ISSN : 2573-4466
Insights in Enzyme Research
Investigating The Inhibition Of 5-LO Enzyme By Main Cannabinoids Contained In Cannabis Sativa And Seized Resin Cannabis By Using Molecular Modeling
Bouchentouf S1,2*, Ghalem S2,3, Allali H2,3 and Missoum N2,3
1Faculty of Technology, Doctor Tahar Moulay University of Saida, Algeria
2Laboratory of Naturals Products and Bioactives- University of Tlemcen, Algeria
3Department of Chemistry, Faculty of Sciences, Aboubekr Belkaid University of Tlemcen, Algeria
- *Corresponding Author:
- Bouchentouf Salim
Faculty of Technology, Doctor
Tahar Moulay University of Saida, Algeria
Tel: 00213557974407
Email: bouchentouf.salim@yahoo.fr
Received Date: June 17, 2017 Accepted Date: September 08, 2017 Published Date: September 18, 2017
Citation: Bouchentouf S, Ghalem S, Allali H, Missoum N. Investigating the Inhibition of 5-LO Enzyme by Main Cannabinoids Contained in Cannabis sativa and Seized Resin Cannabis by Using Molecular Modeling. Insights Enzyme Res. 2017, Vol. 1 No. 1: 10.
Copyright: © 2017 Bouchentouf S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Therapeutic cannabis is a subject which many researchers are very interested in all over the world. The effect of main compound of Cannabis is investigated to treat many diseases. Arachidonate 5-lypoxygenase (5-LO) is implicated in cancer progression, inflammation, tissue damage and other sickness. Commonly to inhibit 5-LO enzyme a famous drug Zileuton is used. In our work we compare inhibition of 5-LO by main compounds of cannabis using tools such as molecular mechanics, molecular dynamics and molecular docking by using MOE software (Molecular Operating Environment) [1]. Obtained results show that main cannabinoids inhibit 5- LO better than Zileuton.
Keywords
Cannabis; 5-LO; Zileuton; Molecular modeling; Docking; Molecular operating environment
Introduction
Cannabis sativa L. is a widespread species in nature. It is found in various habitats ranging from sea level to the temperate and alpine foothills of the Himalayas, from where it was probably spread over the last 10,000 years [2]. As a plant, it is valued for its hallucinogenic and medicinal properties, more recently being used for pain, glaucoma, nausea, asthma, depression, insomnia, and neuralgia. Derivatives are used in HIV/AIDS and multiple sclerosis [2].
Since the last decade of the twentieth century, evidence for medical uses increased considerably. In different ways, various countries recognized a role for safe and effective medicinal use of cannabis. Currently, medical use of cannabis is allowed in a number of countries. In the past twenty years, its (legal) medical consumption has gone up from almost non-existent to 23.7 tonnes in 2011 and 77 tonnes in 2014. The United Kingdom produces cannabis for the production of a dronabinol-cannabidiol combination preparation for the treatment of spasticity due to multiple sclerosis (Sativex) that is prepared using cannabinoids extracted from plant material. This preparation has been approved as a medicine in twentyfour countries (including Austria, Australia, Belgium, Canada, the Czech Republic, Finland, Germany, Hungary, Iceland, Italy, the 20 Netherlands, New Zealand, Norway, Poland, Portugal, Slovakia, Spain, Sweden, Switzerland, and the United Kingdom) [3].
The 5-LO-derived arachidonic acid metabolites have been shown to be potent mediators of inflammatory reactions [4]. During the last decades, many investigations demonstrated that the 5-LO pathway plays a role in the development of allergic diseases such as asthma and inflammatory disorders. Moreover, there is some evidence that the 5-LO pathway could be involved in tumorigenesis, especially in prostate cancer and certain forms of leukaemia. Up to now, only one 5-LO inhibitor is on the market, zileuton for the treatment of asthma [4]. Zileuton [R, S (±) N-(1-(benzo [b]-thien-2-yl) ethyl)- Nhydroxyurea] is a racemic mixture having approximately equal therapeutic activities which selectively and reversibly inhibits 5-lypoxygenase potentiating leukotrienes (LT’s-LTA4, LTB4, LTC4, LTD4 and LTE4; mostly indicated in inflammatory diseases akin to 35 psoriasis, rheumatoid arthritis, asthma, multiple sclerosis, uveitis and inflammatory bowel syndrome. Zileuton is a very slightly soluble compound without any ionizable functional group. Recently, the molecule has been reported in the literature as a new efficient and safe antiacne drug and employed in United States of America to treat asthma [5].
Up to now, the iron-ligand type-5-LO inhibitor zileuton is the only 5-LO inhibitor that came on the market for the treatment of asthma. So, how to come up with novel inhibitors? There are several different strategies in drug design, divided mainly in (I) the improvement of already known compounds by medicinal chemistry approaches to cope with, for example, a poor pharmacokinetic profile and (II) the identification of novel scaffolds. For the latter, high-throughput screening of large databases of synthetic compounds or natural product extracts can be applied to find active compounds [6,7]. Alternatively, computational methods are quite helpful tools to speed up the drug discovery process. The latter consist consists of ligand-based and structure-based techniques dependent on the data at hand, that is, the availability of a three-dimensional structure of the drug target.
In our work we study inhibition of 5-LO enzyme by main cannabinoids present in cannabis and cannabis street (marijuana) by computational helpful tools (Molecular docking). Molecular Operating Environnement software (MOE) is used.
Materials and Methods
Main chemical molecules in Cannabis sativa L and Cannabis resin seized
The following cannabinoids (Table 1) were found in Cannabis plant and resin Cannabis seized using recent analytical methods. Main provenance of seized cannabis is Morocco, India and Lebanon [8,9].
| Δ9-Tetrahydrocannabinol | Δ8 -Tetrahydrocannabinol |
|---|---|
| Cannabichromene | Cannabicyclol |
| cannabilesoin | cannabigerol |
| Tetrahydrocanabivarain | cannabicitran |
| Cannabidiol | Cannabitriol |
| Cannabinodiol | - |
Table 1: Main cannabinoids in cannabis and resin cannabis [2,9,10].
Arachidonate 5-lypoxygenase
The 5-LO protein consists of a C-terminal catalytic domain and an N-terminal C2-like β-barrel domain. The catalytic domain contains a non-haem iron in the active site that acts as an electron acceptor or donator during catalysis [10]. During enzyme activation, for example by lipid hydroperoxides, the iron is oxidized from the ferrous state (Fe2+) into the ferric (Fe3+) form, so that the enzyme can enter the catalytic cycle. The C2-like domain has a regulatory function. It interacts with various lipids (phosphatidylcholine, diacylglycerides or lipid membranes) and Ca2+ or Mg2+ ions. Ca2+ as well as glycerides stimulate 5-LO activity by the reduction in the requirement for activating lipid hydroperoxides.
Preparation of 5-LO enzyme, cannabinoids and Zileuton
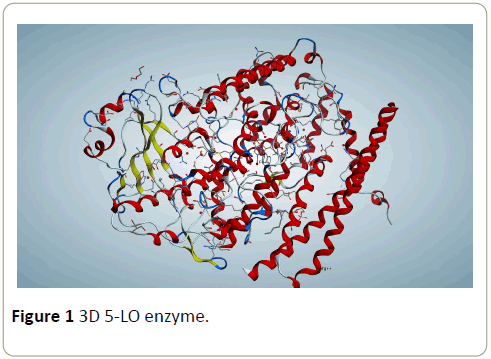
Arachidonate 5-lypoxygenase X-ray crystal structure with resolution of 1.48 Å was retrieved from Protein Data Bank with PDB ID, code (5IR4) (Figure 1). 3D protonation and energy minimization of the X-ray enzyme structure was executed by (MOE) to keep the complex structure ready for downstream analyses. The add in the right geometric orientation of the hydrogen atoms is present in the enzyme as well as in the environment. The calculation was performed using the MMFF 94x force field to parameterize a system comprise of large proteins and small organic molecules.
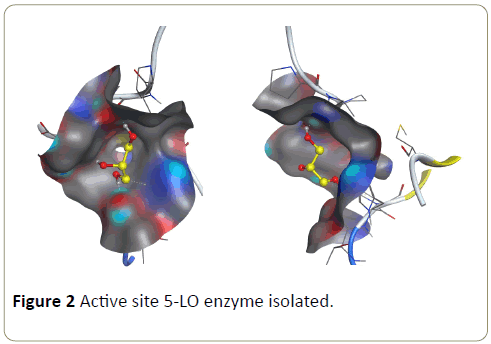
Once a proper orientation of the hydrogen atoms in the enzyme and environment are obtained, the complex structure then kept under the PDB extension to maintain proper connectivity and guidance [11]. Cannabinoids (ligands) (Table 1) and Zileuton structure were downland from PubChem data base. We selected the active site in the enzyme and we minimized the energy of both enzyme and ligands. Energy minimizing was done under following conditions: Temperature=3000 K, pH=7 and Hamiltonian AM1. Figure 2 shows the active site of the enzyme with molecule of cocrystallization in different orientation.
Docking and building complexes
After optimization of both molecules (ligands) and enzyme, we proceed to positioning of ligands in to active site of the enzyme (5IR4) using Dock module (Molecular Docking) with MOE software (Molecular operating environment).
Docking of Zileuton was done to permit comparison. The purpose of the Dock application is looking at favorable conformational binding between medium size ligands and a not so soft macromolecular target, which is usually a protein. The site finder implemented in MOE used for prediction a nearby pocket or active site able to anchor molecule. For each ligand, a number of conformations called poses were generated, and energy of the built complex (score) is done in Table 2.
| Molecules | Toxicity | Poses | Score |
|---|---|---|---|
| Zileuton | No | 9 | -4.54582 |
| Δ9-tetrahydrocannabinol | No | 6 | -4.56957 |
| Δ8-tetrahydrocannabinol | No | 8 | -4.87163 |
| Cannabichromene | No | 7 | -5.14138 |
| Cannabicyclol | No | 9 | -4.48549 |
| Cannabigerol | No | 10 | -5.58683 |
| Cannabilesoin | No | 10 | -4.83303 |
| Cannabicitran | No | 8 | -4.40216 |
| Tetrahydrocannabivarain | No | 7 | -4.43522 |
| Cannabidiol | No | 7 | -4.97139 |
| Cannabitriol | No | 10 | -4.55872 |
| Cannabinodiol | No | 7 | -5.02914 |
Table 2: Energy balance of 12 complexes (Kcal/mol).
Results and Discussion
Given results in Table 2 show balance interaction between ligands and 5-LO enzyme (code 5IR4). It’s clear that many molecules contained in cannabis give more stable complexes with low energy than the complex given by interaction between enzyme and Zileuton drug. The best inhibition comparing to Zileuton is given in order by; cannabigerol (-5.58682823 Kcal/mol), cannabichromen (-5.14138079 Kcal/ mol), cannabinodiol (-5.02914 Kcal/mol), cannabidiol (-4.97138882 Kcal/mol), Δ8 -tetrahydrocannabinol (-4.87162733 Kcal/mol) and cannabilesoin (-4.83302832 Kcal/ mol).
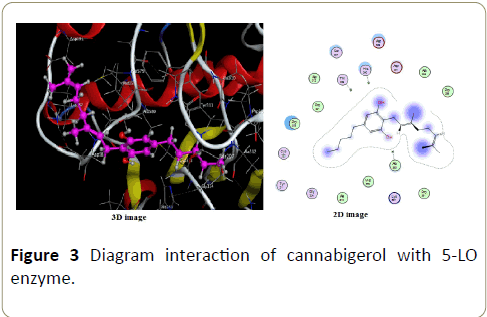
Interactions in complex formed with cannabigerol is shown in Figure 3, there are non-perceptible interaction, only Vanderwals interaction are perceptible. Vanderwals interactions are the residual attractive or repulsive forces between molecules or atomic groups that do not arise from covalent bonds, nor ionic bonds.
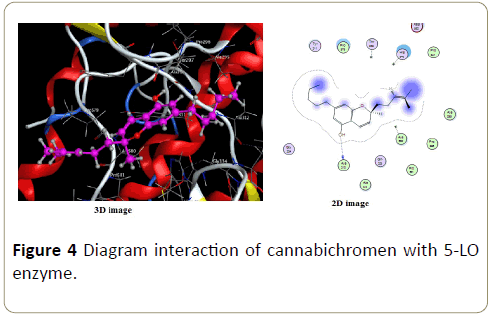
For cannabichromen ligand only one interaction is possible with hydrogen (Figure 4). The distance is about 3.05 Å and energy about -1.1 Kcal/mol. A hydrogen bond is the electrostatic attraction between two polar groups that occurs when a hydrogen (H) atom covalently bound to a highly electronegative atom.
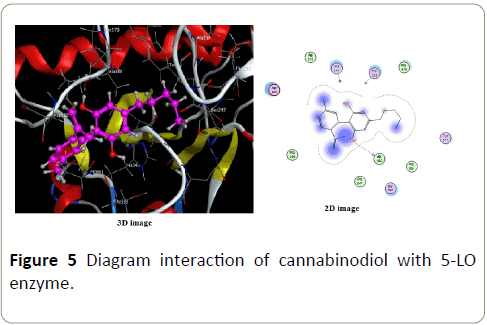
For complex formed with cannabinodiol (Figure 5) only one interaction with hydrogen is possible with distance about 3.06 Å and energy of -2.0 Kcal/mol. The small difference between complexes formed with cannabichromen and cannabidiol can be interpreted by difference in distance of hydrogen bonds.
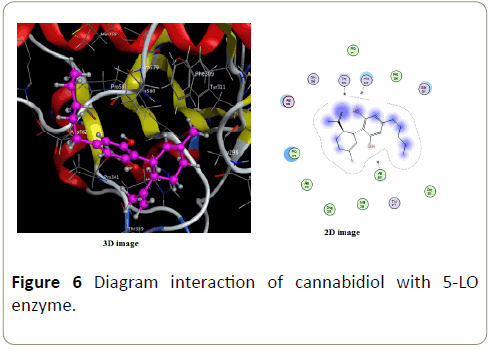
In cannabidiol interaction with 5-LO enzyme (Figure 6) there are non-perceptible interactions, only Vanderwals interactions are perceptible.
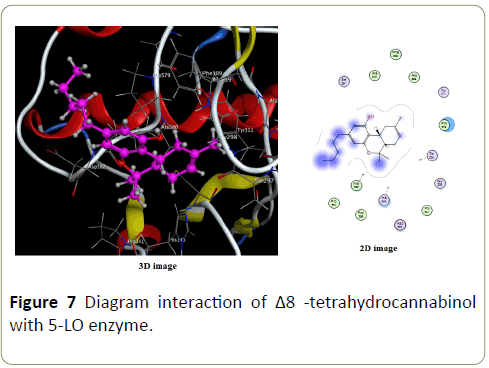
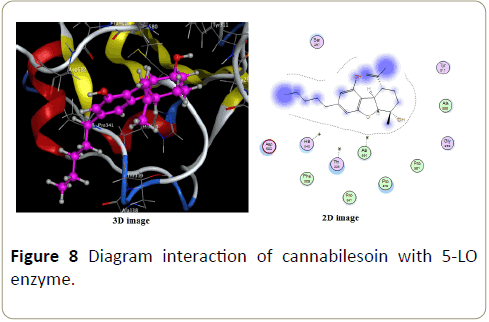
Only Vanderwals interactions are perceptible in complex formed with Δ8-tetrahydrocannabinol (Figure 7) and cannabilesoin (Figure 8).
Differences in energy complexes given by cannabinodiol, Δ8 THC and cannabilesoin can be neglected.
Conclusion
In this work we performed a study using molecular docking technique to determine the inhibition effect of main cannabinoids on 5-Lo enzyme.
More than five cannabinoids from Cannabis sativa L. plant and seized cannabis resin inhibit Arachidonate 5-lypoxygenase enzyme better than Zileuton drug. Cannabigerol is the best inhibitor according to the obtained results by molecular docking. Clinical tests are highly recommended. Regarding role of 5-LO enzyme in pain and inflammation we can also explain why in many regions of the world cannabis is consumed not only for its hallucinogenic effect but also for its painkiller properties. The obtained results encourage investigation on cannabinoids in inhibition of other enzymes.
References
- Ishii A, Shioda M, Okumura A, Kidokoro H, Sakauchi M, et al. (2013) A recurrent KCNT1 mutation in two sporadic cases with malignant migrating partial seizures in infancy. Gene 531: 467-471.
- Thomas BF, ElSohly M (2015) The analytical chemistry of cannabis: Quality assessment, assurance, and regulation of medicinal marijuana and cannabinoid preparations. Elsevier.
- WHO Expert Committee on Specifications for Pharmaceutical Preparations: Forty-eighth Report (2014) World Health Organization.
- Steinhilber D, Hofmann B (2014) Recent Advances in the Search for Novel 5‐Lipoxygenase Inhibitors. Basic Clin Pharmacol Toxicol 114: 70-77.
- Ganorkar SB, Dhumal DM, Shirkhedkar AA (2014) Development and validation of simple RP-HPLC-PDA analytical protocol for zileuton assisted with Design of Experiments for robustness determination. Arabian J Chem.
- Gao L, Lee SS, Chen J, Sun H, Zhao Y, et al. (2016) High-throughput screening of substrate specificity for protein tyrosine phosphatases on phosphopeptide microarrays. Microarray Technology Methods. Mol Biol 1368: 181-196.
- Liqian G, Sun H, Yao S (2010) Activity‐based high‐throughput determination of PTPs substrate specificity using a phosphopeptide microarray. Biopolymers 94: 810-819.
- Nascimento IR, Costa HB, Souza LM, Soprani LC, Merlo BB, et al. (2015) Chemical identification of cannabinoids in street marijuana samples using electrospray ionization FT-ICR mass spectrometry. Anal Methods 7: 1415-1424.
- Hanus LO, Levy R, Vega DDL, Limor Katz MR, Tomicek P (2016) Israel J Plant Sci.
- Radmark O (2002) Arachidonate 5-lipoxygenase. Prostaglandins and Other Lipid Mediators 68: 211-234.
- Khan A, Munir M, Aiman S, Wadood A, Khan AU (2017) The in silico identification of small molecules for protein-protein interaction inhibition in AKAP-Lbc–RhoA signaling complex. Comput Biol Chem 67: 84-91.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences