ISSN : 0976-8505
Der Chemica Sinica
Insight of Spectrophotometric Determination Using 4-(2-Pyridylazo)- Resorcinol: Application of Stop Flow Injection Analysis
S.Chandramouleeswaran, Jayshree Ramkumar*
Analytical Chemistry Division, Bhabha Atomic Research Centre, Mumbai-85, India
Abstract
Metal complexation and its kinetics are very crucial in analytical chemistry for the selective determination of ions of interest. In this study, the kinetics of reactions between 4-(2-pyridylazo)-resorcinol and UO22+, Cu2+ and mixture of UO22++ Cu2+ were examined. The role of pH of solution on the process was evaluated. Spectrophotometric studies revealed the possible existence of cation-cation complexes in solution. Stop flow single flow injection analysis method was used to understand the effect of pH on the stability of the complexes.
Keywords
Spectrophotometric, Resorcinol, Stop flow injection analysis.
Introduction
Since 1960, pyridylazo derivatives like 4-(2- pyridylazo)-resorcinol, (PAR) were all-embracing analytical reagent. PAR is commercially available as the free dye in the protonated form (H2L) or as the monosodium (NaHL.H2O) or disodium salt (Na2L.2H2O) forms. The dissociation constant pK1, pK2 and pK3 corresponding to nitrogen protonation, proton removal from para hydroxyl group and deprotonation of ortho hydroxyl groups respectively and the equivalent values for the three reactions being 3.1, 5.6 and 11.9 respectively. The species, H4L2+ and H5L3+ exist only under high acidic conditions. PAR forms red or red-violet coloured (ter / bi dentate) complexes with most of the metal ions but the selectivity can be achieved by controlling the pH of the metal ion solution [1,2].
Reactions in solutions are of great interest especially when there are more than one metal ions in solution competing with the ligand to form complexes. The presence of cation-cation interactions and its effect on extraction properties had been discussed extensively in our earlier paper [3].
In this present study, an attempt has been made to understand the interactions in solutions using uranyl and copper metal ions and PAR as the ligand. The results obtained could possibly help in the understanding the interferences from other cations by controlling the kinetics of the reaction.
Experimental Study
The metal ion solutions were prepared in distilled water using the corresponding nitrate salts. All reagents used were of analytical grade. KCl was used to maintain a constant ionic strength (1.00 mol L-1) in all the working solutions. Buffer solutions of different pH were prepared using suitable reagents [4]. Solution of PAR indicator was prepared daily by weighing of the monosodium salt. Spectrophotometric determinations were carried out using JASCO 650 UV-Vis double beam Spectrophotometer with a wavelength range of 190 nm -900 nm. The reaction kinetics were monitored using the simplest flow injection analyzer (Single flow injection) consisting of a peristatic pump for pushing the carrier containing PAR reagent through a narrow tube; an injection port for reproducible delivery 20μL of sample solution (metal or mixture) into the carrier stream and a reaction coil which assists the formation of a coloured Metal- PAR complex that is detected using a UV Vis flow detector at 525 nm. The flow injection analysis was carried out using JASCO UV 2070 Plus flow detector.
Results and Discussion
The studies in aqueous solutions provide scope for research. The interactions between different species, can have an effect on the kinetics of the process. In our earlier study, the formation of cation-cation complex in the aqueous phase between uranyl and iron had been established for the first time [3]. Hence, encouraged by the results, it was thought useful to examine the present system before studying the complexation with PAR.
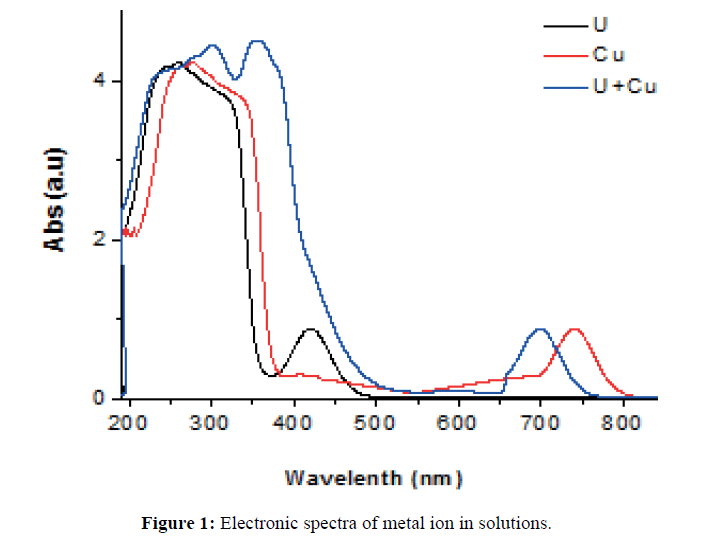
The spectrophotometric evaluation of aqueous solutions of pure and the binary mixture of copper and uranyl ions in the absence and presence of PAR were carried out. The spectra of the metal ion solutions in the absence of PAR is given in Figure 1. The spectra of pure Cu2+ shows a main peak around 270 nm and a well defined characteristic peak at 742 nm. The peak at 742 nm corresponds to the absorption peak of pure Cu2+ metal ion in solution due to the d-d transitions. The peak at 270 nm is due to the presence of copper-nitrate ion pair as reported earlier [5]. The spectrum of pure uranyl ion revealed huge broad peak in the range of 236-325 nm with a maximum at 252 nm and a small peak at 419 nm respectively. The broad peak from 236 - 325 nm indicates the presence of monomeric and polymeric divalent cationic forms of uranium which are dependent on the pH of the system. The presence of trinitro complexes of uranyl ion also leads to the presence of a very broad peak [6]. The peak in the visible region at 419 nm also suggests that the uranyl ion is not present as simple UO22+. However in the mixture, the peak maxima at 240, 308, 352 and 701 nm respectively clearly indicate the alteration of the individual peak maxima. These changes in spectra are an indication of presence of interactions between the two cations viz copper and uranyl in solution. This is in accordance with our earlier observation using uranyl and iron ions [3].
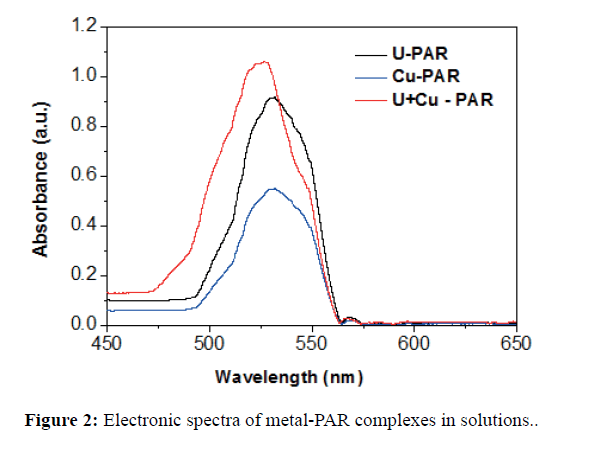
The electronic spectra of metal complexes with PAR are given in Figure 2. From Figure 2, it is seen that the maximum absorption for the PAR complexes of uranyl and Cu2+ ions are very nearly similar (530 and 532 nm respectively). However, the peak maxima for the mixture of ions in presence of PAR are shifted to 525 nm. This clearly indicates that the mixture is quite different from that of the individual ion solutions. This is probably due to the presence of cation-cation complexes which will interact differently with PAR. The values of molar absorptivity, €, were calculated at 525 nm for the different complexes is given in Table 1. This suggests that UO22+ plays a more dominant role on the spectral behaviour of the mixture containing UO22+ and Cu2+ as compared to Cu2+. Hence it is expected that kinetics will also be dominated by uranyl ion.
| Metal-PAR | Molar Absorption coefficient (ε) (104*mol*L-1*cm-1) |
|---|---|
| UO22+ | 4.2 |
| Cu2+ | 2.6 |
| UO22+ - Cu2++ | 5.2 |
Table 1: Molar absorptivity of UO22+, Cu2+ and UO22+ - Cu2+ complexes with PAR (pH=8).
The kinetics of metal ion complexation (uranyl and copper respectively) with PAR was evaluated using single flow injection analysis in the stop flow mode. The reaction rate constants were determined for both the complex formation as well as the decay. The rate of formation of complex was evaluated from the surge in the values within a short time frame but the stability of the complex could be obtained from the rate of decrease of the colour with time. For the calculations, the linear portions were considered. The studies were carried out at different pH to get an idea of the changes occurring during complexation. The results rate of change of detector response with unit time has been given in Table 2. This gives us an exact idea of the changes occurring at a fixed concentration of metal ion for different pH values. From Table 2, it is seen that both the formation as well as the decay is pH dependent for both copper and uranyl ions. It is seen that the stability of the copper-PAR complex was maximum at pH 4 and the rate of decay was also the least. Thus it was seen that pH of 4 was suitable for the determination of copper ions. For uranyl ion, it is seen that the rate of formation was the maximum at pH 8. Normally the determination of uranyl ion by spectrophotometry is carried out at this pH of 8. However, stop flow analysis showed that the rate of decay was also the maximum at this pH. Therefore it can be understood that the determination of uranyl ion can be carried out at the lower pH wherein the rate of decay is comparatively lower.
| pH | Rate of formation (*105 min-1) | Rate of decay (*104 min-1) | ||
|---|---|---|---|---|
| Cu2+ | UO22+ | Cu2+ | UO22+ | |
| 4 | 21.1 | 24.6 | 0.32 | 0.46 |
| 6 | 13.3 | 25.1 | 1.05 | 0.97 |
| 8 | 11.1 | 29.6 | 2.3 | 1.82 |
Table 2: Kinetic parameters obtained from the stop flow method.
Conclusion
The use of Pyridyl azo resorcinol in determination of metal ions like copper and uranyl ions are well known. However, the deeper insight into the complexation has been carried out in the present study. The spectrophotometric evaluation of the metal ion systems in the absence and presence of PAR have been carried out. The spectral features of the UV Visible studies of copper and uranyl ions both in free form and in their mixtures reveal the presence of cation-cation interactions between the two ions in the mixture. The complexes with PAR also showed that the mixture behaved very differently as compared to the free ions. The values of molar absorption coefficients indicate that UO22+ plays a more dominant role on the spectral behaviour of the mixture containing UO22+ and Cu2+ as compared to Cu2+. The kinetics of metal ion complexation (uranyl and copper respectively) with PAR was evaluated using single flow injection analysis in the stop flow mode. From the studies it was seen that the pH had a very strong effect on the formation as well as decay of the complex. The studies showed that unlike in normal spectrophotometric determination of uranium, flow injection analyses can be carried out at lower pH values.
Acknowledgement
The authors thank Dr. P.D.Naik (Associate Director, Chemistry Group, BARC and Head Analytical Chemistry Division) for his constant support.
References
- Karpińska J, Kulikowska M (2002) Simultaneous determination of zinc(II), manganese(II) and iron(II) in pharmaceutical preparations. J Pharm Biomed Analy 29: 153-158.
- Gavazov K, Simeonova Z, Alexandrov A (2000) Extraction spectrophotometric determination of vanadium in natural waters and aluminum alloys using pyridyl azo resorcinol (PAR) and iodo-nitro- tetrazolium chloride (INT). Talanta 52: 539-544.
- Chandramouleeswaran S, Ramkumar J, Basu M (2016) Insight of solvent extraction process: Reassessment of trace level determinations. Analytica Chimica Acta 938: 123-128.
- Vogel AI. Vogel's Text-book of Quantitative Inorganic Analysis, Including Elementary Instrumental Analysis. Fourth Edtn. Longman Scientific and Technical London 1980.
- Gvozdić V, Tomišić V, Butorac V, Simeon V (2009) Association of nitrate ion with metal cations in Aqueous Solution: A UV-Vis spectrometric and factor-analytical study. Croat Chem Acta 82: 553–559.
- Smith AN, Cerefice SG, Czerwinski RK (2013) Fluorescence and absorbance spectroscopy of the uranyl ion in nitric acid for process monitoring applications. J Radioanal Nucl Chem 295: 1553–1560

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences