ISSN : 2348-9502
American Journal of Ethnomedicine
Phytochemical Screening on Euphorbia milii Red Flowers ÃÆâÃâââ¬Ãâââ¬Å Isolation of Terpenoids, Flavone and Phenols
Hemalatha Kamurthy1*, Sunitha Dontha2 and Rajani. K2
1Department of Pharmaceutical Chemistry, Acharya & BM Reddy College of Pharmacy, Acharya Dr.Sarvepalli Radhakrishnan Road Soldevanahalli, Hesarghatta Road, Achit Nagar Post, Bangalore-560 107, India
2Malla Reddy College of Pharmacy, Maisammaguda, Dhulapally, Secunderabad- 14, Hyderabad, Andhra Pradesh, India
Abstract
Objective: To learn the phytochemical screening of terpenoids, flavone and phenols from different extracts ofEuphorbia milii Red flowers.
Materials and Methods: Euphorbia milii (Euphorbiaceae) is an ornamental plant plays a role in folk medicine. The Chinese use it as a cure for cancer, and some Brazilians believe that it can cure warts.The flowers were extracted with the three different solvents like petroleumether, ethyl acetate and 70% ethanol &the yield were found to be 21 g, 15 g and 12 g respectively, concentrations of 50 % and 100% ethanol respectively.
Results: Two triterpenoids, Taraxerol(1), 28-hydroxyfriedelan-1,3-dione-29-oic acid (2) as well as one flavone, Quercetin 3-O-(2"-O-galloyl)-a-Larabinofuranoside (3) and two phenoilccompounds, 7 7'- dihydroxy, 8, 6'- bicoumarin (4), 9-acetyl-3'4'-dimethoxy dehydroconiferyl-3-alcohol (5) were isolated for the first time from the flowers of Euphorbia milii. The structures of the isolated compounds (1–5) were realized on the basis of the spectral data (IR, 1H and 13C NMR and mass).
Conclusion: The obtained compounds of Euphorbia milii flowers are effective pharmaceutical compounds which will serve as a better alternative to chemical based pharmaceuticals.
Keywords
Euphorbia milii, Euphorbiaceae, triterpenoids, flavones, coumarins, phenolic compounds.
Introduction
The genus Euphorbia is the largest genus of medicinal plants widely distributed in tropical countries. Different species of Euphorbia are used for the treatment of various ailments such as skin diseases, intestinal parasites and warts. It has been reported that Euphorbia possesses antiarthritis, anticancer [1], anticonvulsant, antidiabetic, anti-eczema, antiinflammatory, antimicrobial, antioxidant, antispasmodic, antitumor, antitussive properties hormonal and myelopoiesis properties [2]. Some species of Euphorbia have been traditionally used for the treatment of skin diseases, gonorrhea, migraine, intestinal parasites and as wart cures [3]. The genus Euphorbia has been studied widely for its antiproliferative [4].Euphorbia milii (Euphorbiaceae), a flowering plant commonly known as “Christ plant” or “Christ thrown”. It is ornamental shrub native to Madagascar and Philippines, widely distributed in India. Euphorbia milii widely used in folk medicine for the treatment of warts (South Brazil), cancer and hepatitis (china). It has been reported that Euphorbia milii possesses antifungal and antinociceptive property, acts as natural molluscicide, can curb the spread of schistosomiasis. Some of the latter diterpene esters of ingenol are potent skin irritants but, in contrast with other closely-related ingenol and phorbol derivatives, they showed no tumour promoting activity [5], Milliamines isolated from E. milii latex exhibited potent molluscicidal activity [6]. Phytochemical studies of Euphorbia milii revealed the presence of β-sitosterol, cycloartenol, β- amyrin acetate, lupeol, euphol, triterpenes, phenols and flavonoids [7]. Euphorbia milli crude latex showed potent plant molluscicide [8], its toxic effect to mammals has been studied. The undiluted latex of E. milli was also found to be irritant to mammalian eyes and skin [9].Inspite of the various researchers on Euphorbia milii, investigation on the chemical constituents was carried out on polar extracts. This paper elucidates the structures of two triterpenoids, one flavone and phenolic compounds from the flowers of E.milii on the basis of various spectroscopic data.
MATERIALS AND METHODS
General
Column chromatography was carried out by using silica gel (60-120 mesh) (Merck, Bombay) and Aluminum sheets and glass-backed TLC plates (20X20 cm; Merck, silica gel 60-F254) were used for isolation of compounds. Analytical grade solvents (Sigma Aldrich 32213) were used. IR spectra was recorded using KBr pellets on Thermo Nicolet Nexus 670; 1H NMR spectra were taken on Varian EM-360 (300 MHZ) NMR spectrometer using CDCl3 as solvent; 13C NMR was recorded on Bruker instrument with CDCl3 as solvent at 300 MHz and Mass spectra were recorded on a EI-MS, data on E:ISO/21184-1.QGD.
Plant material
Flowers of Euphorbia milii were collected from the local gardens of Hyderabad. The plant was authenticated by Dr. B. Bhadraiah (HOD, Dept. of Botany, Osmania University, Hyderabad). Voucher specimen (EM/2013/0067) was kept at Malla Reddy College of Pharmacy, Dhulapally, Hyderabad, Telangana, India.
Extraction of plant material
Around 4 kg of Euphorbia milii red flowers were shade dried, coarsely powdered and subjected for successive extraction process with three different solvents (petroleum ether, ethyl acetate and ethanol (70 %)) into 15 batches of each 200 g in Soxhlet extractor for 48 hours. After complete extraction, the solvents were distilled off and concentrated under reduced pressure to the dryness in a flash evaporator. The yield was found to be 21 g, 15 g and 12 g respectively.
Isolation and purification of compounds
The concentrated petroleum ether extract (20 g) was eluted by gradients of petroleum ether/ethyl acetate, then ethyl acetate, followed by a gradient of ethyl acetate/methanol and finally with methanol to afford a total of 35 fractions (each 200 mL).
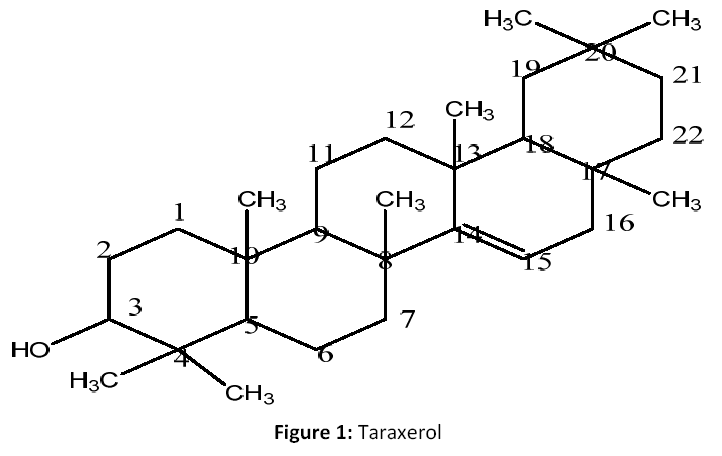
Fractions 02 to 15 (7.2 g) on silica gel column with Petroleum ether: EtOAC (85:15) gave six subfractions F1a- F1f. Fraction F1b-F1e (929 mg) was rechromatographed over a silica gel eluting with petroleum ether: EtOAC(83:17) to yield compound 1 on TLC (peteroleum ether: EtOAC; 8.2:1.8), the compound was further purified with acetone to give pure compound (1) (142 mg) [Fig 1].
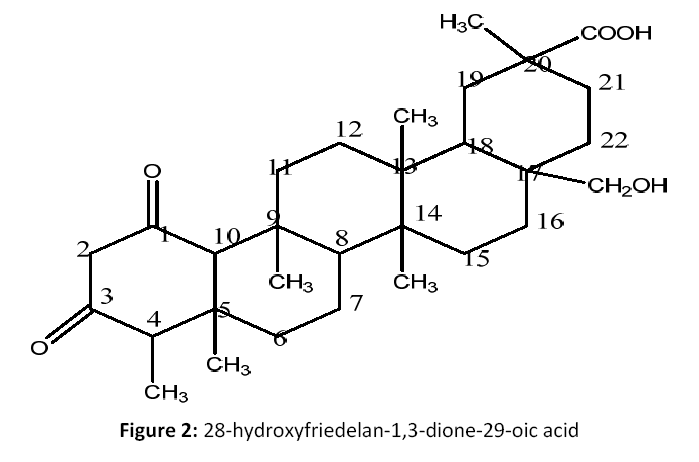
Fractions 17 to 33 (6.5 g) was rechromatographaed on silica gel column with, Petroleum ether: EtOAC(gradient) to afford 8 new subfractions F2a-F2f. Fractions F2a-F2h(845 mg) was rechromatographed on silica gel with Petrolem ether: EtOAC (70:30 to 50:50) to yield compound 2on TLC (petroleum ether: EtOAC ; 6.5:4.5), ascertained as pure compound (2) (79 mg) [Fig 2].
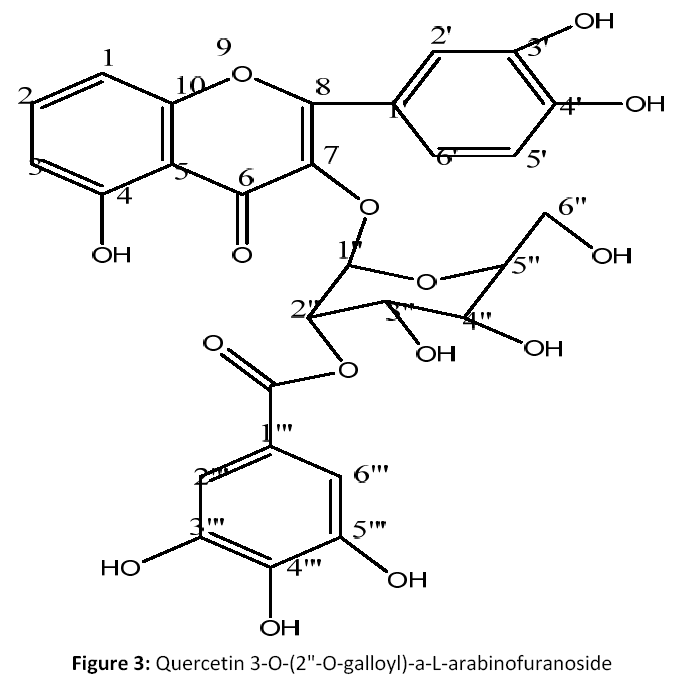
The ethyl acetate fraction (12 g) was fractionated by column chromatography over a silica gel G (60-120 mesh, Merck) with ethyl acetate: n-hexane (gradient) to afford 15 factions. Fractions 3 to 12 (9.3 g) on silica gel column with Et OAC: n-hexane (80:20) gave eleven subfractions F3a-F3k. Fraction F3b-F3j (3.2g) was rechromatographed over a silica gel eluting with EtOAC: MeOH (90:10) to yield compound 3 on TLC (Toluene: ethyl acetate: formic acid ; 6:2:0.8) and sprayed with Aluminum chloride reagent as pure compound (3) (1.42 mg) [Fig 3].
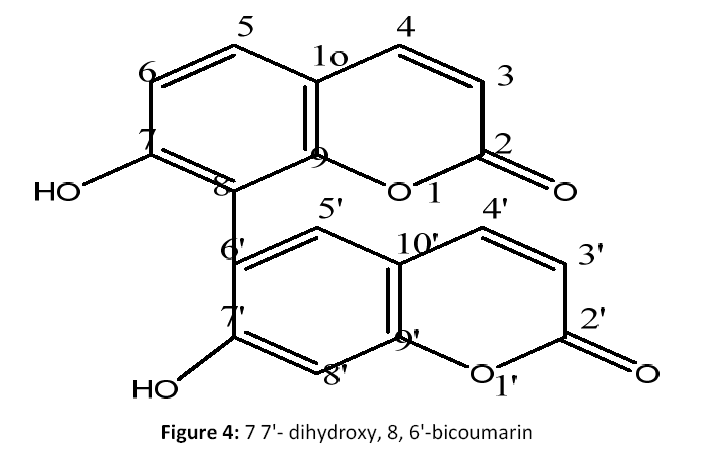
The ethanolic fraction (11 g) was fractionated by column chromatography over a silica gel G (60-120 mesh, Merck) was eluted with CHCl3-MeOH (95:05, to 20:80) to afford 14 fractions [10, 11]. Fractions 02 to 07 (3.7 g) on silica gel column with CHCl3- MeOH (70:30) gave five subfractions F4a- F4f. Fractions F4a-F4d (757 mg ) was rechromatographed over silica gel eluting with CHCl3-MeOH (85:20) to yield 4 on TLC (BuOH-HOAc-H2O ; 6.3:2.7:01),ascertained as pure compound (4) (92 mg) [Fig 4].
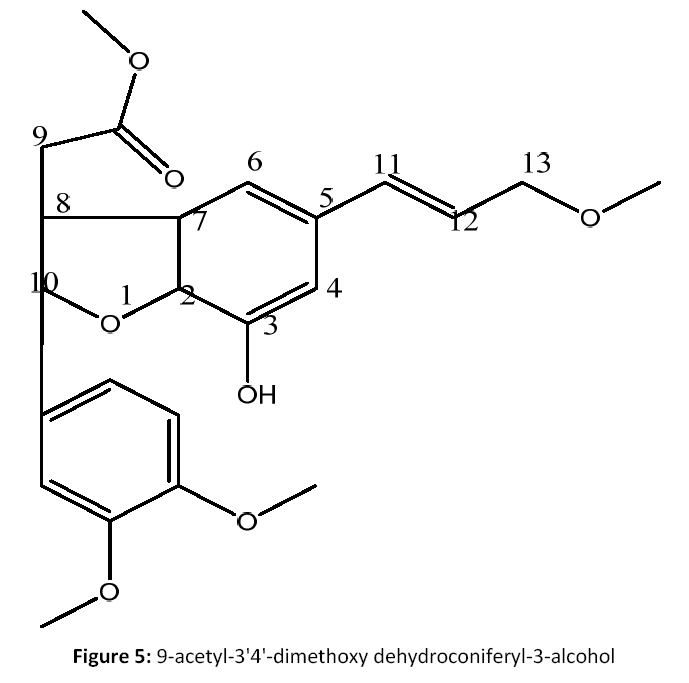
Fractions 8 to 12 (2.7 g) was rechromatographed on silica gel column with CHCl3: EtoAC (70:30 to 40: 60) to give four subfractions F5a-F5d. Fractions F5a-F5c (656 mg) was rechromatographed over silica gel eluting with CHCl3: EtoAC (50:50) to yield 5 on TLC (CHCl3: EtoAC; 1:1), the compound was further purified with methanol to give pure compound (5) (66 mg) [Fig 5].
RESULTS
Taraxerol(1): Pale yellow amorphous powder; (M.P: 283°C), (KBr, cm-1): 3321,2944, 2871, 1715, 1642, 1463, 1380 cm-1; 1H NMR (CDCl3, 500 MHz):δ5.32 (d,1H,olefinic proton, H-14), 3.39 (d,1H, OH group, H-3), δ2.35 to 1.27(m,24H,CH2 & CH Protons), δ 1.32 to 1.00 (m, 12H,4XCH3 groups,H-2,24,25,26), δ0.99 to 0.88(m,12H,4X CH3 group H- 27,28,29,30), 13C NMR (CDCl3, 125 MHz): Table 1; C30 H50O; EIMS m/z: 426[M]+, 449[M+ Na]+.
Table 1. 13C-NMR Spectral data of triterpenoids (1& 2), flavone (3)& phenols (4&5)
| C | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 37.8 | 217.7 | C-O | C-O | C-O |
| 2 | 29.2 | 63.6 | 155.6 | 160.8 | 76.7 |
| 3 | 33.1 | 215.2 | 136.7 | 115.3 | 188.6 |
| 4 | 206.9 | 57.5 | 177.8 | 154.5 | 116.3 |
| 5 | 49.2 | 37.2 | 160.1 | 130.9 | 131.3 |
| 6 | 18.9 | 46.9 | 115.4 | 113.5 | 145.7 |
| 7 | 35.2 | 18.3 | 139.6 | 163.3 | 60.9 |
| 8 | 36.5 | 55.1 | 105.7 | 117.5 | 35.1 |
| 9 | 36.9 | 38.1 | 155.6 | 157.2 | 33.3 |
| 10 | 47.6 | 63.9 | 113.3 | 119.3 | 89.9 |
| 11 | 17.1 | 37.3 | 121.3 | ||
| 12 | 31.2 | 30.1 | 133.3 | ||
| 13 | 31.3 | 41.2 | 82.3 | ||
| 14 | 156.6 | 41.3 | |||
| 15 | 116.9 | 30.3 | |||
| 16 | 35.5 | 31.6 | |||
| 17 | 41.5 | 37.2 | |||
| 18 | 36.3 | 33.9 | |||
| 19 | 50.1 | 40.2 | |||
| 20 | 30.3 | 33.7 | |||
| 21 | 29.2 | 32.2 | |||
| 22 | 34.1 | 32.2 | |||
| CH3 | 26.0 | 9.9 | |||
| CH3 | 25.8 | 16.9 | |||
| CH3 | 15.4 | 17.2 | |||
| CH3 | 19.2 | 19.6 | |||
| CH3 | |||||
| CH3 CH3 CH3 |
26.2 | 18.8 33.7 |
|||
| CH2OH | 29.3 | ||||
| COOH OCH3 OCH3 |
31.8 31.9 |
70.1 181.2 |
56.5 56.6 |
||
| OCH3 | 61.9 | ||||
| OCH3 | 55.5 | ||||
| C=O | 177.1 | ||||
| 1ˈ | 116.9 | C-O | 120.3 | ||
| 2ˈ | 144.1 | 166.8 | 113.3 | ||
| 3ˈ | 149.9 | 116.3 | 151.2 | ||
| 4ˈ | 145.2 | 151.3 | 153.2 | ||
| 5ˈ | 129.9 | 128.8 | 109.9 | ||
| 6ˈ | 121.2 | 125.3 | 137.7 | ||
| 7' | 159.9 | ||||
| 8' | 101.3 | ||||
| 9' | 157.3 | ||||
| 10' | 117.2 | ||||
| 1˝ | 105.5 | 159.9 | |||
| 2˝ | 73.5 | 101.3 | |||
| 3˝ | 74.1 | 157.3 | |||
| 4˝ | 72.9 | 117.2 | |||
| 5˝ | 81.3 | ||||
| 6˝ | 65.6 | ||||
| 1"' | 109.5 | ||||
| 2"' | 143.5 | ||||
| 3"' | 141.2 | ||||
| 4"' | 147.7 | ||||
| 5"' | 143.5 | ||||
| 6"' | 166.6 |
28-hydroxyfriedelan-1,3-dione-29- oic acid (2):Acicular crystals;(M.P: 296° C); (KBr, cm-1): 3349, 1767, 1696 cm-1; 1H NMR (CDCl3, 500 MHz):δ 3.79, 3.77(d,d, 2H, CH2OH group, H-28), δ 2.89 to 1.47 (m, CH2 and CH group of skeleton moiety), δ 1.43 to 0.88 (m, 18H, 6XCH3groups, H-23, 24, 25, 26, 27, 30). 13C NMR (CDCl3, 125 MHz): Table 1; C30 H46O5; EIMS m/z: 486[M]+, 519[M+ Na]+.
Quercetin 3-O-(2"-O-galloyl)-a-Larabinofuranoside (3): Orange yellow powder;(M.P: 319°C); (KBr, cm-1): 3462, 2915, 2847, 1738, 1653, 1472, 1432, 1234 cm-1; 1H NMR (CDCl3, 500 MHz): δ 7.45 to 6.32, (dd, 8H, Ar-protons, H-6,7, 8,2',5',6', 2"', 6'"), δ 6.12 (d, 1H, CH group of pyranose moiety, H-1"), δ 5.35 (s, br, 6H, OH group of aromatic ring), δ 4.72 to 3.32 (m, 4H, pyranosemoeity), δ 3.69, 3.51 (s, 2H, OH group of pyranose moiety, H-6"),δ 3.58 & 3.63 (s,OH group of pyranose moiety, H-3", 4"), δ 3.63 (s, OH group of pyranose moiety, H-6") .
13C NMR (CDCl3, 125 MHz): Table 1; C28 H24O15; EIMS m/z: 600[M]+, 623 [M+ Na]+.
7 7'- dihydroxy, 8, 6'-bicoumarin (4): Red sticky mass; (M.P: 270°C);(KBr, cm-1): 3484, 2916, 2849, 1734, 1605, 1376, 1242 cm-1; 1H NMR (CDCl3, 500 MHz): δ 7.29 (d, 2H, Ar-proteins, H-4,4'), δ 7.61 to 6.59 (d, 4H, d, Ar-proteins, H-5,6,5', 8'), δ 5.58(d, 2H, Ar-proton, H-3,3'), δ 5.41 (s, 2H, OH groups, H-7,7'). 13C NMR (CDCl3, 125 MHz): Table 1; C18 H10O6; EIMS m/z: 322[M]+, 345[M+ Na]+.
M]+, 345[M+ Na]+. 9-acetyl-3'4'-dimethoxy dehydroconiferyl- 3-alcohol (5): Brown sticky mass; (M.P: 274°C); (KBr, cm-1): 3333, 2932, 2817, 1516, 1337, 1203 cm-1; 1H NMR (CDCl3, 500 MHz): δ7.55 to 6.63 (d, 5H Arprotons), δ 6.48 (d, 2H oleifinic protons, H- 11), δ 6.45 (m, 1H, OH group, H-3), δ 4.07 (m, 2H, CH2 group, H-13), δ 3.87 & 3.41 (m 2H, CH2 group, H-9). δ3.61 (s, 6H, 2XOCH3 group, H-3', 4'), δ 3.56 (s, 3H, -COOCH3 group, H-9), δ 3.21 (s, 3H, OCH3 group, H- 13). ). 13C NMR (CDCl3, 125 MHz): Table 1; C18 H10O6; EIMS m/z: 322[M]+, 325[M+ Na]+.
DISCUSSION
The above obtained isolated compound (1-5) was realized on the basis of spectral evidence (IR, 1H &13C NMR and Mass).
Compound (1) was obtained as pale yellow amorphous powder. Its molecular formula was determined to be C30 H50O on the basis of positive-ion peak EIMS (m/z) 426 [M+]. IR spectral data showed absorption bands for hydroxyl group (3321 cm-1), C-H stretching (2944& 2871 cm-1), for C=C stretching (1642 cm-1) functionalities. 1H NMR data of 1 indicated the presence of double doublet centered at δ 5.32 attributable to the olefinic proton at C- 14. The broad doublet centered at δ 3.39 could be assigned to the oxymethine proton at C-3. The large coupling of this proton (H- 3) with the vicinal methylene protons suggested a β (beta) orientation of the hydroxyl group at C-3 and eight three proton singlets at δ 0.80, 0.82, 0.90, 0.94, 0.92, 0.964, 0.97, and 0.99. These were attributed to the methyl group protons at C-17, C-13, C-4α, Me-4β, C-20α, C-10, C-8 respectively. The above 1 H-NMR signals suggested the presence of a typical pentacyclictriterpene skeleton [12]. 13C NMR spectrum of 1 exhibited the presence of 30 carbon signals are there in respective δ ppm (See Table 1). Olefinic carbons of double bonds at δ 156. 6 and 116.9 attributed to C- 14& C-15 respectively, in addition to one oxymethane group at δ 33.1 for C-3 postion1 [3].
Compound (2) was obtained as pale yellow amorphous powder. Its molecular formula was determined to be C30 H46O5 on the basis of positive-ion peak EIMS (m/z) 486 [M+]. IR spectral data at showed absorption one broad absorption band due to a hydroxyl function at (3349 cm-1) and another intense band at (1696 cm-1) corresponding to a carboxylic acid group. The skeletal basis of a friedelanetriterpenoid skeleton [14]. 1H NMR data of 2 indicated the presence of six methyl group signals at δ 0.88, 0. 95, 1.04, 1.24, 1.43 (s, 3H each) as singlet and δ 0.99 as doublet for H-3 position. The δ 2.21, 2.07 for H-2, 4 positions respectively and doublet of doublet signals at δ3.79, 3.77 for carbonly carbon H- 28 were consistent with a type friedelanetriterpene.13C NMR spectrum of 2 exhibited the presence of 30 carbon signals are there in respective δ ppm (see Table 1). Two carbonyl carbons at δ 70.1 and other at δ 181.2 for C-28, 29 position respectively, six carbon signals at δ9.9, 16.9, 19.2, 17.2, 19.6 and 33.7 for C-23, 24, 25, 26, 27 and 30 positions, specifically a downfield shift at δ 40.2 for C-20 in agreement with terpenoid skelton [15].
Compound (3) was obtained as pale yellow amorphous powder. Its molecular formula was determined to be C30 H46O5 on the basis of positive-ion peak EIMS (m/z) 600 [M+]. IR spectral data showed absorption bands for OH stretching for phenol (3462 cm-1), C=O Aryl ketonicstretch at (1653cm-1), C-O stretch for phenol (1232cm-1), C-CO-C stretch & bending in ketone & out of plane C-H bending of hydrocarbons (945, 822, 576 cm- 1) groups of alcohols respectively [16].
1H NMR data of 3 indicated the presence of hydrogen-bonded hydroxyl signal at δ 5.35 indicated the presence of a 5-hydroxy A ring system in flavonol. A spin system of three aromatic signals at δ 7.72 1H, d, δ 6.93, 7.29, 1H, dd, indicated the presence of a 3',4'-dihydroxy B ring system in flavonol, an aromatic methine correlated at δ 6.97 for 2"' & 6"' positions. Four anomeric protons of pyranose ring were resonated as doublets in between δ 4.45 to 3.73 for H-2", 3", 4" and methylene signals at δ 3, 79, 3.54 for H-6" was observed. 13C NMR spectrum of 2exhibited the presence of 28 carbon signals are there in respective δ ppm (see Table 1). Significant flavonol signals at δ 155.6, 136.7, 177.8 (C-4) were observed for C-2, 3, 4 position, the signals at δ 149.9 for c-3', δ 145.2 for C-4', δ 121.2 for C-6' and δ 144.1 for C-2', 116.9 for C-1' were found. From these data, the aglyconeof 3 was determined to be quercetin [17]. In addition, an aromatic methineδ 109.5 for C- 1", δ 143.5 for C-2"' & 6'", δ 141.2 for C-3"' & 5'" and δ 147.7 for C-4"' and δ 166.6 for carbonyl (C=O), indicating the presence of a galloyl moiety. From these observations, the sugar moiety was determined to be L-aarabino- pyranose. Thus, the structure of 3 was determined to be Quercetin 3-O-(2"-Ogalloyl)- a-L-arabinofuranoside.
Compound (4) was obtained as pale yellow amorphous powder. Its molecular formula was determined to be C18H10O6 on the basis of positive-ion peak EIMS (m/z) 322 [M+].IR spectral data showed absorption bands at (3484, 2916 & 2849 cm-1) compatible with the presence of OH groups and two carbonyl carbon functionalities and it also further demonstrated peak at 1734cm- 1)for lactone carbonly group.1H NMR data of 4 signal peak atδ 5.41indicates the presence of basic bicoumraol unit with OH groups of either bridge linking the umbelliforne unit, two protons are all most identical chemical shifts at δ 7.55 for H-4,4' positions18.It alsoreveals the presence of hydroxylic group as broad singlet at δ 5.41 for 7, 7' position integrating for two protons. 13C NMR spectrum of 4 exhibited the presence of 18 carbon signals are there in respective δ ppm (see Table 1). Showing signals at δ160.8, 115.3 for aromatic carbons account for their coumarin skeleton, showed a long range correlation signals at δ117.5for C-8 position.
Compound (5) was obtained as pale yellow amorphous powder. Its molecular formula was determined to be C18H10O6 on the basis of positive-ion peak EIMS (m/z) 600 322[M+]. IR spectraldata showed absorption bands forhydroxyl group (3333 cm-1), C-H functional group and stretching vibration (2932& 2817 cm-1) suggesting the presence of benzene ring, C=C stretching for conjugated carbonly group (1516cm-1).NMR data of 3 indicated the presence of doublet of doublet signals at δ4.04 as doublet of doublet for H-13 position revealed the presence of diastereotopic methylene protons, A doublet signal at δ 4.46 (d, 1H) showed the presence of methine proton attached to a heteroatom and following evidence this methine was also connected to a methine at δ2.55 (m, 1H) forming an furan ring.The presence of doublet of doublet signal at δ6.81, 6.83, 7.03 for H-1', 2’, 5’ positions respectively and doublet of doublet at δ5.09 for H-4 position suggested the presence of spin system on the aromatic ring. 13C NMR spectrum of 2 exhibited the presence of 18 carbon signals are there in respective δ ppm (see Table 1). The three aromatic proton at δ116.3 for C-4, 6.86 (H-8) with δ108.1, methylene protons at δ76.7 for C-2 and oxymethylene protons were observed.Theaceytocy (-COOCH3) group at δ 171.1, whereasmethoxy group were observed at peak δ 56.5, 56.1 for C-3', 4' positions respectively [19].
CONCLUSION
The investigation of chemical compounds from Natural products is fundamentally important for the development of new drugs, especially in view of the vast worldwide flora. Total five compounds include, two triterpenoids, one flavone and two phenolic compounds were isolated from the petroleum ether, ethyl acetate and ethanolic extracts of E. milii flowers. This study is considered as the first report of these compounds from E. milii flowers which could be helpful and can contribute in the chemotaxonomic analysis of this complex genus.The structures of isolated compounds were characterized by IR, 1H-NMR, 13C-NMR and Mass spectral studies. Based on the results, the obtained compounds of E. milii flowers are effective pharmaceutical compounds which will serve as a better alternative to chemical based pharmaceuticals.
Over all conclusion, triterpenoids, flavonoids and phenolic compounds are of great biological properties. Their physiological, bacteriostatic, antioxidant and cardiovascular activities etc, makes these compounds attractive for further derivatization and screening as novel therapeutic agents, which will be related to various beneficial effects exerted on human health.
ACKNOWLEDGMENT
Authors are thankful to Malla Reddy College of Pharmacy, Hyderabad, for providing necessary laboratory facilities. Authors also thankful to Dr. Prathibha, Dept. of Botany, Osmania University, Hyderabad, for the identification of plant material. Head, IICT, Hyderabad and NISHKA Labs, Hyderabad for recording various spectral data.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES
- Bani S, Kaul A, Khan B, Gupta VK, Satti NK, Suri KA, Qazi GN. Anti-arthritic activity of a biopolymeric fraction from Euphorbia tirucalli. Journal of Ethnopharmacology. 2007; 110(1): 92-98.
- Milica P, Jasna B, Ivana SA, Nina M, Todorovic, Milka J, Vlatka E. Vajs, Vele VT, IvanV, Miljana M, Ivanka D, Markovic, Nikola T, Sabera R. New anticancer characteristics of jathrophanediterpenes from Euphorbia dendroides. Food Chemistry and Toxicology. 2011; 49(12): 3165-3173.
- Mwine TJ, Van Damme P. Why do Euphorbiaceae tick as medicinal plants: a review of Euphorbiaceae family and its medicinal features. Journal of Medicnal plant Research. 2011; 5(5): 652-662.
- Chaabi M, Freund-Michel V, Frossard N, Randriantsoa A, Andriantsitohaina R, Lobstein A. Anti-proliferative effect of Euphorbia stenoclada in human airway smooth muscle cells in culture. Journal of Ethnopharmacol. 2007; 109(1):134-139.
- Opferkutch HJ, Hecker E. Active Principles of the Euphorbiaceae. Journal of Cancer Ressearch Clinical Oncology. 1982;103: 255-68.
- Rauf A, Khan A, Uddin N, Akram N, Arfan M, Uddin G, Quasir M. Priliminary Phytochemical screening, antimicrobial and antioxidant activities of Euphorbia milii. Pakisthan Journal of Pharmceutical Science. 2014; 27(4):947-51.
- Qaisar M, Gilani SN, Farooq S, Rauf A, Naz R, Shaista, Perveez S. Preliminary Comparative Phytochemical Screening of Euphorbia Species. American-Eurasian Journal of Agriculture & Envirormental Science. 2012; 12 (8): 1056-1060.
- Delgado IF, De-Carvalho RR, De-Oliveria AC, Kuriyama SN, Oliveira-Filho EC, Souza CA, Paumgarteen FJ, Absence of tumor promoting activity of Euphorbia milii latex on the mouse back skin. Toxicology Letters. 2003; 145: 175-180.
- Freitas JCBR, Presgrave OAF, Fingola FF, Menezes MAC, Vasconcellos MC, Schall VT, Paumgartten FJR 1991. Toxicological study of the molluscicidal latex of Euphorbia splendens. Irritant action on skin and eye. MemInstOswaldo Cruz.1991; 86: 87-88.
- Khan R, Shawl AS, Tantray M, Alam MS. New coumarin glycosides from Rhododendron lepidotum. Fitoterapia. 2008;79: 232-233.
- Zhang W, Shen YH, Liu RH, Zhang C, Chen HS, Fu P, Shan L, Zhang WD. Coumarins from the Stem bark of Daphne marginata. Chemistry of Natural Compounds.2007;43: 317-318.
- Fakir Shahidullah Tareq, Md. Hossain Sohrab AM. Sarwar Uddin Chowhdury, FarhanaAfroz, M. Al-Mansur and Choudhury M. Hasan. Phytochemical Studies on the Leaves of Xyliadolabriformis. Journal of Pharmaceutical Science.2009; 8(2): 171-172.
- Ahmed Al-Harrasi, Liaqat Ali, JavidHussain, Ahmed Al-Rawahi.Two New and Four Known Triterpenoids from Boswellia sacra Fluckiger. Records of Natural Products. 2014; 8(4): 407-411.
- Mahabir P. Gupta, DionisioA. Olmedo, José L. López-Pérez, Esther del Olmo, YelkairaVásquez, Arturo San Feliciano and Mahabir P. Gupta . A New Cytotoxic Friedelane Acid – Pluricostatic Acid – and Other Compounds from the Leaves of Marilapluricostata. Molecules. 2008; 13: 2915-2924.
- Cui-Rong Sun, He-Jiao Hu, Run-Sheng Xu, Jie-Hong Yang and Hai-Tong Wan. A New Friedelane Type Triterpenefrom Euonymus hederaceus. Molecules. 2009; 1: 2650-2655.
- AartiChourasiya, Anil Upadhayay, R.N. Shukla.Isolation of Quercetin-from the leaves fromAzadirachtaindica and anti- diabetic studies from crude extracts. Journal of Pharmaceutical & Biomedical Science. 2012; 25(25): 179-181
- Keiichi Matsuzaki, Rie Ishii, Kaori Kobiyama, Susumu Kitanaka. New benzophenone and quercetingalloyl glycosides from Psidiumguajava L. Journal of Natural Medicine.2010; 64:252–256.
- SheelaTandon and Rastogi. Wikstrosin. A Tricoumarin from Wikstroemiaviridiflora. Phytochemistry, 1977; 16(1): 1991-1993.
- Shakeel-u-Rehma, Reehana Khan, Khursheed A. Bhat, Alsaba F. Raja, Abdul S. Shawl, Mohd S. Alam. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall, Ericaceae. Brazilian Journal of Pharmacognosy 2010; 20(6): 886-890.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences