ISSN : 2348-9502
American Journal of Ethnomedicine
Impact of Cooked Tribulus Terrestris Fruit Extract on Lead Induced Hepato and Renal Toxicity
1Department of Clinical Nutrition and Dietetics, PSG College of Arts and Science, Coimbatore, Tamil Nadu 641014, India
2PSG College of Pharmacy, PSG Institute of Medical Sciences and Research, Tamil Nadu, India
3Department of Biochemistry, PSG Institute of Medical Sciences and Research, Tamil Nadu, India
Abstract
Aim: The present study was undertaken to explore the effect of cooked Tribulus terrestris fruit extract in lead acetate induced toxicity in female Sprague dawley rats.
Methods: Rats ageing (6 months) were grouped into three in a randomized mode that average weight of animals in each group would be 150-250 gms. Normal feed pellets and water ad libitum were provided to animals throughout the experiment. As Group 1-Experimental Group was fed with cooked Tribulus terrestris fruit extract and lead acetate (100mg/kg/bwt + 15mg/kg/bwt/ day) respectively, Group 2-Negative Control Group was administered only with lead acetate (15mg/kg/bwt/ day) and no other treatment was given, Group 3- Control Group was on normal standard diet. After the treatment period of 30 days the animals were weighed and blood was taken by cardiac puncture. Further, liver and kidneys were surgically removed for histopathological examination.
Result: After the treatment period, it was observed that the negative control had a significant abnormality in their hepatic enzyme markers (P< 0.01) namely ALT, AST, ALP whereas the experimental group treated with the fruit extract did not have a deterious increase in the enzymes but significantly different from negative control group. Total protein, albumin and globulin were also analogous with normal control group.
Conclusion: However, histopathological study showed no gross change in the liver morphology but the treatment with cooked Tribulus terrestris fruit extract showed no deposition of lead precipitates in the kidney but deposits were found in negative control group. Therefore it is imperative that cooked Tribulus terrestris fruit extract is potential in protecting lead induced toxicity.
Keywords
Cooked Tribulus terrestris fruit extract, Hepatic markers, Renal markers, Lead acetate, Histopathology.
INTRODUCTION
Lead is ever-present pollutant and hazardous heavy metal, unsafe even in small amounts [1]. This metal is known to provoke a broad range of physiological, biochemical, and behavioural dysfunctions in laboratory animals and humans [2], together with central and peripheral nervous systems [3], haemopoietic system [4], cardiovascular system [5], hepatic [6] and female reproductive systems [7].
One of the chief mechanisms behind heavy metal toxicity has been ascribed to oxidative stress. Toxic metals increase production of free radicals and decrease availability of antioxidant reserves to respond to the resultant damage. A mounting amount of data provide evidence that metals are capable of interacting with nuclear proteins and DNA causing oxidative deterioration of biological macromolecules [8].
Considering that lead toxicity is currently one of the serious problems Worldwide, there is still no specific, reliable and safe treatment. As a matter of fact, among all the heavy metal toxicities, lead toxicity is widely common and highly possible as not only industrial waste, effluents or chemicals used for various purpose lead to the accumulation of this noteworthy toxicity but very much from commonest debris and demolishment of old buildings, interiors and furniture with paint give way for huge accretion of lead toxicity. This is the reason why lead toxicity is taken into account and how economically and ecohealth friendly treatment could alleviate this menace of lead toxicity is keenly considered in the present study.
Despite the fact that several metal chelators (CaNa2EDTA and DMSA) have been used to manage lead toxicity in the event of exposure but none are suitable in reducing lead body burden [9]. Additionally these chelators in turn are potentially toxic [10] and often fail to remove Pb burden from all body tissues [11].
Investigation for hepatoprotective and Renal protective agents has made man turn to substitute sources viz. Indigenous system of medicine. It is a well-documented fact that most medicinal plants are enriched with bioflavonoids, which have hepatoprotective property. Tribulus terrestris (L.) (Zygophyllaceae) is one such plant containing a number of bioactive compounds. Tribulus terrestris is used in folk medicine as tonic, aphrodisiac, analgesic, astringent, stomachic, antihypertensive, diuretic, litho-triptic and urinary anti-infectives [12]. Recently, antitumoural activity and effects on cardio vascular system have been stated by Tomova (1981) [13], Wu (1999) [14], Xie (1988) [15] and Xu (2000) [16]. With these corroborative findings, it was conceptualized that Tribulus terrestris grown in Coimbatore District would also possess salubrious health effect. Hence, an effort was taken in the present study with the hypothesis that Tribulus terrestris fruit holds antioxidant capacity that would mitigate oxidative damage due to any heavy metal induced toxicity. In the light of above mentioned medical properties of Tribulus terrestris, this study was carried out to investigate the possible protective properties of Tribulus terrestris fruit extracts on toxicity related biochemical parameters in liver and kidney of lead intoxicated rats. Usually any potential herbal or plant extracts will be made with solvents like ethyl acetate, ethanol, methanol, petroleum ether, aqueous ( with water) etc., but nevertheless any one has pondered whether the same phytochemical containing herb or plants If they are edible enough, how the cooked extract will impart its health benefits. Hence, as a novel idea, the investigators intended to explore particularly the cooked extract of Tribulus terrestris fruit and thus the study was carried out. Of course, the investigators have also carried out selected screening tests for various phytonutrients and has reported that cooked i.e., boiled extracts too are comparatively safer [17]. (See figure 1.)
The objective for the present study is to explore the protective effect of cooked fruit extract of Tribulus terrestris (L) on lead induced hepato-renal toxicity in rats.
MATERIALS AND METHODS
Collection of plant material
The healthy plant samples of Tribulus terrestris (L) plant were collected from Coimbatore District, Tamil Nadu, India. The plant material was authenticated by a skilled Taxonomist, at Botanical Survey of India and a voucher specimen (No.:BSI/SRC/5/23/2013- 2014/Tech/1024) was deposited at the Tamil Nadu Agricultural University, Coimbatore. The Fresh fruits of Tribulus terrestris (L) was collected and were dried in the shade, powdered and stored in a sterile air tight opaque container for further use, which was later taken into lab for screening tests.
Extraction of plant material
Crushed coarse powder of Tribulus terrestris (L) fruit was subjected to exhaustive extractions using a soxhlet apparatus. Powder weighing 10g was extracted with 100ml of boiled distilled water (Boiled at 100°C). Then the extraction was agitated vigorously for 3 hours in an agitator and kept for 72 hours. Then, the extracts were filtered through Whatman no.1 filter paper to remove the particles, in a Buchner funnel.
Animals and experimental design
Female sprague dawley rats (weighing 150 ± 10 g) were acclimatized for 7 days prior to lead treatment and housed under standard condition of temperature 24 ± 1 ºC and 55 ± 5% relative humidity with regular 12 hour light: dark cycle and allowed free access to standard laboratory feed (Pranav agro industries, Vadodara) and water ad libitum. Animals were maintained in accordance with the guidelines of National Institute of Nutrition Indian Council of Medical Research (Hyderabad, India) and protocol was approved by animal research ethical committee of PSG Institute of Medical Sciences and Research (Approval number198/2013/IAEC).
The rats were divided into three groups of six rats per group that was treated as follows:
Group 1 (Experimental Group)
This group was given 100 mg in 10 ml of cooked Tribulus terrestris fruit extract by oral gavage and 15 mg/kg b.wt./ day of lead acetate diluted with normal saline which was intoxified intraperetonially.
Group 2 (Negative Control Group)
Negative Control Group was injected intraperitoneally with lead acetate diluted in normal saline at a dose of 15mg/ kg body weight. However, no treatment was intended to be given except normal chow and water adlibitum.
Group 3 (Control group)
This group of rats was maintained at normal conditions neither inducing toxicity with lead acetate nor administering test extract.
Bio-Chemical Analysis
Rat blood samples were centrifuged at 2,500 g for 15 min at 4°C (Sorvall RC6, NC, US). Sera were obtained and the levels of the urea, creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) [18], Alkaline phosphatase (ALP), Serum total protein, Total bilirubin [19], Serum Globulin and Albumin/Globulin ratio were measured by chemistry autoanalyzer (COBAS 6000, Roche Diagnostics, New York)
Histopathology/Histological Analysis
Liver and Kidneys were removed, washed in saline were fixed in buffered 10 % formalin at room temperature for 72 h. After fixing the tissue, it was thoroughly washed under running water and dehydrated in ascending grades of ethyl alcohol, cleared and then embedded in soft paraffin. Tissue sections of about 6 μm were obtained, stained by Haematoxylin and Eosin and examined under light microscope [20].
Statistical Analysis
All quantitative measurements were expressed as means ± SD for control and experimental animals. The data were analysed using one sample t test with the help of SPSS 16.V, the results were considered statistically significant if the P value is less than 0.05 and 0.01.
RESULTS
Hepatic marker enzymes and proteins
See table 1.
Table 1: Changes in the concentration of hepatic marker enzymes of AST, ALT, Alkaline Phosphatase, Total protein, Albumin, Globulin, Albumin: Globulin, Bilurubin
| Groups | AST | ALT | Total Protein | Albumin | globulin | Alkaline phosphat ase | Albumin: globulin | Bilurubin direct | Bilurubin indirect |
|---|---|---|---|---|---|---|---|---|---|
| experimental | 137.30± 3. 38** | 39. 93 ± 2.21 | 5. 83 ± 0.30* | 1. 20 ± 0.10 | 4. 36 ± 0. 15 | 136. 33 ± 10. 5** | 0.27 ± 0.01 | 0.10 ± 0. 00 | 0.10 ± 0. 00 |
| control | 118.70 ± 10.30 | 38.46 ± 2.38 | 6. 86 ± 0.11 | 1. 50 ± 0.10 | 5. 43 ± 0. 15* | 120. 73 ± 8. 54** | 0. 26 ± 0. 05 | 0.10 ± 0. 00 | 0.10 ± 0. 00 |
| Negative control | 185.19 ± 5. 82** | 100.00 ± 3.60** | 5. 53 ± 0. 41* | 1. 03 ± 0.11* | 4. 50 ± 0. 40 | 282. 37 ± 5. 10** | 0. 23 ± 0. 02 | 0.10 ± 0. 00 | 0.10 ± 0. 00 |
Values are expressed as mean ± SD for 6 rats in each group. Values with astrix (*) show significant difference as *P< 0.05 and **P<0.01
AST
The treatment with cooked Tribulus terrestris fruit extract on experimental animals that were induced with lead toxicity resulted in AST levels that was near close to control group levels. And of course, the AST levels were significantly low (P< 0.01) in comparison with control group and counter negative control group.
ALT
There was a significant elevation of ALT (P<0.01) in negative control group, which indicates that the lead acetate administration has induced damage on the hepatic cells. However, there was a significant retention of ALT enzymes in experimental rats that was treated with cooked Tribulus terrestris fruit extract which is clearly evident that the extract has controlled the oxidative damage and kept the ALP concentration almost at par with control rats levels.
ALP
There was an exorbitant increase (P<0.01) in ALP concentrations of serum of negative control group which was induced with lead toxicity but not treated with any drug or herb. In this case also, the experimental group who were on cooked Tribulus terrestris fruit extract administration, resulted in appreciable control over the elevation of ALP levels in comparison with normal control group.
Bile pigment (Bilurubin)
Both direct and indirect bilirubin of all the groups was stable and had an equal level 0. 10, which denotes that 30 days administration of lead acetate did not make them icteric.
Proteins (Total Proteins)
In comparison with the total protein of normal control group, both the experimental rats eated with cooked Tribulus terrestris fruit extract and the negative control group had slightly less levels of total protein that was significant at 0.05%.
Albumin
Only the negative control group had a significant reduction in their serum albumin levels (P<0. 05). However, there was only a significant reduction in albumin level (11.1%) of experimental group when compared with control group.
Globulin
With regard to globulin concentration, the levels were almost similar in both the lead acetate administered group i.e. in experimental and negative control rats. However, the normal control rats maintained a normal range of globulin levels.
Renal parameters
Lead toxicity may affect kidney function more predominantly than the liver function. In the present study, of course negative control rats had a significantly elevated levels (P<0.05) of blood urea but surprisingly their creatinine levels were pretty lesser (P<0.01) than the other two groups. However, all the three groups despite their toxicity and treatment and particularly normal control groups without any treatment did not show up any abnormal concentration of creatinine. (See table 2.)
Table 2. Changes in the concentration of renal marker of urea and creatinine
| Groups | Urea | Creatinine |
|---|---|---|
| Experimental | 26. 73 ± 1. 77 | 0. 73 ± 0.05* |
| Control | 27.66 ± 1.33 | 0.96 ± 0.01* |
| Negative control | 35. 50 ± 3. 98* | 0.63 ± 0.05** |
Histological examination
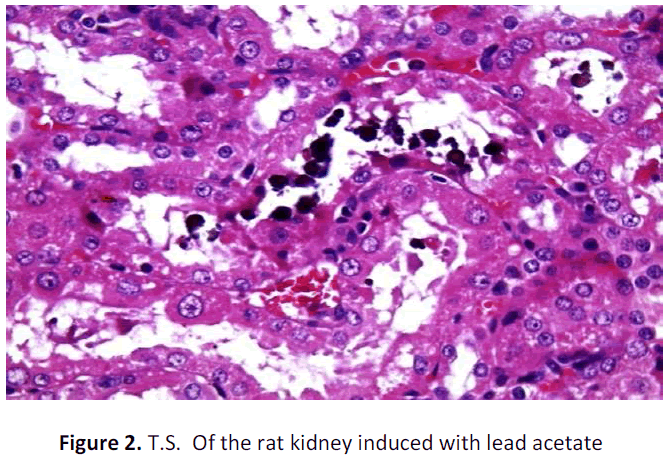
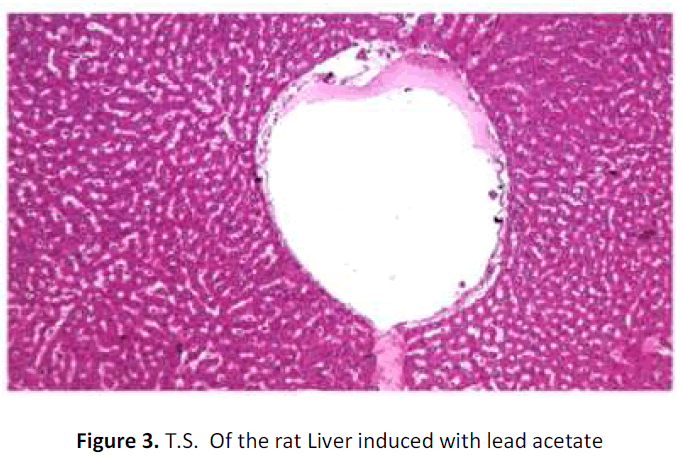
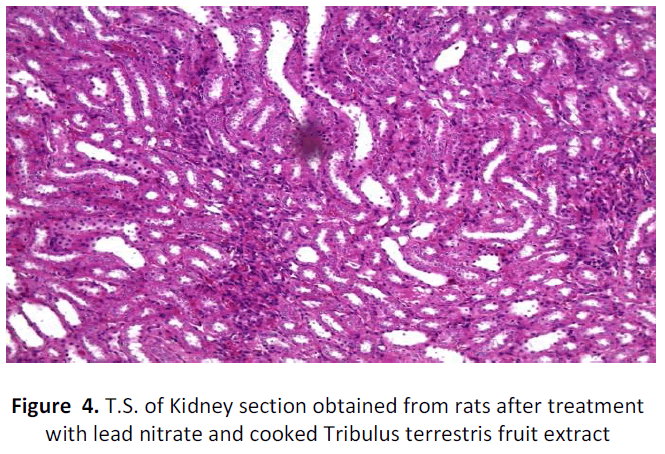
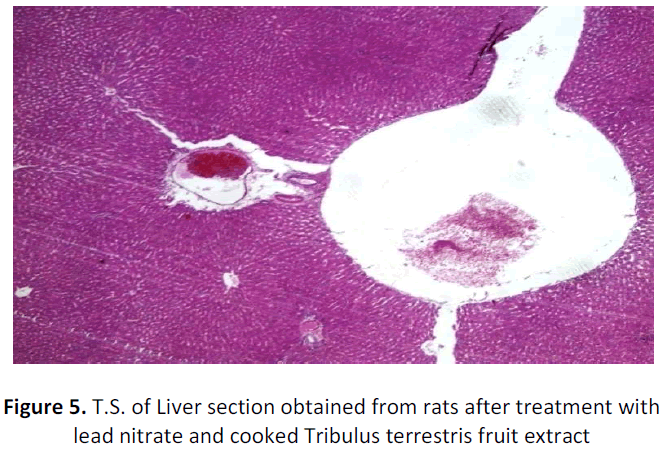
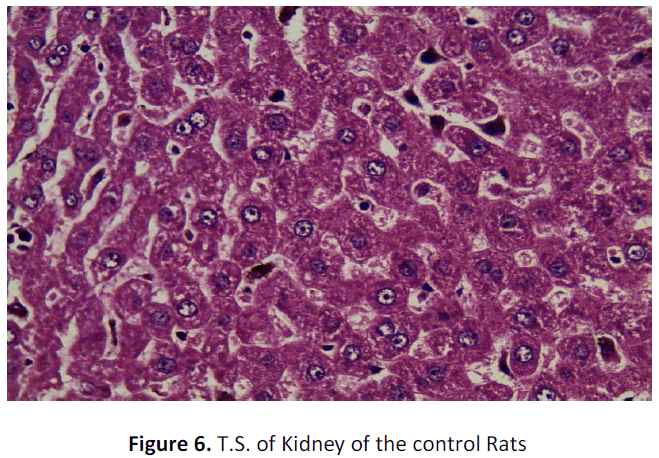
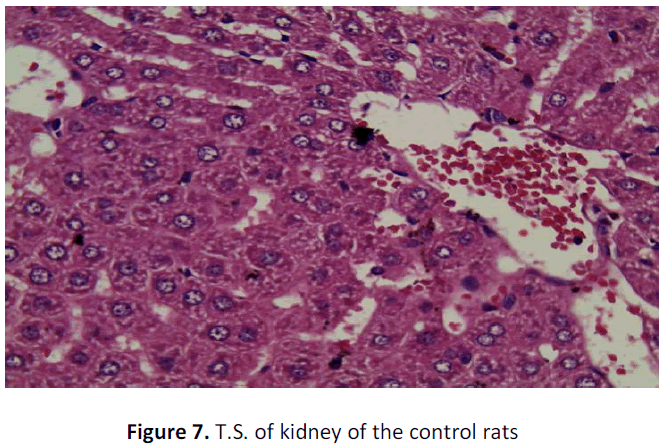
See figure 2-7.
Kidney of rat (negative control) administered with lead acetate shows blackish brown precipitates in the renal tubular epithelium (Arrows); H&E; X 400
Liver of rat (negative control) administered with lead showing a dilated central vein and normal hepatocytes and sinusoids. H&E; X 100
Kidney of rat(Experimental group) administered with lead and treated with cooked Tribulus terrestris fruit extract showing absence of blackish brown precipitates in the renal tubular epithelium H&E; X 100
Liver of rat (Experimental group) administered with lead and treated with cooked Tribulus terrestris fruit extract showing dilated central veins and normal hepatocytes and sinusoids. H&E; X 40
Liver of the control group rat shows occasional dilated central vein. Also intracellular and canalicular bile stasis is noticed. These changes may generally ally be attributed H&E; X 40
Kidney of the control group rat shows only congestion of intestinal blood vessels may be due to abnormal clamping which otherwise does not slow any abnormality
DISCUSSION
Lead toxicity and mitigation by phytonutrients
Liver
Generally heavy metals may exert their cytotoxic effects by damaging cell membranes. When liver cell plasma membrane is damaged, mixture of enzymes normally located on the cytosol is released into the blood stream. The present result indicates that lead acetate ingestion induced a significant rise in serum ALT, AST and ALP in lead acetate treatment. Their estimation in serum is a useful quantitative marker of the extent and type of hepatocellular damage [21].
Liver is the major organ of metabolism and is highly exposed to both indigenous and exogenous chemicals which can lead to hepatocellular damage. Studies have documented that liver is one of the primary target of lead associated toxicity. There have been several reports that state lead induced liver damage may be mitigated by phyto chemicals and bioflavonoid from plant origin.
Corroborative study of korien et al (2009) [22] has proven that the rats administered with 0. 5mg/g concentration of lead acetate for 60 days brought a significant increase in lipid per oxidation and transaminases. While Superoxide dismutase (SOD), Glutathione Peroxidase (GPx) and other biochemical parameters were decreased. These abnormalities were significantly normalised when 8mg/ 100g of rat between of methanol extract of C. sempervirens, 0.3 mg/ 100g of quarcetin and 0.1 mg/ 100g of rat between of rut in were administered prior to lead acetate administration.
Similarly Anuradha and krishnamoorthy (2012) [23] have also demonstrated that 150kg bwt/ day of methanolic extract of Pongamia pinnata flowers administrated for 90 days ameliorated elevated liver transaminases and lipid per oxidation in lead induced male albino rats. Falah (2012) [24] has also reported that Ficus carica too rendered hepato protective effect in animal studies. Further, Waggas (2012) [25] noticed that sub acute dose of 100mg/kg/day of lead acetate by intraperitoneal injection to rats significantly elevated SGOT, SGPT and LDH levels. However, pre-treatment with grape seed extract (Vitis vinifera) 100mg/kg/bwt/day normalized these biochemical parameters.
Identical to these studies, in the present study also it was observed that the elevation of transaminases like AST, ALT and ALP were significantly mitigated by the treatment with cooked Tribulus terrestris fruit extract in the experimental rats. Such amelioration of these liver enzymes may be attributed to the plausible presence of bio flavonoids or phenols of the cooked Tribulus terrestris fruit extract which would have prevented the cell membrane rupture and as well as by reducing oxidative stress that could increase these enzymes. Further, this augmentation may be explained as follows: Actually elevated liver enzymes may indicate inflammation or damage to liver cells. Such injured or inflamed liver cells leak out higher than normal amounts of certain metabolites and chemicals, including liver enzymes, into the blood stream, which in turn can result in elevated liver enzymes of blood. However, the presence of antioxidants via polyphenols in the cooked Tribulus terrestris fruit extract could have helped in the mitigation of these liver enzymes.
The increase in liver enzymes may also be attributed to binding of lead to plasmic proteins, where it causes alterations in a high number of enzymes. Treatment with cooked Tribulus terrestris fruit extract significantly reduced the activities of the liver marker enzymes in lead acetate treated rats. This indicates that the cooked Tribulus terrestris fruit extract tends to prevent liver damage by maintaining the integrity of the plasma membrane, thereby suppressing the leakage of enzymes through the membranes, exhibiting hepatoprotective activity. It can also agitate protein synthesis in hepatocytes [26]. The observed decrease in protein content of plasma of rats treated with Pb may be due to decreased hepatic DNA and RNA [27]. The decrease in the level of total protein observed in lead acetate treated rats may be associated with the decrease in the number of hepatocytes, which in turn may result in decreased capacity to synthesize protein.
El-Zayat et al. (1996) [28] and Hassanin, (1994) [29] reported that the decline in hepatic total protein content is in response to lead intoxication and the serum bilirubin is one of the most sensitive tests employed in the diagnosis of hepatic diseases. It provides useful information on how well the liver is functioning [30]. Bilirubin, a chemical breakdown product of hemoglobin, is conjugated with glucuronic acid in hepatocytes to increase its water solubility. However, in the present study there was no change in the both direct and indirect bilirubin levels, which leads to question whether the present dose of lead intoxication (15mg/bwt/day) was not sufficient enough to render its impact on liver damage and thereby its conjugation capacity with glucoronic acid needs further in depth clarity.
With regard to histopathological studies, in the present research exposure to lead acetate (15mg/bwt/day) did not bring out a gross change in the histo architecture of the liver except for dilated central vein and congestion of portal blood vessels. Similarly, it is well understood that except for the marked changes in the liver enzymes, liver was not subjected to any abnormality in their hepatic cellular damage. Hence, it is inconclusive to declare that the cooked Tribulus terrestris fruit extract had any beneficial effect on hepatotoxicity as may be duration and dosage of lead exposure is too short.
Kidney
Kidney is the most predominantly targeted organ for any heavy metal toxicity. There are reports on lead induced nephrotoxicity in experimental animals and human studies [31]. A number of studies have laid credence to lead induced renal toxicity, also a quite number of studies have documented the abilities of some chemical or herbal substances and extracts to mitigate lead induced renal functional and structural impairments. The study of Abdel-Aal and Hussein (2008) [32] has also highlighted on the beneficial impact of lipoic acid and dimercaptosuccinic acid on the renal functions in lead treated rats. Ishiaq et al (2011) have also clearly stated that lycopene; the natural antioxidant from tomatoes can improve antioxidant enzymes of lead poisoned kidneys of wistar rats [33].
In the present research, though the antioxidant enzyme activities were not estimated from kidney tissues, yet blood renal function tests were carried out. The lead acetate administration for 30 days showed a significant increase in the urea levels (P<0.05) of negative control group but the experimental rats who were induced lead toxicity but treated with cooked Tribulus terrestris fruit extract did not alter urea levels and it was just close to the urea levels of the normal control group. Similarly, the creatinine levels of both experimental and normal control groups were within normal limits. However, similar to Ibrahim et al (2012) [34] study, the creatinine level of negative control group had significantly gross reduction (P< 0.01) than the normal and cooked Tribulus terrestris fruit extract treated animals. Lead induced with respect to histopathological study of lead induced renal toxic tissues, it was found to have remarkable changes. Though 30 days of lead exposure did not bring a significant change in histopathology of liver tissues, yet it has lead to dramatic change in that of kidney tissue which was confirmed by the presence of black refractive deposits in the tubules along with cloudy swelling and hyaline changes in the tubules of the lead intoxicated rats. The blackish or dark brownish deposits may be indicative of heavy metal lead acetate. However, there was no such blackish refractile deposits were found in the tubules of the lead intoxicated but treated with cooked Tribulus terrestris fruit extract. Whereas, the absence of such deposits in the kidneys of experimental animals may be attributed to the plausible mechanisms of enhanced glomerular filtration in turn due to the diuretic effect of the cooked Tribulus terrestris fruit extract. The effect of dieresis due to Tribulus terrestris has been explained by Devi et al (2008) [35] and in the present study the washing away of heavy metal lead may be conversely be attested by this phenomenon. Yet, hyaline changes and cloudy swelling of tubules were found to be similar to that of negative control. In addition, interstitial patchy lymphoid aggregates were present in the case of negative control but that was not found in the cooked Tribulus terrestris fruit extract treated rats. In a way, blood urea and creatinine levels also go with this finding.
In corroboration to the present findings, many studies declare that some extracts of plant and chemical substances of animal origin have been reported to confer protection against lead induced renal impairement both structure and function wise [31]. The study of Kensal et al (2011) has proved that aqueous extract of Coriander sativum (500mg/ kg/bwt) inhibited lipid per oxidation, restored antioxidant status and kidney architectures after inducing oxidative stress by administrating 40mg/ kg body weight of dose lead nitrate [36].
Nevertheless, all the studies cited here and generally found in the literature have focussed only on various solvents and aqueous extracts but, exotically our study has addressed all the impact by using cooked extract.
CONCLUSION
Toxicity due to lead exposure have been attributed to its ability to induce oxidative stress by generating reactive oxygen species (ROS). Plant extracts are also observed to protect against lead induced toxicity in experimental animals. The potentiality of such extract to mitigate toxicity is attributed to the antioxidant properties of the phyto chemicals present in these extracts. Actually saying, some of these mitigating agents may need extensive exploration and evaluation if they could be clinically and therapeutically applied.
In conclusion, it is clearly evident that lead acetate administration leads to a significant alteration in the hepatorenal functions and structure. Yet, even the cooked Tribulus terrestris fruit extract with lead acetate has substantially proven to impart its ability to provide a considerable protective effect on hepato renal function and tissue structure.
Conflict of interest
None.
ACKNOWLEDGMENTS
The authors are thankful to the authorities of PSG institution of medical sciences and research for providing support to the study.
REFERENCES
- Gidlow, D; 2004. Lead toxicity. Occup. Med. (Lond) 54, 76-81.
- Flora, S.J.S., Flora, G., Saxena, G. 2006. Environmental occurrence, health effects and management of lead poisoning In: Cascas SB, Sordo J, editors. Lead chemistry, analytical aspects, environmental impacts and health effects. Netherlands: Elsevier Publication. p. 158-228.
- Bressler, J., Kim, K.A., Chakraborti, T., Goldstein, G. 1999. Molecular mechanisms of lead neurotoxicity. Neurochem. Res 24, 595-600.
- Lanphear, B.P., Dietrich, K., Auinger, P., Cox, C. 2000. Cognitive defcits associated with blood lead concentrations <10 μg/ dl in US children and adolescents. Public Health Rep 115, 521-9.
- Khalil-Manesh, F., Gonick, H.C., Weiler, E.W., Prins, B., Weber, M.A., Purdy, R.E. 1993. Leadinduced hypertension: possible role of endothelial factors. Am J Hypertens 6, 723-9.
- Patra, R.C., Swarup, D., Dwidedi, S.K. 2001. Antioxidant effects of α-tocopherol, ascorbic acid and L-methionine on lead- induced oxidative stress of the liver, kidney and brain in rats. Toxicol 162, 81-8.
- Ronis, M.J.J., Bedger, T.M., Shema, S.J. 1998. Endocrine mechanism underlying the growth effects of developmental lead exposure in rat. J. Toxicol. Environ. Health 54, 101-20.
- Leonard, S.S., Harris, G.K., Shi, X.L. 2004. Metal-induced oxidative stress and signal transduction. Free Rad Biol Med 37, 1921- 42.
- Osweiler, G.D; 1999. Williams and Wilkins Philadelphia. Veterinary Toxicology.
- Gilman, A.G., Rall, T.W., Nies, A.S., Taylor, P. 1991. Goodman and Gilman’s. The Pharmacology basis of therapeutics. Pergamon: New York.
- Coryslechta, D.A., Weiss, B., Cox, C. 1987. Mobilization and redistriobution of lead over the course of calcium disodium ethylenediamine tetraacetae chelation therapy. J. Pharmacol. Exp. Ther 243, 804- 813
- Majeed SH, Mahmood MJ, 1988. Herbs and Medicinal Plants in Iraq between Traditional Medicine and Scientific Research. 1st Ed. Baghdad: Dar Al-Thaowra for Publishing, 40.
- Tomova, M; Gjulemetova, R; Zarkova, S; Peeva, S; Pangarova, T; Simova, M;1981. Steroidal sa-po-nins from Tribulus terrestris l. With a stimulating action on the sexual functions. First intern conf chem biotechnol biol active natural products, proceed-ings, varna. 3,299-303.
- Wu T.S; Shi L.S; Kuo SC; 1999. Alkaloids and other con-stituents from Tribulus terrestris. Phytochemistry.; 50, 1411-1415.
- Xie Z.F.S; Huang X.K; Dictionary of traditional chinese medicine, Hong Kong: commercial press, ltd. 1988;205-206
- Xu, Y.X; Chen, H.S; Liang, H.Q; Gu, Z.B; Lui, W.Y; Leung, W.N; Li T.J; 2000. Three new saponins from tribulus terre-stris. Planta medica. 2000; 66(6):545-550.
- Sasikala, S; Kannan, E; Brindha; 2014. Qualitative characterization of solvent and cooked extracts of Tribulus terrestris l. fruit European Journal of Medicinal Plants 4(8), 907-919.
- King EJ, Wotton ID. Microanalysis in medical biochemistry, 4 th ed. London: Churchill Ltd; 1964. p. 86-101.
- Mallay HT and Evelyn KA. Estimation of serum bilirubin level with the photoelectric colorimeter. J Biol Chem. 1937; 119: 481– 484.
- Mayer P, (1896), Mitt. zool. Stn. Neapel., 12, 303.
- Mitra, S.K., Venkataranganna, M.V., Sundaram, R. and Gopumadhavan, S. 1998. Protective effect of HD – 03, a herbal formulation against various hepatotoxic agents in rats. J. Ethanopharmacol 63, 181- 186.
- Koriem, K.M., 2009. Lead toxicity and the protective role of Cupressus sempervirens seeds growing in Egypt. Rev. Latinoamer. Quím 37(3), 230-242.
- Anuradha, R. and P. Krishnamoorthy, 2012. Impact of pongamia pinnata extract on lead acetate mediated toxicity in rat liver. Int. J. Pharm. Tech. Res 4(2), 878-882.
- Falah, A.M., 2012. Protective effects of latex of Ficus carica L. against lead acetate- induced hepatotoxicity in rats. J. J. Biol. Sci 5(3), 175.
- Waggas, A.M., 2012. Grape seed extract (Vitisvinifera) alleviate neurotoxicity and hepatotoxicity induced by lead acetate in male albino rats. J. Behav. Brain Sci 2, 176- 184
- Georing, P.L; 1993. Lead-protein interaction as a basis for lead toxicity, Neurotoxicol 14, 45–60.
- Shalan, M. A., Mostafa, M. S., Hassouna, M. M. El-Nabi, S. E. and El-Refale, A; 2005. Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicol 206, 1-15.
- El-Zayat, E.M., El-Ymany, N.A., Kamel, Z.H; 1996. Combined supplementation of zinc and vitamin C as protective agents against lead toxicity in growing male albino rats. Liver functions, J. Egypt Ger. Soc. Zool 20, 115–139.
- Hassanin, L.A.M; 1994. The effect of lead pollution on the susceptibility of rats to anticoagulants rodenticides, M.Sc., Thesis, Zoology Department, Faculty of Science, Cairo University, Giza, Egypt. P.123.
- Harper, H.A; 1961. The functions and tests of the liver. In: Review of physiological chemistry. Lange Medical Publishers, Los Atlos, California, P. 271-283.
- Elias A; Oputiri D; Oru-Bo; Precious G; Akuegbe E; 2013. Lead Organ and Tissue Toxicity: Roles of Mitigating Agents. British Journal of Pharmacology and Toxicology 4(6), 232-240.
- Abdel-Aal, K.M; M.R. Hussein; 2008. Therapeutic efficacy of alpha lipoic acid in combination with succimer against lead- induced oxidative stress, hepatotoxicity and nephrotoxicity in rats. Ass. Univ. Bull. Environ. Res 11(2), 87-99.
- Ishiaq, O., A.G. Adeagbo and H. Nta; 2011. Effect of a natural antioxidant fruit-tomatoes (Lycoperscion esculentium) as a potent nephroprotective agent in lead induced nephrotoxicity in rat. J. Pharmacog. Phytotherap 3(5), 63-66.
- Ibrahim, N.M., E.A. Eweis, H.S. El-Beltagi and E. Yasmin, 2012. Effect of lead acetate toxicity on experimental male albino rat. Asian Pac. J. Trop. Biomed 2(1), 41-46.
- Devi, D. J; Ramesh, C. U Different chemo types of Gokhru (Tribulus terrestris): A herb used for improving physique and physical performance: International Journal of Green Pharmacy; july sep 2008, Pp: 158- 161
- Kansal, L., V. Sharma, A. Sharma, S. Lodi and S.H. Sharma, 2011. Protective role of Coriandrum sativum (coriander) extracts against lead nitrate induced oxidative stress and tissue damage in the liver and kidney in male mice. Int. J. Appl. Biol. Pharm. Technol 2(3), 65-83.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences