Metformin Challenges in Advanced Chronic Kidney Disease: A Promising Therapeutic Strategy
M. Fidalgo Díaz*, R. Alonso Valente, V. Becerra Mosquera, I. Abuward, S. Puello Martínez, N. Ardha, M. Durán Beloso, D. Novoa García, T. Cordal Martínez, D. Güimil Carbajal, C. Díaz Rodríguez
Department of Nephrology, Hospital Clínico Universitario de Santiago de Compostela, Spain
- *Corresponding Author:
- Manuel Fidalgo Díaz
Department of Nephrology
Hospital Clínico Universitario de Santiago de Compostela, Spain
E-mail: manuel.fidalgo.diaz@sergas.es
Received Date: July 12, 2017; Accepted Date: August 16, 2017; Published Date: August 18, 2017
Citation: Díaz MF, Valente RA, Mosquera VB, Abuward I, Martínez SP, et al. (2017) Metformin Metformin Challenges in Advanced Chronic Kidney Disease. A Promising Therapeutic Strategy. Jour Ren Med. Vol.1 No.2: 12.
Abstract
Background: Metformin is the first-line treatment in type 2 diabetes mellitus because the beneficial effects respect to other antidiabetic drugs on hypoglycemia, obesity, dyslipidemia and cardiovascular morbidity and mortality and even on renal cancer incidence. However, the accumulation of metformin in cases of impaired renal function may lead to a type B lactic acidosis, which has led to its contraindication in patients with chronic kidney disease (CKD), initially to glomerular filtration less than 60 ml/min and subsequently less than 30 ml/min. The dosedependent toxicity, the low rate of onset of metforminassociated lactic acidosis (MALA) and lack of knowledge of pharmacokinetics in CKD, have motivated the development of studies which could support metformin use in advanced stages of CKD.
Methods: We did a literature review compiling recent, more relevant and impact articles, to conclude about the current situation of metformin safety in advanced stages of CKD as well as try to offer a concise future perspective. The analysis has been structured about pharmacokinetics and pharmacodynamics studies, mortality, rate of metabolic acidosis and potential benefits.
Findings: Several studies have demonstrated to provide accurate pharmacokinetics and pharmacodynamics of metformin in advanced CKD stages allowing us to predict a possible behavior. It has been necessary to perform plasma levels and adjustments due to high variability concentrations in hemodiafiltration. MALA has a high mortality rate but incidence rate remains very low, clearly dose dependent among other modifiable factors.
Conclusions: We believe in the need to reconsider the current contraindications of metformin in CKD, especially in stage 4. A single dose of 500 mg per day with some recommendations it seems appropriate. Clinical trials are urgently needed having a possible impact on diabetic nephropathy progression. More data are necessary to validate its use on stage 5 of CKD, which gain in complexity and seems prohibitive.
Keywords
Type 2 diabetes mellitus; Metformin; Lactic acidosis; Hemodiafiltration; Cardiovascular outcomes; Advanced chronic kidney disease; Therapeutic strategy
Introduction
Metformin is the first-line treatment in type 2 diabetes mellitus (DM), belongs to biguanides group, oral hypoglycemic agents that have been used in treatment of DM for many years. Already known from medieval times (french lilas), in the 1920s guanidine were discovered as the active ingredient. During the 1950s and 1960s 3 biguanide compounds were available for clinical use in diabetes: phenformin, buformin and metformin. In 1976, phenformin was withdrawal in many countries due to the high rate of B lactic acidosis [1]. Metformin never had that rate of acidosis but his renal elimination provoked that regulatory agencies contraindicated it in patients with chronic kidney disease (CKD) as his accumulation could develop in lactic acidosis. Metformin was initially restricted to filtered glomerular functions greater than 60 ml/min (FDA in 1995) and subsequently to greater than 30 ml/min [1,2].
Metformin has proved in many studies in recent years to be beneficial versus other anti-diabetic drugs on hypoglycemia, cardiovascular morbidity and mortality [3-6] as described, for example, a pivotal study by the United Kingdom Prospective Diabetes (UKPDS) 34, a trial of 1,704 overweight patients and type 2 DM [6]. Also has been described renal protection [7] and even on incidence of renal cancer [8]. Another analysis from the UKPDS group stratified patients according to their total daily dose of metformin showed that there were no significant differences in the observed reduction in the risk of coronary artery between the groups, this provides suggestive evidence that the cardiovascular benefits of metformin observed in the UKPDS may be independent of the dose of metformin [9]. Concomitant and simultaneous treatment of diabetic dyslipidemia and metformin have been shown to reduce urinary albumin excretion in obese subjects and ultimately reduction in microvascular and macrovascular complications independent of tight glycaemic control [10-12]. Its low cost makes it a very profitable drug and taking into account the number of people with CKD and type 2 DM, there are millions of people currently not taking metformin and who could be benefit. Suspension of metformin in CKD patients often leads to poor glycemic control and may accelerate the progression of diabetic nephropathy, in addition to the loss of cardiovascular effects in a population already at high risk [2].
The metformin absorption is incomplete and variable (25-75%), occurs in the small intestine and decreases slightly with increasing doses. Metformin is a highly ionized, watersoluble substance absorbed, distributed and eliminated by transporters; the best studied transporters are the organic cation transporters (OTC). Metformin renal clearance is 4-fold greater than creatinine clearance, reflecting a significant tubular secretion; in addition, renal and total clearance may also depend on another factor such as low-function transporters variants [13]. Action mechanism consists of decreasing hepatic glucose production through the transient inhibition of the hepatocyte mitochondria cycle [1,14]. This energy decrease will activate the cAMP-activated protein kinase (AMPK), a metabolic cell sensor, which will provoke increased expression of “SHP” transcription factor, which in turn inhibits the expression of hepatic gluconeogenic genes PEPCK and GLC-6-Pass [15]. These mitochondrial effects of metformin explain the theoretical risk of lactic acidosis: reduction of gluconeogenesis and glycogenolysis, increased glycolysis and activation of intestinal anaerobic metabolism [1,2,14]. Lactic acid occurs when there are hypoperfusion and tissue hypoxia. Additional risk factors for the development of lactic acidosis would be: overdose, sepsis, shock, myocardial infarction, renal, cardiac, respiratory or hepatic failure and hypoxemia. Acidosis would occur when production exceeds its elimination and if severe, could have repercussions on the cardiovascular system (ventricular dysfunction, arrhythmia, hypotension), respiratory (pulmonary edema), and central nervous system (delirium and coma) [1,2].
Lactic acidosis secondary to metformin has been described with a frequency ranging from 1 to 47 cases per 100,000 person-years and with a mortality rate around 50%, Therefore, despite the widespread use of metformin worldwide, the number of cases described appears to be small [2].
There is also a great deal of debate about whether metformin is associated with lactic acidosis. There are epidemiological studies suggesting that the use of metformin is associated with an increased risk of lactic acidosis while others do not show such association, even in CKD populations. In the majority of cases the additional risk factors previously described are involved [1,2].
Experience with lactic acidosis of phenformin has probably sensitized metformin to regulatory organisms leading to the approval of CKD restrictions in the absence of clear data assessing the risk [2].
The pharmacokinetics of metformin in patients with CKD has been described with studies investigating metformin in hemodialysis or hemodiafiltration lacking detailed pharmacokinetic or therapeutic doses or are in the context of overdose of metformin and lactic acidosis [16,17]. The therapeutic range of plasma concentrations of metformin is not entirely clear, however, concentrations higher than 5 mg/L are considered high [13].
We think, as has been happening in recent years, that it is necessary again reconsider the current contraindications of metformin in CKD [18,19] and develop studies that support the new strategies emerged.
Methods
We did a literature review, compiling recent, more relevant and impact articles, including systematic reviews, retrospective observational studies, original research, research letters, pharmacokinetic and pharmacodynamic studies, opinions about the current state and safety of metformin employment on advanced CKD. The analysis has been structured about pharmacokinetic and pharmacodynamic data, potential benefits, mortality and rate of metforminassociated metabolic acidosis (MALA).
We believe that any approach to expand metformin use must begin and be sustained with pharmacokinetics and pharmacodynamics data. To describe the pharmacokinetics, dosage and safety of metformin in advanced stages of chronic renal disease we focused on two pivotal studies.
A pharmacokinetic model of metformin in the general population developed by K. Duong et al. [13] describes the factors that influence the variability between healthy subjects and patients with type 2 diabetes mellitus (DM2) as well as the profile of concentrations in renal patients. They grouped the metformin data of plasma values from three studies: patients with type 2 DM (study A, n = 120), healthy Caucasian subjects (study B, n = 16) and healthy subjects from Malaysia (study C, n = 169). In Study 52 patients (43%) had renal insufficiency: creatinine clearance (CLCr) 60 ml/min, including 13 patients (11%) with advanced chronic kidney disease (CLCr below 30 ml/ min) [Table 1]. In addition 33 patients (28%) were taking at least one diuretic (thiazide, furosemide or potassium-sparing diuretic). Approximately half of patients in the type 2 DM group were taking metformin in combination with another oral anti-diabetic agent, mainly sulfonylureas. Pharmacokinetics were developed using the NONMEM Version VI software for both immediate release (IR) and prolonged release (XR) formulations. Potential variables investigated were: total body weight (TBW), lean body weight (LBW), creatinine clearance (CLCr, estimated using TBW and LBW) and 57 polymorphisms (SNPs) of metformin transporters (OCT1, OCT2, OCT3, MATE1 and PMAT). A nonparametric bootstrapping method (n = 1,000) was used to evaluate the 95% confidence interval. This model was also used to simulate 1,000 concentration time profiles to evaluate metformin dose ranges (500, 1,000, 2,000 and 3,000 mg) at varying levels of renal function (CLCr: 15, 30, 60 and 120 ml/min) to ensure that Cmax 95th percentile of metformin was kept below 5 mg/L. The impact of variability in the simulations was analyzed. Each simulation generated 1000-time profiles of concentration for all subjects per dose and renal function group. The mean steady-state metformin plasma concentration was used to investigate correlations with HbA1c and lactate. Statistical analyzes were performed.
| Characteristics | Study A | Study B | Study C | Total |
|---|---|---|---|---|
| n | 120 | 16 | 169 | 305 |
| No. of blood samples | 2 (1-18) | 120(52-120) | 15 (10-18) | 15(1-120) |
| Age (years) | 65 (39-86) | 27 (19-40) | 23 (18-47) | 28(18-86) |
| BMI (kg/m2) | 29.7 (18.6-56.0) | 23.3 (20.2-27.7) | 20.9(17.1-28.7) | 23.4 (17.1-56.0) |
| TBW (kg) | 86 (53-165) | 70(53-103) | 58 (41-88) | 65(41-165) |
| CLCr(ml/min) | 67 (15-127) | 81 (63-102) | 90 (57-178) | 81(15-178) |
| Metformin doses (mg/day) | 1500 (250-3000) | 1000 (500-2000)a | 500 (500-1000)b | - |
| HbA1C (%) | 7.2 (4.7-14.5) | - | - | - |
| Lactic acid (mmol/l) | 1.7 (0.5-5) | - | - | - |
Subjects in study B, all received the same doses bSubjects received single doses BMI body mass index, TBW total body weight. CLCr<creatinine clearance (using lean body weight). HbA1C glycosylated haemoglobin.
Table 1: Study characteristics of patients and healthy subjects in the population pharmacokinetic model. Data are presented as median and range.
Another recent model for pharmacokinetic on Hemodiafiltration were developed by O. Day et al. Four patients with type 2 DM in Hemodiafiltration (HDF) 20 three times per week during a 3-month follow-up period who initially received treatment with 500 mg of immediate release metformin after each HDF session (total 1500 mg/week). Pre- HDF blood samples were taken at the end of weeks 1, 4, 8 and 12. To determine metformin clearance during the session preand post-filter samples were collected in each procedure.
Three venous blood samples were collected from 4 to 42 hours post dose 3 times to determine metformin concentrations between the HDF sessions. A protocol was followed whereby the metformin dose would be reduced if the metformin plasma concentrations were close to the safety threshold, considered at 5 mg/L and/or if the lactate concentrations were close to 45 mg/dL. The methods, biochemistry, demographic characteristics and HDF sessions are summarized in Table 2.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Mean HDF Parameters | ||||
| Duration of HDF sessions, h | 6.0 | 5.5 | 4.5 | 5.0 |
| Blood flow rate, mL/min | 297 | 294 | 303 | 303 |
| Flow rate of HDF fluid, mL/min | 364 | 369 | 346 | 371 |
| Pre- or post-HDF dilution | Post-dilution | Post-dilution | Pre-dilution | Post-dilution |
| Effect of HDFa | ||||
| Reduction in plasma metformin concentrations during HDF, % | 63 ± 3 (6) | 58 ± 10 (6) | 74 ± 6 (6) | 52 ± 8 (5) |
| Extraction ratio | 0.84 ± 0.14 (4) | 0.90 ± 0.04 (4) | 0.64 ± 0.21 (4) | 0.56 (1) |
| Clearance, mL/min | 176 ± 24 (4) | 179 ± 5 (4) | 135 ± 41 (4) | 123 (1) |
| Dose removed, % | 28 ± 14 (4) | 22 ± 4 (4) | 11 ± 6 (4) | 2 (1) |
Note: All data for individual HDF sessions are summarized in Item S1. a Values are mean ± standard deviation for each patient across all HDF sessions (i.e., study visits) when data collection was complete and all concentrations of metformin were above the lower limit of assay (0.05 mg/L). Number in parentheses corresponds to number of qualifying sessions
Table 2: Mean HDF conditions and metformin removal from plasma, by patient.
MALA
Trying to characterize the onset of MALA and clarify a true impact, several pivotal studies were analyzed, of which we would emphasize on a review by Inzucchi et al [20]. From January 1950 to June 2014 they tried to determine the risk of metabolic acidosis, employing Medline and Cochrane databases searching for articles related to metformin, renal disease and lactic acidosis between 1950 and June 2014. (Reviews, letters, editorials, clinical cases, and small series of cases). Of a total of 818 articles, 65 were selected including pharmacokinetic-metabolic studies, large case series, retrospective studies and meta-analysis.
Another recent international retrospective study in diabetic patients with renal disease performed by Hung et al. [21] using historical data from Taiwan, was published in The Lancet Endocrinology and Diabetes, providing information with a unique insight about metformin potential toxicity. Until 2009, metformin was prescribed in all patients irrespective of their renal function. They examined 12,350 stage 5 CKD type 2 diabetic patients from the Taiwan National Health Insurance database between 1 January 2000 and 30 June 2009, with follow-up up to 31 December 2009. In this population of origin 1,005 patients were using metformin and 11,345 who did not. A metformin dose unit was defined: daily cumulative dose (DDD), categorized as ≤ 15, 16 to 40 and> 40 DDD (1 DDD equals 2000 mg of metformin over a 90-day exposure period), And prescribed daily dose, categorized as ≤ 500, 501-1,000, and> 1,000 mg / day. The main objective was to analyze all causes of mortality.
Results
Pharmacokinetics
In Duong et al. study patients with type 2 DM were older and had a lower renal function than healthy population. Glycemic control was variable in patients with type 2 DM and the daily dose of metformin was higher in patients with poor control (HbA1C> 7.5%, p <0.05). The mean daily dose of metformin was 1,500 mg (range 250-3000 mg). Most patients with type 2 DM had normal plasma lactate concentrations, except for 13 patients with lactate concentrations above the reference range ([2.7 mmol /L]). For patients with type 2 DM no correlation was found between the steady-state plasma concentration of metformin and the concentrations of HbA1C and lactate.
Among the renal results, it should be noted that with the inclusion of CLCr (calculated using LBW) on model’s parameters, the interindividual variability was reduced and its elimination together with that of the total body weight caused a greater weakness of the model. The patients' age was not a significant variable, neither the lean weight, nor any of the polymorphisms studied. In addition the use of diuretics did not influence the clearance of metformin.
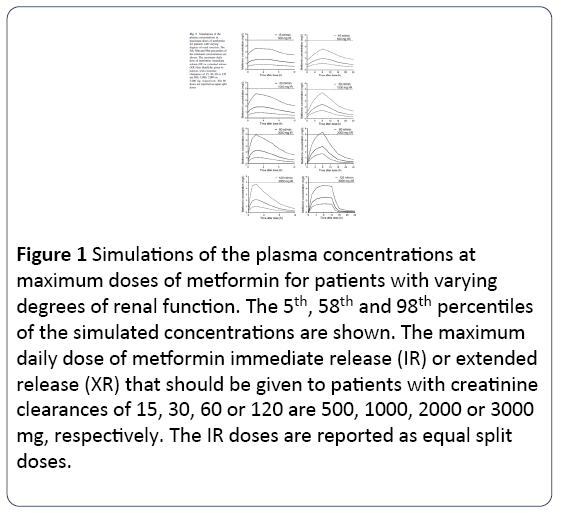
The mean clearance of metformin for studies A, B and C was 760 ml/min (range 156-2307 ml/min), 1,201 ml/min (800-1,762 ml/min) and 1066 ml/min (564-3,048 ml/min) respectively. The mean concentration of Metformin in studies A and B was 1.28 mg / L (range 0.2-7.7 mg/L) and 0.9 (0.6-1.1 mg/L), respectively. One patient with end-stage renal disease (CLCr = 15 ml/min) presented the highest concentration (7.7 mg/L) and received high doses of metformin per day (2000 mg). Dose simulations revealed that to ensure that the 95th percentile of metformin Cmax is less than 5 mg/L, maximum doses of 500, 1,000, 2,000 and 3,000 mg daily should be prescribed to patients with a respective CLCr of 15, 30, 60 and 120 ml/min. Metformin concentrations were greater than 5 mg/L (5.4 mg/L, 95th percentile) in patients with CLCr of 60 ml/min at doses of 2000 mg for the delayed formula but not for the immediate one. The maximum recommended dose of metformin in the delayed form is 2,000 mg daily, but patients with good renal function may receive doses of 3,000 mg daily without exceeding metformin concentrations of 5 mg/L. All this is shown in Fig.3. The half-life was similar for both formulations and higher with lower CLCr: for CLCr of 15, 30 and 60, ml/min, mean half-lives were around 13.8, 4.5 and 3 hours respectively: For CLCr of 120 ml/min were approximately 3 hours for the immediate formulation, and biphasic for the delayed formulation with 1.2 hours for the initial phase and 7 hours for the late formulation.
In the hemodiafiltration study 20 metformin concentrations did not exceed 5 mg/L however, pre-HDF concentrations approached 5 mg/L at week 4 in patients 1 and 2, forcing the dose to 500 mg once week. The metformin dose was also reduced to 250 mg after each HDF (750 mg/week) in patients 3 and 4. These dose changes not made possible to achieve plasma concentrations of metformin equilibrium or steady state.
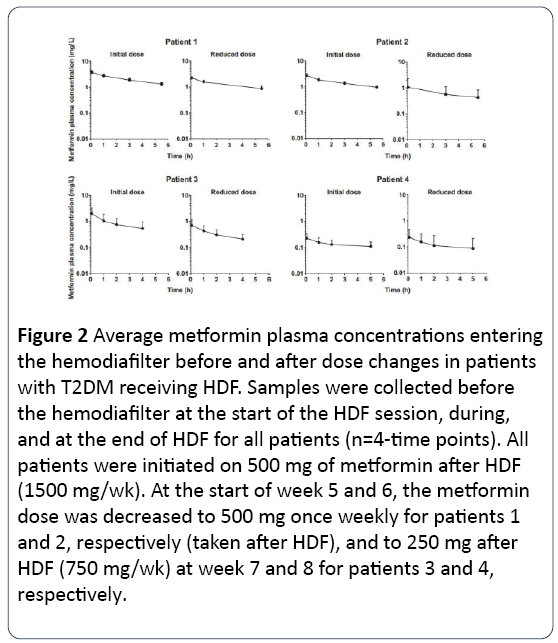
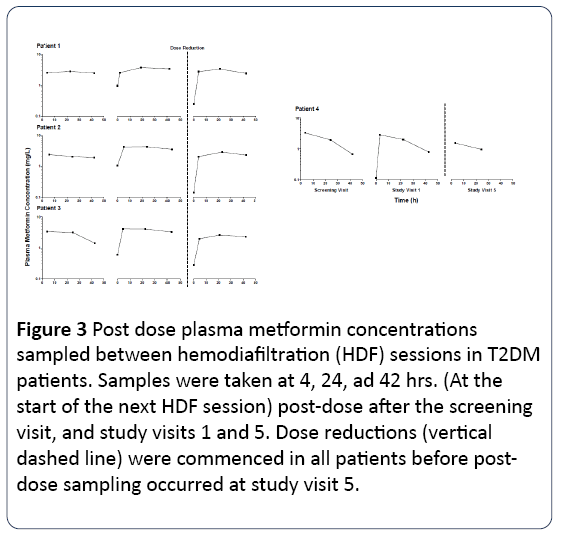
Metformin plasma concentrations were significantly reduced (52% -74%, Figure 1) during HDF session. The extraction fraction in plasma ranged from 0.56 to 0.90 as well as free spaces ranging from 123 to 179 ml/min, consistent with the physical properties of metformin (low molecular weight and lack of binding to plasma proteins), which make it easily removable by HDF. This metformin clearance was relatively low expressed as a percentage of administered dose (patients 1-3, 11-28%, Table 1), probably reflecting incomplete metformin absorption (about 25-75% in healthy individuals) and very low clearance of erythrocytes. Metformin plasma concentrations at 4 to 42 hours after dosing were generally constant (Figure 2); due to almost exclusively renal elimination, patient 4 preserved significant residual renal function, as demonstrated by the decrease in plasma metformin between sessions of HDF (Figure 3).
Figure 1: Simulations of the plasma concentrations at maximum doses of metformin for patients with varying degrees of renal function. The 5th, 58th and 98th percentiles of the simulated concentrations are shown. The maximum daily dose of metformin immediate release (IR) or extended release (XR) that should be given to patients with creatinine clearances of 15, 30, 60 or 120 are 500, 1000, 2000 or 3000 mg, respectively. The IR doses are reported as equal split doses.
Figure 2: Average metformin plasma concentrations entering the hemodiafilter before and after dose changes in patients with T2DM receiving HDF. Samples were collected before the hemodiafilter at the start of the HDF session, during, and at the end of HDF for all patients (n=4-time points). All patients were initiated on 500 mg of metformin after HDF (1500 mg/wk). At the start of week 5 and 6, the metformin dose was decreased to 500 mg once weekly for patients 1 and 2, respectively (taken after HDF), and to 250 mg after HDF (750 mg/wk) at week 7 and 8 for patients 3 and 4, respectively.
Figure 3: Post dose plasma metformin concentrations sampled between hemodiafiltration (HDF) sessions in T2DM patients. Samples were taken at 4, 24, ad 42 hrs. (At the start of the next HDF session) post-dose after the screening visit, and study visits 1 and 5. Dose reductions (vertical dashed line) were commenced in all patients before postdose sampling occurred at study visit 5.
MALA
A review by Inzucchi et al. [21] from January 1950 to June 2014 determine that although metformin is eliminated by renal route, drug levels, therapeutic range, and lactate concentrations do not increase substantially when used in patients with mild to moderate chronic kidney disease (estimated glomerular filtration (eGFR) between 30- 60 and 60-90 ml/min per 1.73m2).
The overall incidence of lactic acidosis in metformin users varies between studies from 3 to 10 per 100,000 person-years and is generally indistinguishable from the background rate in the whole population with diabetes. Data suggesting an increased risk of lactic acidosis in patients with metformin are therefore poorly reproducible.
In studies where metformin was associated with increased lactate levels, none increased to a significant level (defined as lactate level 5 mmol/L and pH, 7.35). A. Frid et al, show that is necessary high levels of metformin to cause MALA [22].
In the reviewed cases, there were other associated causes to metabolic acidosis such as infection, hepatic insufficiency, renal failure or cardiovascular event. Therefore, the use of metformin was concomitant but not clearly causal. Patients with type 2 DM have not been associated with higher rates of lactic acidosis.
Several observational studies also suggest a metformin benefit on macrovascular outcomes, even in patients with contraindication criteria for renal failure. Range and lactate levels are not substantially increased when metformin is used in those with reduced GFR and It also suggest that in the current prescription of metformin there is no good adherence to the guidelines for its use in renal failure [Table 3] [23-26].
| Renal Contraindication | |||||
|---|---|---|---|---|---|
| Source | No. | Setting | Frequency, No. (%) | Definitiona | Frequency of Lactic Acidosis |
| Kosmalski et al. [20] | 335 | Hospital | 56 (16.7) | eGFR<60 | No cases |
| Vasisht et al. [21] | 234 | Outpatient | 36 (15.4) | eGFR<60 | No cases |
| Scotton et al. [22] | 283 | Hospital | 17 (6.0) | SCr>1.5 (men) | Not reported |
| SCr>1.4 (women) | |||||
| Kamber et al. [23] | 425 | Outpatient | 78 (8.4) | eGFR<60 | No casesb |
| Runge et al. [24] | 92 | Outpatient | 4 (4.4) | SCr ≥ 1.5 (men) | Not reported |
| SCr ≥ 1.3 (women) | |||||
| Sweileh et al. [25] | 124 | Outpatient | 34 (27.4) | Renal impairment | Not reported |
| Warren et al. [26] | 11 297 | Outpatient | 880 (25.5) | eGFR<60 | Unknown |
| Kennedy et al. [27] | 4838 | Outpatient | 219 (4.5) | eGFR<60 | Not reported |
| 290 (13.4) [men] | SCr ≥1.5 (men) | ||||
| 362 (17.7) [women] | SCr ≥1.4 (women) | ||||
| Millican et al. [28] | 83 | Hospital | 12 (14.5) | eGFR<50 or | Not reported |
| SCr>1.7 | |||||
| Horlen et al. [29] | 22 | Outpatient | 8 (36.4) | SCr ≥1.5 (men) | Not reported |
| SCr ≥1.4 (women) | |||||
| Calabrese et al. [30] | 263 | Hospital | 32 (12.2) | SCr ≥1.5 (men) | 3 cases (metformin could not be ruled out as the cause) |
| SCr ≥1.4 (women) | |||||
| Emslie-Smith et al. [31] | 1347 | Outpatient | 63 (4.7) | SCr ≥1.7 | 1 case, unrelated (extensive myocardial infarction, renal function previously normal) |
| Holstein et al. [32] | 308 | Hospital | 59 (19.2) | eGFR<60 | No cases |
| Selby et al. [33] | 9875 | Outpatient | 128 (1.3) | SCr ≥1.5 | 1 case, likely unrelated (renal function normal) |
| Sulkin et al. [34] | 89 | Outpatient | 2 (2.3) | SCr ≥1.4 | Not reported |
Abbreviations:eGFR:Estimated Glomerular Filtration Rate; SCr:Serum Creatinine. SI conversion factor: To convert serum creatinine values to μmol/L, multiply by 88.4.
aEstimated glomerular filtration rate values reported in mL/min per 1.73 m2; serum creatinine values reported in mg/dL.
bDuring study follow-up (1993 to 2001); authors reported 3 patients with metformin-associated lactic acidosis during extended follow-up via data linkage through 2006, each of whom had at least 1 major comorbidity associated with lactic acidosis [estimated incidence similar to that of patients not treated with metformin (P=0.4)].
Table 3: Retrospective studies examining the frequency of metformin use in patients with active renal contraindications.
In another observational study of 51 675 Swedish [42] men and women with type 2 diabetes who were followed up for 4 years, no increased risk of acidosis or serious infection was recorded in metformin users with an eGFR of 30–45 mL/min per 1.73 m2 compared with metformin non-users.
In Taiwan registry because of initial difference between groups they did a new division in two comparable groups: 813 patients with metformin versus 2,439 without metformin, there were no significant differences compared to the 30 clinical and socioeconomic variables analyzed. The mean age was 67.2 years, the mean eGFR was 10 mL/min per 1, 73 m² for men and 7mL/min for 1.73 m² for women.
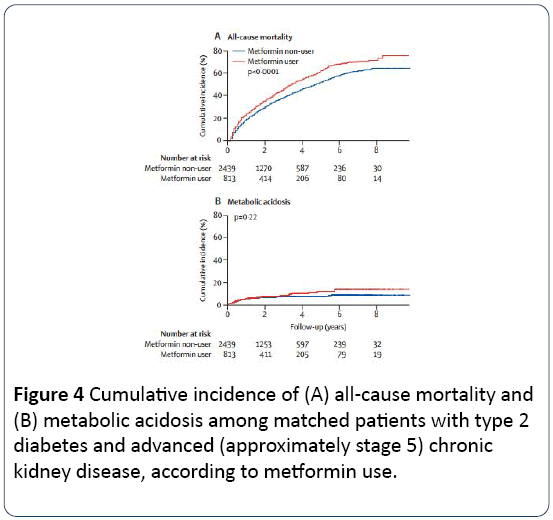
All-cause mortality was 53% (413 of 813) in the metformin group and 41% (1012 of 2439) in the other group. After multivariate adjustment, metformin was an independent risk factor for mortality (adjusted risk ratio 1.35, 95% CI 1.20-1.51, p <0.0001). The increased risk of mortality was dosedependent and was consistent across all subgroup those taking a dose of between 500-1000 mg/day did not have a risk of death significantly higher than patients who did not take metformin. The metabolic acidosis incidence was slight higher in the metformin group although without significant risk (incidence of 4% in both groups). The metformin group was less susceptible to progression of CKD to dialysis. [Tables 4 and 5, Figure 4]
Figure 4: Cumulative incidence of (A) all-cause mortality and (B) metabolic acidosis among matched patients with type 2 diabetes and advanced (approximately stage 5) chronic kidney disease, according to metformin use.
| Variables | All-cause mortality | Metabolic acidosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Events(n/N) | Incidence (per 100 patient- years) | Crude hazard ratio (95% CI) | Adjusted hazard ratio* (95% CI) | Events(n/N) | Incidence (per 100 patient- years) | Crude hazard ratio (95% CI) | Adjusted hazard ratio* (95% CI) | |
| Matched cohort | ||||||||
| Non-users | 1012/2439 | 15·8 | 1·00 | 1·00 | 86/2439 | 1·3 | 1·00 | 1·00 |
| Users | 434/813 | 20·1 | 1·29 (1·15-1·45) | 1·35 (1·20-1·51) | 36/813 | 1·6 | 1·27 (0·86-1·88) | 1·30 (0·88-1·93) |
| Deï¬ÂÂned daily dose | ||||||||
| ≤15 DDD | 129/252 | 16·7 | 1·12 (0·85-1·32) | 1·16 (0·91-1·45) | 8/252 | 1·7 | 1·31 (0·93-2·41) | 1·36 (0·98-2·33) |
| 16–40 DDD | 141/269 | 22·1 | 1·27 (0·99-1·50) | 1·31 (1·02-1·52) | 12/269 | 1·3 | 1·21 (0·77-2·12) | 1·25 (0·74-2·29) |
| >40 DDD | 164/292 | 28·7 | 1·52 (1·21-1·75) | 1·58 (1·25-1·80) | 16/292 | 1·5 | 1·26 (0·80-2·20) | 1·28 (0·81-2·24) |
| p for trend | ·· | ·· | 0·044 | 0·051 | ·· | ·· | 0·667 | 0·503 |
| Prescribed daily dose | ||||||||
| ≤500 mg/day | 93/193 | 15·1 | 1·10 (0·81-1·40) | 1·14 (0·85-1·44) | 4/193 | 1·8 | 1·33 (0·98-2·98) | 1·35 (0·97-2·88) |
| 501–1000 mg/day | 129/255 | 17·2 | 1·23 (0·87–1·37) | 1·30 (0·93–1·45) | 8/255 | 1·4 | 1·22 (0·95-2·45) | 1·27 (0·96-2·32) |
| >1000 mg/day | 212/365 | 28·2 | 1·52 (1·24–1·72) | 1·57 (1·29–1·83) | 24/365 | 1·5 | 1·27 (0·98–2·44) | 1·29 (0·96–2·38) |
| p for trend | ·· | ·· | 0·064 | 0·048 | ·· | ·· | 0·633 | 0·488 |
Data before propensity score matching are presented in the appendix (p 5). DDD=defined daily dose. *Adjusted for all covariates in table.
Table 4: Risk of death and metabolic acidosis in patients with type 2 diabetes and advanced (approximately stage 5) chronic kidney disease after propensity score matching (n=3252).
| Events (incidence per 100 patient-years) | Crude hazard ratio (95% CI) | Adjusted hazard ratio* (95% CI) | Competing risk- adjusted hazard ratio (95% CI) | ||
|---|---|---|---|---|---|
| Metformin users (n=813) | Metformin non-users (n=2439) | ||||
| Chronic dialysis | 478 (59.3) | 1616 (82.3) | 0.76 (0.68-0.84) | 0.75 (0.67-0.83) | 0.76 (0.69-0.84) |
| Death before E SRI) | 261 (32.4) | 528 (26.9) | 1.21(1.04-1.41) | 1.3 (1.11-1.51) | 1.55 (1.33-1.80) |
| Death after ESRD | 173 (8.5) | 484 (7.5) | 115 (1.03-1.39) | 1.25 (1.08-1.48) | NA |
| ESRD=end-stage renal disease, NA= not applicable, *Adjusted for all covariates in table | |||||
Table 5: Risk of chronic dialysis, death before ESRD, and death after ESRD
Discussion
Metformin in CKD therefore represents an exciting challenge related to it contradictory behavior: mortality data versus clear excellent cardiovascular outcomes, among them a possible lower progression of diabetic nephropathy as described the taiwanese data. Patients with advanced CKD and dialysis are at very high risk of cardiovascular disease, however they currently do not have the opportunity of metformin benefits, making this one of our first duties of this current times in the nephrological community.
If we are looking at the simulation model for metformin plasma levels proposed by Duong et al. according to the degrees of CLCr, we can see an interesting behavior of metformin with CLCr less than 30 ml/min. The simulation model for dose of 500 mg day at 15 ml/min of CLCr has the best stability in comparison with the others, as never approaching the marked limits and even presents the most distance to plasma levels of 5mg/L considered dangerous. This behavior is not observed with higher doses and even with higher CLCr levels. This would provide a relative safety to encourage us if we want to tackle the issue of metformin in advanced CKD.
The incidence of MALA is very low, even in patients with moderate and advanced chronic kidney disease. The study of the Taiwanese population relies on a number of strengths such as national representativeness, prolonged follow-up period, detailed information on the level of comorbidities, medications, and laboratory parameters. It is clearly shown how the risk of mortality is associated with dose dependence and finding no greater risk with doses of 500-1000 mg/day. The incidence of metformin-associated lactic acidosis remains very low in consistency with all previous literature and presents a high mortality, near the unacceptable, in glomerular filtrations around 10 ml/min. We should not forget there are other associated causes to metabolic acidosis such as dehydration, infection, hepatic insufficiency, renal failure or cardiovascular event.
With all of the above we simply consider this as the continuation work has so far been to find the correct dose for the level of CKD, as happened with the previous stages.
An approximation to metformin use in patients on HDF has been showed an important dose variability, being necessary to individually reduce dose after 4 or 5 weeks, motivated in big part by the residual renal function and probably also by dose of HDF, this will lead to an individual and not fixed-dose and will establish a frequently monitoring blood levels in our clinical practice. It is important to note that metformin plasma levels do not correlate consistently with lactate levels, which in part is due that MALA need the presence of other elements: acute gastroenteritis, shock, sepsis...so we are not yet in a position to strong adequately monitor toxicity in a technique where metformin levels presents a high variable behavior. If we add the high mortality rate described on glomerular filtration rates around 10 ml/min, it can be concluded that the use of metformin in ERSD patients it seems to remain prohibitive and complex with a needing of continue expanding studies with a longer follow-up period with an extra difficult ethical approval. We do not encourage further exploration in this regard.
On the other hand, what happens with patients in stage 4 of CKD. This patients have enough renal function, especially those who may be stable for a considerable period of time, could maintain a relative safe fixed dose of 500 or even 250 mg/day. It would be necessary accompanied by mandatory advices like to instruct in metformin withdrawal during any intercurrent illness or risk of dehydration: gastroenteritis, diarrhea, hypotension or before any surgical operation... also would be necessary perform a periodical study of blood levels and note that, especially in rapid progressors, metformin should be withdrawn as eGFR approaches to 15 ml/min. The cardiovascular benefits are independent of dose, and in addition to these effects there also appears to be improvement on renal and other types of cancer: pancreas, breast and prostate [42-49]. If we add that diabetic nephropathy could be delayed, stage 4 of the CKD population has a more interesting current application than dialysis or HFD, with all the savings that would entail reducing the annual incidence of ESRD.
We believe, with all the necessary precautions and with meticulous patient follow-up, that reconsideration of the current contraindications of metformin in CKD should continue. The previously described strategy seems appropriate and should encourage to perform a trial to confirm the use and benefits of metformin in eFG between 15 and 30 ml/min.
Conclusion
The incidence of MALA is very low, even in patients with moderate and advanced chronic kidney disease. The increased risk of mortality of metformin is dose-dependent, and due to other factors and comorbidities.
A possible strategy to confirm, with adjusting doses and perhaps, simple tips, in case of patients in stage 4 of CKD may be appropriate. Metformin treatment could be continued by lowering dose to 500 mg/day with frequent and strict controls of renal function, plasma levels and providing a careful written information about treatment discontinuation. A pharmacokinetic simulation model of levels of metformin provides a reasonable safety to confirm. We could provide a benefit at this stage and perhaps more interesting than for stage 5 of CKD, since the progression of nephropathy could be delayed.
The use of metformin on stage 5 of CKD gains in complexity and faces a difficult perspective: the important variations in concentrations, that will be necessary to perform plasma levels periodically on clinical practice, lack of adequately monitor toxicity and the high mortality data on glomerular filtration rate around 10 ml/min. Continuing to expand pharmacokinetic data with more powerful studies and longer follow-up periods is necessary, although approving these studies in renal replacement therapy poses an ethical and very challenging difficulty.
Therefore, as evidences accumulate, it seems that the more conservative positions should be abandoned in some cases, with the necessary cautions, so allow us we should be encouraged to perform randomized studies.
References
- Rhee CM, Kovesdy CP, Kalantar-Zadeh KK (2016) Risks of metformin in type 2 diabetes and chronic kidney disease: Lessons learned from Taiwanese data. Nephron 135: 147-153
- Chowdhury TA, Srirathan D, Abraham G, Oei EL, Fan SL, et al. (2017) Could metformin be used in patients with diabetes and advanced chronic kidney disease? Diabetes ObesMetab 19: 156-161.
- Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, et al. (2008) Cardiovascular outcomes in trials of oral diabetes medications: A systematic review. Arch Intern Med 168: 2070-2080.
- Schramm TK, Gislason GH, Vaag A, Rasmussen EN, Folke F, et al. (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: A nationwide study. Eur Heart J. 32: 1900-1908.
- Morgan CL, Mukherjee J, Jenkins-Jones S,Holden SE, Currie CJ (2014) Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: A retrospective, observational study. Diabetes ObesMetab. 16: 957-962
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, et al. (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510: 542-46.
- Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, et al. (2013) Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: Simulation of doses according to renal function. ClinPharmacokinet 52: 373-384
- Kim YD, Park KG, Lee YS, Park YY, Kim DK, et al. (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57: 306-314.
- Lalau JD, Andrejak M, Moriniere P, Coevoet B, Debussche X, et al. (1989) Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: A study of metformin elimination. Int J ClinPharmacolTherToxicol 27: 285-288.
- Calello DP, Liu KD, Wiegand TJ, Roberts DM, Lavergne V, et al. (2015) Extracorporeal treatment for metformin poisoning: Asystematic review and recommendations from the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med 43: 1716-1730.
- LuWR, Defilippi J, Braun A (2013) Unleash metformin: Reconsideration of the contraindication in patients with renal impairment. Ann Pharmacother 47: 1488-1497.
- Stanton RC (2015) Metformin use in type 2 diabetes mellitus with CKD: Is it time to liberalize dosing recommendations? Am J Kidney Dis. 66: 193-195
- Smith FC, Kumar SS, Furlong TJ, Gangaram SV, Greenfield JR, et al. (2016) Pharmacokinetic of metformin in patients receiving regular hemodialfiltration. Am J Kidney Dis. 14: 30416-30424.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK (2014) Metformin in patients with type 2 diabetes and kidney disease a systematic review. JAMA 312: 2668-2675.
- Tseng CH (2016) Use of metformin and risk of kidney cancer in patients with type 2 diabetes.Eur J Cancer 52: 19-25.
- Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC Jr, et al, (2010) Reduction of Atherothrombosis for Continued Health (REACH) registry investigators. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 170: 1892-1899.
- Eppenga WL, Lalmohamed A, Geerts AF, Derijks HJ, Wensing M, et al. (2014) Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: A population-based cohort study. Diabetes Care. 37: 2218-2224.
- Duong JK, Roberts DM, Furlong TJ, Kumar SS, Greenfield JR, et al. (2012) Metformin therapy in patients with chronic kidney disease. Diabetes ObesMetab. 14: 963-965.
- Ekstrom N, Schioler L, Svensson AM (2012) Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: A cohort study from the Swedish National. Diabetes Register. BMJ Open 2: e001076.
- Kosmalski M, Drozdowska A, Sliwinska A, Drzewoski J (2012) Inappropriate metformin prescribing in elderly type 2 diabetes mellitus (T2DM) patients. Adv Med Sci. 57: 65-70.
- Vasisht KP, Chen SC, Peng Y, Bakris GL (2010) Limitations of metformin use in patients with kidney disease: Are they warranted? Diabetes ObesMetab. 12:1079-1083.
- Scotton DW, Wierman H, Coughlan A, Walters M, Kuhn C (2009) Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy-based measures to improve guideline adherence. QualManag Health Care 18: 71-76.
- Kamber N, Davis WA, Bruce DG, Davis TM (2008) Metformin and lactic acidosis in an Australian community setting: The Fremantle Diabetes Study. Med J Aust188: 446-449.
- Runge S, Alte D, Baumeister SE, Völzke H (2008) Prevalence of risk determinants for metformin-associated lactic acidosis and metformin utilization in the study of health in Pomerania. HormMetab Res 40: 491-497.
- Sweileh WM (2007) Contraindications to metformin therapy among patients with type 2 diabetes mellitus. Pharm World Sci29: 587-592.
- Warren RE, Strachan MW, Wild S, McKnight JA (2007) Introducing estimated glomerular filtration rate (eGFR) into clinical practice in the UK: implications for the use of metformin. Diabet Med 24: 494-497.
- Kennedy L, Herman WH (2005) GOAL A1C Study team renal status among patients using metformin in a primary care setting. Diabetes Care. 28: 922-924.
- Millican S, Cottrell N, Green B (2004) Do risk factors for lactic acidosis influence dosing of metformin? J Clin Pharm Ther 29: 449-454.
- Horlen C, Malone R, Bryant B, Dennis B, Carey T, et al. (2002) Frequency of inappropriate metformin prescriptions. JAMA 287:2504-2505.
- Calabrese AT, Coley KC, Da-Pos SV, Swanson D, Rao RH (2002) Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy. Arch Intern Med 162: 434-437.
- Emslie-Smith AM, Boyle DI, Evans JM, Sullivan F, Morris AD (2001) DARTS/MEMO Collaboration Contraindications to metformin therapy in patients with type 2 diabetes-A population-based study of adherence to prescribing guidelines. Diabet Med 2001 18483-488.
- Holstein A, Nahrwold D, Hinze S, Egberts EH (1999) Contra-indications to metformin therapy are largely disregarded. Diabet Med 16: 692-696.
- Selby JV, Ettinger B, Swain BE, Brown JB (1999) First 20 months’ experience with use of metformin for type 2 diabetes in a large health maintenance organization. Diabetes Care 22: 38-44.
- Sulkin TV, Bosman D, Krentz AJ (1997) Contraindications to metformin therapy in patients with NIDDM. Diabetes Care 20: 925-928.
- Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH (2015) Metformin use and mortality in patients with advanced chronic kidney disease: National, retrospective, observational, cohort study. Lancet Diabetes Endocrinol 3: 605-614.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences