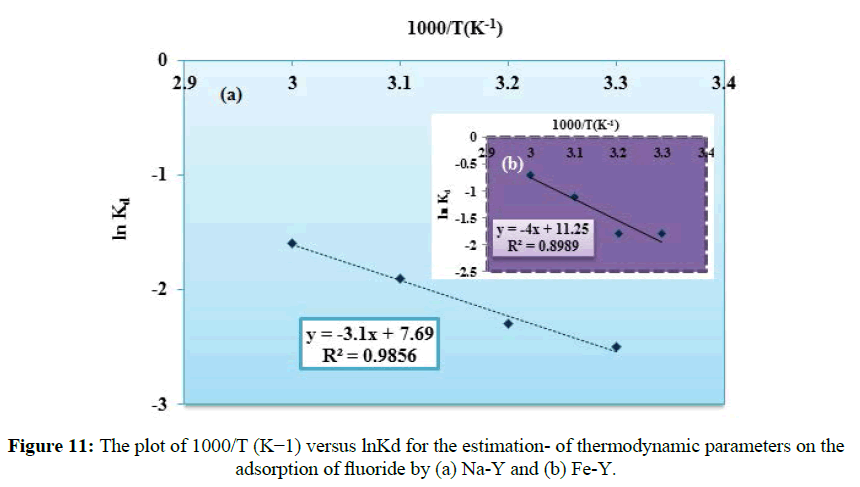
Thermodynamic parameters were evaluated using Equations (12) To (14).
Kd=qe/Ce (12)
ln Kd=(ΔS°/R)-(ΔH°/RT) (13)
ΔG°=ΔH°−TΔS° (14)
Where Ce is the amount of fluoride ion adsorbed per unit mass of the adsorbent and Ce is the concentration of fluoride in aqueous phase. The distribution coefficient Kd reflects the binding ability of the surface for the ingoing species. T (K), R is the ideal gas constant (8.314 J mol-1 K-1); ΔGo and ΔHo are in Jmol-1 and ΔSo is in J mol-1K-1. According to equation (13), the plot of T-1(K-1) versus lnKd gave a straight line (Figure 11). The value of ΔHo and ΔSo obtained respectively from the slope and intercept of the plot and given in (Table 3). The positive values of ΔGo confirmed that the sorption of fluoride by the adsorbents was unspontaneous indicating the reversible nature of the adsorption under experimental conditions. The positive value of the entropy change (ΔSo) reflected that the increase of randomness which occurred with increasing in the number of fluoride species at the solid-liquid interface, because the hydrated fluoride from the aqueous phase before distribution on to the solid surface released their hydrated spheres. The positive values of ΔHo confirmed the endothermic nature of sorption process. Similar results were also reported for the removal of fluoride onto conducting polymer/alumina composites.
| Thermodynamic parameters | |||||||
|---|---|---|---|---|---|---|---|
| Adsorbents | ΔH° (J/mol) | ΔS° (J/mol.K) | ΔG° (J/mol) | R2 | |||
| 298K | 308K | 318K | 328K | ||||
| Na-Y | 25.8 | 64 | -19046 | -19686 | -20326 | -20966 | 0.9856 |
| Fe-Y | 33.3 | 93.5 | -27830 | -28765 | -29700 | -30635 | 0.8989 |
Table 3: Thermodynamic parameters of F-adsorption by Na-Y and Fe-Y adsorbents.
Conclusion
The Fe-Y adsorbent prepared by modification of Na-Y zeolite was used for removal of fluoride from aqueous solutions. The performance of the modified zeolite compared to the parent zeolite indicated that Fe-Y adsorbent possesses higher capability for showed for removal of fluoride from aqueous solutions. The methods used for characterization of the modified zeolite including XRD and FTIR indicated that the structure of zeolite remained intact during the exchange process. The adsorption capacity of Fe-Y sample was much higher than the reported values for the previous studied adsorbents. The fluoride uptake was kinetically fast and affected by temperature, contacting time, pH and initial concentration of fluoride. The maximal adsorption capacity was obtained at fluoride initial concentration of 3500 mgL- 1, pH=4, contact time of 60 min. Fluoride adsorption on Fe-Y followed Freundlich model and on Na-Y followed the Langmuir model. The adsorption process was unspontaneous and endothermic. Evaluation of the adsorption kinetics showed that the adsorption of fluoride on the Na-Y and Fe-Y followed pseudo second-order kinetics.
References
- Mohapatra M, Anand S, Mishra BK, Giles DE, Singh P (2009) Review of fluoride removal from drinking water. J Environ Manage 91: 67-77.
- Water, S. and World Health Organization (2006) Guidelines for drinking-water quality [Electronic Resource]: Incorporating First Addendum, in: W.H.O. edn., pp. 375–377.
- Paulson EG (1977) Reducing fluoride in industrial wastewater. Chem Eng 84: 89-94.
- Banks D, Reimann C, Røyset O, Skarphagen H, Sæther OM (1995) Natural concentrations of major and trace elements in some Norwegian bedrock ground waters. Appl Geochem 10: 1-16.
- Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geochem 33: 13-24.
- Reddy NB, Prasad KSS (2003) Pyroclastic fluoride in ground waters in some parts of Tadpatri Taluk, Anantapur district, Andhra Pradesh. Indian J Environ Health 45: 285-288.
- Reardon EJ, Wang Y (2000) A limestone reactor for fluoride removal from wastewaters. Environ Sci Technol 34: 3247-3253.
- Shen F, Chen X, Gao P, Chen G (2003) Electrochemical removal of fluoride ions from industrial wastewater. Chem Eng Sci 58: 987-993.
- Teutl-Sequeira A, Solache-Rios M, Balderas-Hernandez P (2012) Modification effects of hematite with aluminum hydroxide on the removal of fluoride ions from water. Water,Air & Soil Pollut 223: 319-327.
- Tor A, Danaoglu N, Arslan G, Cengeloglu Y (2009) Removal of fluoride from water by using granular red mud: batch and column studies. J Hazard Mater 164: 271-278.
- Sundaram CS, Viswanathan N, Meenakshi S (2008) Defluoridation chemistry of synthetic hydroxyapatite at nano scale: equilibrium and kinetic studies. J Hazard Mater 155: 206-215.
- Sujana MG, Thakur RS, Das SN, Rao SB (1997) Defluorination of waste waters. Asian J Chem 4: 561-570.
- Ku Y, Chiou HM (2002) The adsorption of fluoride ion from aqueous solution by activated alumina. Water Air Soil Pollut 133: 349-360.
- Li YH, Wang SG, Zhang XF, Wei JQ, Xu CL, et al. (2003) Adsorption of fluoride from water by aligned carbon nanotubes. Mater Res Bull 38: 469-476.
- Yang M, Hashimoto TN, Myoga H (1999) Fluoride removal in a fixed bed packed with granular calcite. Water Res 33: 3395-3402.
- Cengeloglu Y, Kir E, Ersoz M (2002) Removal of fluoride from aqueous solution by using red mud. Sep Purif Technol 28: 81-86.
- Suna Y, Fang Q, Dong J, Cheng X, Xu J (2011) Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe(III). Desalination 277: 121-127.
- Foo KY, Hameed BH (2011) The environmental applications of activated carbon/zeolite composite materials. Adv Colloid Interface Sci 162: 22-28.
- Bowman RS (2003) Applications of surfactant-modified zeolites to environmental remediation. Micropor Mesopor Mat 61: 43-56.
- Tokunaga S, Wasay SA, Park SW (1997) Removal of arsenic (V) ion from aqueous solutions by lanthanum compounds. Wat Sci Tech 35: 71-78.
- Sujana MG, Thakur RS, Rao SB (1998) Removal of fluoride from aqueous solution by using alum sludge. J Colloid Interface Sci 206: 94-101.
- Flanigen EM, Khatami H, Szymanski HA (1974) Molecular sieve zeolites, Advances in Chemistry Series, American Chemical Society,Washington, DC, USA, 101.
- Chaturvedi AK, Yadava KP, Pathak KC, Singh VN (1990) Defluoridation of water by adsorption on fly ash. Water Air Soil Pollut 49: 51-61.
- Bhaumik M, Leswifi TY, Maity A, Srinivasu VV, Onyango MS (2011) Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic Nanocomposite. J Hazard Mater 186: 150-159.
- Dey S, Goswami S, Ghosh UC (2004) Hydrous ferric oxide (HFO) – a scavenger for fluoride from contaminated water. Water Air Soil Pollut 158: 311-323.
- Elizalde-González MP, Mattusch J, Wennrich R, Morgenstern P (2001) Uptake of arsenite and arsenate by clinoptilolite rich tuffs. MicroporMesoporMat 46: 277-286.
- Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J Colloid Interface Sci 279: 341-350.
- Karthikeyan M, Kumar KKS, Elango KP (2009) Conducting polymer/alumina composites as viable adsorbents for the removal of fluoride ions from aqueous solution. J Fluorine Chem 130: 894-901.
- Venkobacher C, Iyengar L (1998) Defluoridation of water using activated alumina-a report, UNICEF Project No. CE/UNICEF/9132.
- Sundaram CS, Viswanathan N, Meenakshi S (2008) Uptake of fluoride by nanohydroxyapatite/chitosan, a bioinorganic composite. Bioresour Technol 99: 8226-8230.
- Li YH, Wang S, Zang X, Wei J, Xu C, et al. (2003) Adsorption of fluoride from water by aligned carbon nano tubes. Mater Res Bull 38: 469-476.
- Bhatt DB, Bhatt PR, Prasad HH, Popat KM, Anand PS (2002) Removal of fluoride ion from aqueous bodies by aluminium complexed amino phosphonic acid type resins. Indian J Chem Technol 11: 299-303.
- Mourabet M, El Boujaady H, El Rhilassi A, Ramdane H, Bennani-Ziatni M, et al. (2011) Defluoridation of water using Brushite: equilibrium, kinetic and thermodynamic studies. Desalination 278: 1-9.
- Kamble SP, Deshpande G, Barve PP, Rayalu S, Labhsetwar NK, et al. (2010) Adsorption of fluoride from aqueous solution by alumina of alkoxide nature: batch and continuous operation. Desalination 264: 15-23.
- Kamga ET, Alonzo V, Njiki CPN, Audebrand N, Ngameni E, et al. (2010) Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water. Carbon 48: 333-343.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences