From Photobiolumination to Optogenerapy, Recent Advances in NIR Light Photomedicine Applications
Department of Biosystems Science and Engineering, ETH Zurich, Switzerland
- Corresponding Author:
- Marc Folcher
Department of Biosystems Science and Engineering
ETH Zurich, Switzerland
Tel: +41 61 387 32 04
E-mail: Marc.Folcher@bsse.ethz.ch
Received date: April 04, 2018; Accepted date: April 06, 2018; Published date: April 15, 2018
Citation: Pierroz V, Folcher M (2018) From Photobiolumination to Optogenerapy, Recent Advances in NIR Light Photomedicine Applications. J Mol Genet Med Vol 2:2
Abstract
The biophysical properties associated with the Near Infrared light (NIR) have fostered the development of many therapeutic applications with high clinical relevance. On the one hand the unique NIR penetration properties enable the clinical monitoring of tissue hemodynamic state; on the other hand, NIR light allows a unique spatiotemporal control over a drug delivery scenario. Furthermore, the optogenetic bioengineering toolbox has now widened the spectrum of possible applications to NIR fluorescing proteins for cell monitoring but also to NIR synthetic optogenetic pathway programming. This review gives an overview of the recent proof of concepts that may broaden the field of NIR light photomedicine application. NIR deepbrain photobiomodulation may hold the promise to offer new options for the treatment of neurodegenerative disorders. Light-activated nanometer-sized drugs and light control vesicles delivering photothermal therapy effectors could circumvent deleterious side effects associated with systemic drugs. Optogenerapy profits from the NIR light optogenetic interface to have a controlled delivery of therapeutic proteins by a bioelectronic implant and opens the road of the tomorrow photomedicine.
Keywords
Near infrared light; Optogenetic; Photomedicine; Photobiomodulation
Introduction
The Near-infrared (NIR) light spectrum spans wavelengths from 700 nm to 1 million nm; it is a low energy electromagnetic radiation in which the spectrum merges with the red/far-red spectrum of visible light. NIR photonic energy can be transmitted in the tissue when it is irradiated with the appropriate NIR fluence rate [1]. Given its unique tissue penetration properties NIR light is used for many medical applications; from in vivo imaging, to light-activated drug-carrier sometime of nanometres size. Modern photomedicine focuses on the development of therapeutic actions that can be localized using light as an inducer of an active principle delivered at a specific location. The recent progresses in optogenetic have offered a toolbox of genetically encoded photosensitizer molecules that can be assembled as a programmable interface to trigger a therapeutic action. These characteristics are an asset for an unmet clinical need: to overcome toxicity associated with the systemic activity of the current untargeted chemotherapeutic drugs. This mini-review gives an overview of the current and future applications of NIR-inducible drugdelivery systems.
Clinical Relevance of NIR Physical Properties
Electromagnetic radiations are used for many medical interventions in the growing field of photomedicine including the Magnetic Resonance Imaging (MRI) and the radiofrequency ablation of tumours for the treatment of cancer. NIR light irradiation is perceived as safe even beneficial for the patient when irradiance is kept under 50 mW/cm2 [2]. Photomedicine capitalizes on the interaction of light photons with photosensitizer molecules naturally present in the patient tissue or that are injected beforehand to the patient. Photon penetration in the skin is dependent on the wavelength but remains limited to few millimetres depth due to the absorption and the scattering of light. Jöbsis [3] identified the optical transmission window of the skin to have an optimal wavelength range from 700 to 1300 nm for in vivo treatment. In the low wavelength region, the optical window is limited by the absorption of main chromophores present in the subcutaneous tissue. The hemoglobin (oxy- and deoxy-hemoglobin) is an oxygen carrier metalloprotein circulating in the bloodstream, they are with the cytochrome c oxidase and the melanin the primary absorbing compounds of the skin. On the one hand NIR absorption can be used to monitor the hemoglobin oxidative state. On the other hand, NIR can also mediate an allosteric control over an enzymatic module associated to the chromophore, and thus the "light-control" of a biological process.
Clinical Monitoring using NIR Light
Given its unique optical properties, NIR imaging represents an excellent asset for non-invasive determination of the hemodynamic state of tissues. Thereby for imaging, pure NIR reflectance can shed light on the vascularization under the skin to help medical staff practicing intravenous injection [4]. The pulse oxymetry allows a non-invasive monitoring of pulse read and blood oxygenation. These low-cost devices should be present in all operating rooms, as a part of the patient checklist edited by the World Health Organization WHO. The enforcement of the technology has contributed in a reduction of mortality by 30% [5] in hospitals.
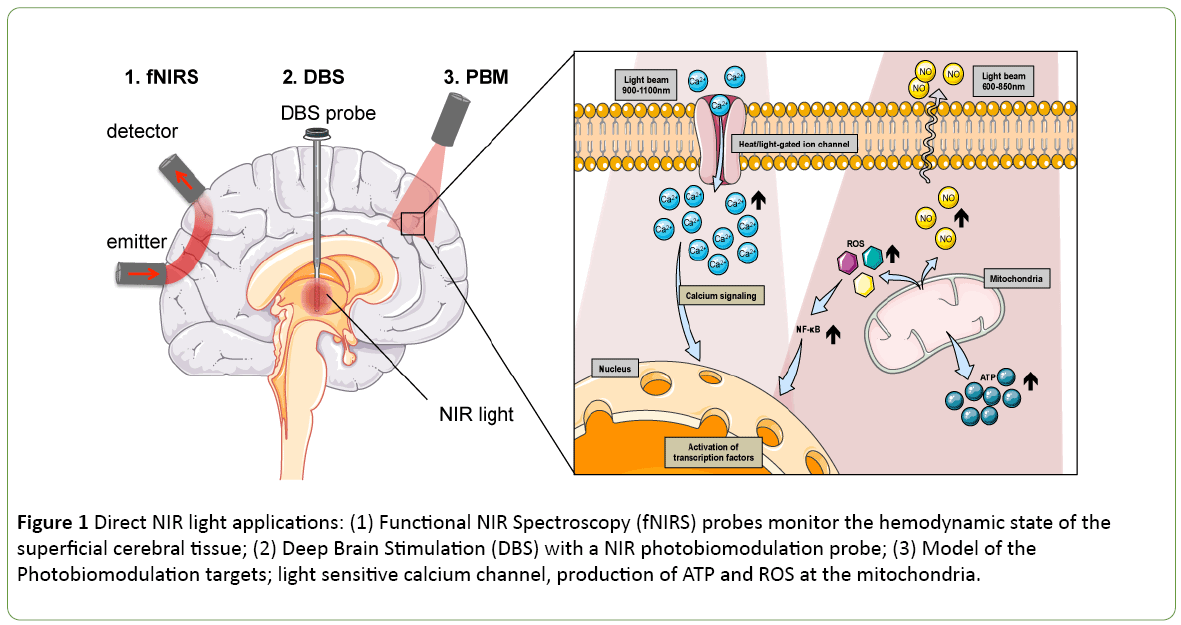
Functional NIR spectroscopy (fNIRS) can be used to noninvasively track neuronal activity by monitoring the associated cerebral hemodynamics in the superficial cerebral tissue (Figure 1). Taking advantage of the changes in NIR absorptions of oxygenated to deoxygenated hemoglobin, a practician could estimate the brain oxygenation during operation in neonates [6] but also evaluate brain activity during cognitive tasks [7]. The progresses of this technology sometimes associated with electroencephalography (EEG) electrodes are driven by the development of new hardware [8]. While the non-invasive fNIRS technology is mostly used to measure haemoglobin oxidative state, it was primarily identified to access the mitochondrial cytochrome c oxidase states. The cytochrome oxidase protein resides in the mitochondria and catalyses the last step of the respiratory chain. Its monitoring offers a direct indication on the metabolic activity in the cell. It is not only possible to measure the cytochrome c oxidative state using NIR light, but it is also possible to modulate its activity.
Figure 1: Direct NIR light applications: (1) Functional NIR Spectroscopy (fNIRS) probes monitor the hemodynamic state of the superficial cerebral tissue; (2) Deep Brain Stimulation (DBS) with a NIR photobiomodulation probe; (3) Model of the Photobiomodulation targets; light sensitive calcium channel, production of ATP and ROS at the mitochondria.
Phototherapies using NIR Light
Photobiomodulation (PBM) sometimes also referred, as low light level therapy (LLLT) is a therapeutic approach where light irradiation is beneficial for tissue healing (Figure 1). During PBM, the activity of the cytochrome c oxidase is activated, leading to a production of energy, i.e., adenosine triphospate (ATP) and reactive oxygen species (ROS) [9,10] (Figure 1). This energy gain proved to be helpful for e.g. wound healing, tissue regeneration, stimulating several pathways such as glucose metabolism. It was found as a potent therapeutic application to control insulin production [11] or improving neurodegenerative disorder conditions like Alzheimer’s or Parkinson’s diseases [12,13]. Historically, the pioneering experience of deep brain electrical stimulation electrode by the Prof. Benabid [14] has brought a real benefit to the patient’s quality of life in the treatment of Parkinson’s [14]. Today the neurosurgeon and its associated research team are tackling another challenge with the development of an intracranial PBM photobiomodulation probe [15-17] (Figure 1). The PBM treatment helps to protect midbrain dopaminergic neuron and increases locomotor activity in animal models of Parkinson's disease [18]. While the primary, and secondary mechanisms of actions are still uncertain [6]. It is accepted that ROS produced during PBM will foster the immune response. The PBM will also promote the activation of the nuclear factor NF-κB pathway that causes expression of stress response genes that ultimately leads to cell survival by blocking apoptosis in mouse embryonic fibroblast [19] (Figure 1). Also the effects of NIR are dependent on the wavelength and on the fluency of the NIR source. Hamblin reports a biphasic dose response for two wavelengths at both 810 nm and 980 nm on adipose-derived stem cells [20]. The study suggests that the 810 nm acts on the cytochrome oxidase while the 980 nm one functions through heat activation. A proposed mechanism of action involves a light sensitive calcium channel known as transient receptor potential (TRP) [20] (Figure 1). Thus it is possible to profit from the PBM technics to control specific photosensitive molecule activation. Using the external NIR light source to trigger a drug action allows a spatiotemporal control over the therapeutic drug delivery and could prevent systemic side effect of the effector.
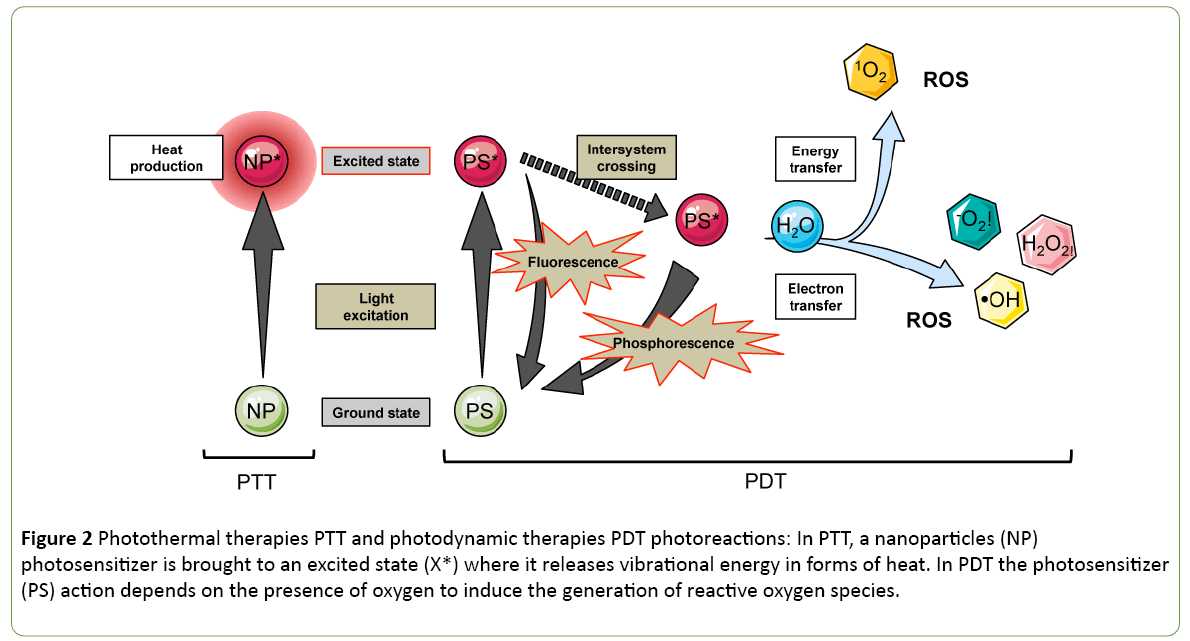
The photothermal and photodynamic therapies (PTT and PDT) capitalize on the excitation by light of nanoparticles or photosensitizers (PS) molecules respectively injected to the target location (Figure 2). In the case of PTT, the excited molecules release heat by vibrational energy to return to their ground state. In contrast to PDT, the energy is transferred finally to a water molecule that turns into its highly reactive singlet state, known as singlet oxygen (Figure 2). The first photothermal sensitizer that reached clinical trials in 2008 for solid tumour treatment is a spherical nanoparticle covered by a thin gold shell called AuroShell gold-nanoshell. The AuroLaser Therapy technic is using NIR irradiation at 808 nm to excite the gold nanoshell that heats up tumour cells.
Figure 2: Photothermal therapies PTT and photodynamic therapies PDT photoreactions: In PTT, a nanoparticles (NP) photosensitizer is brought to an excited state (X*) where it releases vibrational energy in forms of heat. In PDT the photosensitizer (PS) action depends on the presence of oxygen to induce the generation of reactive oxygen species.
However, ROS produced via the photosensitizing process have potent cytotoxic activities to neighbouring tissues. Recently, the PDT sensitivity spectrum was widened to the red of the visible spectrum. The photofrin is a porphyrin-based photosensitizer, that was tested in the clinics in 1993; first for the treatment of bladder cancer and then its application was extended to oesophageal cancer. A lot of effort is applied to find photosensitizers with excitation spectrum in the NIR range, sometimes taking advantage of 2-photons for activation. Promisingly, LUZ11, a new PS candidate developed by a spin-off of the University of Coimbra, has entered clinical trials (Clinical trial Identifier: NCT02070432) in 2017. The active principle is a nontoxic derivative of bacteriochlorophyll, the bacteriochlorin that is having an absorption band at a wavelength of 748 nm.
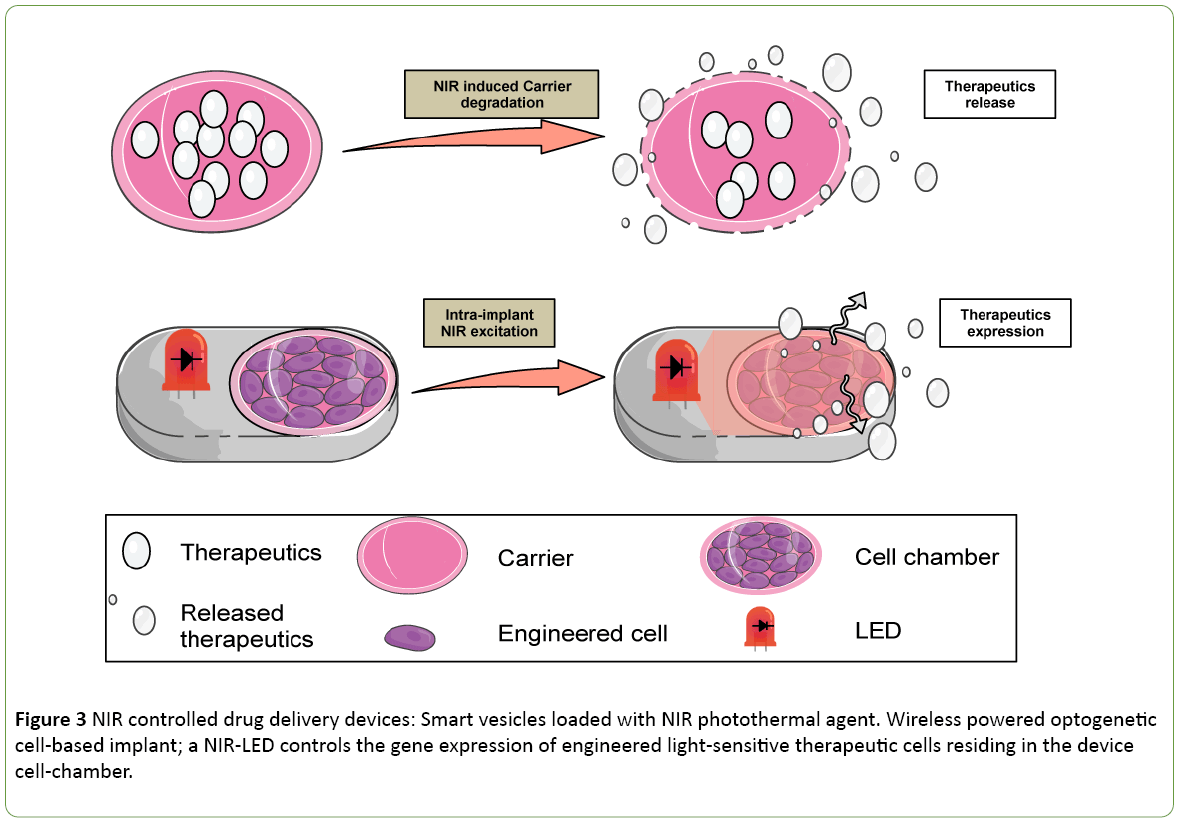
The development of NIR-sensitive molecules with larger Stokes shift and higher fluorescent quantum yield is the focus of many researches both in the field of photosensitizer and imaging [21,22]. Some applications have been developed to support the delivery of multiple contrasting agents for multimodal non-invasive imagine technics like; computed x-ray tomography (CT); MRI magnetic resonance imaging and fluorescence imaging [23]. These imaging technics play a key role in the establishment of a diagnostic. Therefore the NIR light triggering the contrasting agent delivery can also act as theranostic (combine diagnostic and therapeutic) approach. Coencapsulating the different contrasting agents in temperature sensitive liposome offers the possibility to capitalize on the NIR fluorescing compound payload for imaging (diagnostic) and for photothermal therapy (Figure 3). Proof of concept was validated by the observation of efficient tumour inhibition during in vivo experiments with rodent animal model [23].
Figure 3: NIR controlled drug delivery devices: Smart vesicles loaded with NIR photothermal agent. Wireless powered optogenetic cell-based implant; a NIR-LED controls the gene expression of engineered light-sensitive therapeutic cells residing in the device cell-chamber.
As most of the tumours are hypoxic in their core, PDT may lack efficiency. Therefore, light-activated molecules displaying toxicity in an oxygen independent manner spark interest. In an ideal Photoactivated Chemotherapy (PACT) scenario a pro-drug is delivered to the tissue, and the doctor controls using NIR radiation the release of the drug active principle in the tumour. Smart vesicles loaded with NIR photothermal agent represent a new therapeutic way to develop an intelligent drug-delivery system. Metal-bis(dithiolene) are strong photostable NIR absorbing compounds. Since they do not emit photon or single oxygen upon illumination, they show a high PTT stable activity [24]. Light-activated liposomes have proven to be an elegant strategy to achieve ocular drug delivery [25]. Indocyanine green is also an approved clinical imaging agent that can be incorporated into a liposome to serve as smart light-sensitive cargo for drug-delivery [26] (Figure 3). The future development of more photothermal stable nanoparticles will support the field of PTT.
NIR Optogenetic Applications
Fluorogenic substrates are a unique class of non-fluorescent compounds that can be activated by an effector protein to turn fluorescent. The fluorogenic molecules can act as a substrate for an enzyme [27] or as fluorophore moiety for a genetically encoded photosensitizer [28,29]. Wang [28] has developed a specific class of a tumour targeting chimeric protein that activates a NIR fluorogenic substrate at the tumour location. These protein-based drugs are carrying a targeted antibody as powerful detection means associated with a fluorogen activated protein supporting high specificity NIR imaging with low background fluorescence.
Recent developments in genetic engineering have extended the spectrum genetically encoded photosensitizer to the far red and NIR range. The red fluorescent proteins are often derived from the discosoma sea anemones (DsRED, mCherry) while the far-red/NIR fluorescent proteins (NIRFP, smURFP) are developed using bacteriophytochrome as a bioengineering template [30]. Among all the phytochrome receptors, bacterial phytochrome domains (BphPs) sensitivity spectrum is especially interesting for biomedical purposes. Rounds of targeted mutations have been used to evolve the protein non-fluorescent photoreceptors protein into a fluorescing one [31]. They found numerous applications for in vitro and in vivo non-invasive imaging [30].
The photoconversion mechanism that is central to sense a specific red wavelength triggers conformational changes and converts a phytochrome from the red-absorbing Pr form to the far red-absorbing Pfr form. The change is reversible, with far-red illumination restoring Pr, but dark reversion is more specific to phytochromes than bacteriophytochromes. They have a natural sense of far-red to NIR light in the presence of their cofactor, an oxidized product of heme called biliverdin IXα (BV), present in mammal’s blood circulation. The bioavailability of the chromophore opens the way to makes multiple in vivo applications possible [26,32]. First, engineering specific mutations and truncations in the protein amino acid sequence gave rise to powerful NIR protein fluorophores, particularly useful for non-invasive in vivo imaging. Overcoming the limitation associated with the visible emission spectra of fluorescing reporter proteins like the GFP; it is now possible to image gene expression directly in living tissues [26,28].
Optogenetic is a technic where genetically encoded optical actuators are used for fast control of cellular functions in excitable cells. The technology has proven to be an essential tool to help to decipher neuronal network. Interestingly, architecture of the bacteriophytochrome associated domains offers bioengineer an interesting template to develop NIR controlled biological process. As example, bacterial phytochrome photorecptors (BphP's) sensor domains are often linked with the output module (OM) encoding enzyme, such as kinase, phosphodiesterase or NTPs cyclase activities [33]. Genetic engineering on these protein sensor modules leads to the development of several synthetic biology applications [34,35].
The hallmark of optogenetic lays in the activation of photosensitive channel rhodopsin ion channels. Optogenetic tools were primarily developed to decipher excitable cells circuit in brain tissue. The technic is now used to tackle new challenges, like immune-modulation or biologic controlled delivery. Traceless modulation of Ca2+ mobilization in immune cells mimics a physiological response resulting in the engagement of an antigen receptor and thus enables the control of an essential step in the immune response [32]. To overcome the limitations associated with the narrow spectral range of blue light sensing channel-rhodopsin a photo-converter nanoparticle can be associated with the genetically modified immune cells expressing the synthetic optogenetic pathway [32]. The photoconverter nanoparticle emits at 470 nm emission when excited with 980 nm [36,37], it renders possible the spatiotemporal activation of the blue light sensitive synthetic optogenetic pathway in deep tissue by a NIR laser.
Optogenerapy: Drug Delivery
Optogenerapy stands for combine optogenetic and gene therapy and holds the promise of a new modern syringe era [38]. The strategy combines the engineering of synthetic NIR optogenetic pathway with a macroencapsulation device equipped with a wireless powered optoelectronic circuit. In the electronic implant, the NIR light is used as a cell-machine interface (Figure 3). The strategy offers the possibility to use genetically modified cell line in confinement that first of all protects the therapeutic cells from the circulating immune cells but also protects the patient from the common risk associated with the cell therapy. As the device is hermetically sealed, it is also possible to explant it. The optogenerapy proof of concept [39] was performed by connecting a brain-computer interface (BCI) to a powering antenna wirelessly powering the bioelectronic cell-based implant place subcutaneously in the back of a mouse. The user connected to a BCI could trigger the activation of the optogenerapy device following a specific mental task and thus could control the secretion of a biomarker in the blood circulation in a rodent animal model. The wireless powered cell based implant method is getting magnitude as researchers have tested its efficacy for the release of insulin in the context of diabetic disorder in rodent animal model [40]. Light sensitive therapeutic delivery cells can be genetically programmed to produce any therapeutic recombinant proteins. NIR light bioelectronic cell-based implant potentially represents a disruptive innovation to support light controlled drug delivery.
Conclusion
Thanks to its unique properties, the NIR light can support the transmission of energy to the targeted tissue. The light can be used to kill cells but also to heal tissue. While the native biological target is not entirely characterized, the design of synthetic light sensitive module has extended the NIR light application portfolio to the synthetic biology and the cell machine interface. A synergy between the field of the photobiology, the synthetic biology and the photochemistry holds great potential to develop the light controlled therapies of tomorrow therapeutics.
Funding Source
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 720694.
Acknowledgement
We are thankful to our colleagues Fanny Michel and Clémence Tournaire who gave scientific guidance and participated in discussions. Figures are adapted from Servier Medical Art (Creative Commons Attribution 3.0 Unported License).
References
- Henderson TA, Morries LD (2015) Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat 11: 2191-2208.
- Lanzafame RJ (2007) Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg Med 39: 534-542.
- Jobsis FF (1977) Non-invasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198: 1264-1267.
- de Graaff JC (2013) Near-infrared light to aid peripheral intravenous cannulation in children: A cluster randomised clinical trial of three devices. Anaesthesia 68: 835-845.
- Kim RY (2015) Sustainability and long-term effectiveness of the WHO surgical safety checklist combined with pulse oximetry in a resource-limited setting: Two year update from Moldova. JAMA Surg 150: 473-479.
- Biallas M (2012) Multimodal recording of brain activity in term new-borns during photic stimulation by near-infrared spectroscopy and electroencephalography. J Biomed Opt 17: 086011-086011.
- Soltanlou M (2018) Reduction but no shift in brain activation after arithmetic learning in children: A simultaneous fNIRS-EEG study. Sci Rep 8: 1707.
- Scholkmann F (2014) A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85: 6-27.
- Salehpour F (2018) Brain photobiomodulation therapy: A narrative review. Mol Neurobiol.
- Hamblin MR (2017) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol.
- Silva G (2017) Infrared photobiomodulation (PBM) therapy improves glucose metabolism and intracellular insulin pathway in adipose tissue of high-fat fed mice. Lasers Med Sci.
- Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L (2017) Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photomed Laser Surg 35: 432-441.
- Hamblin MR (2016) Shining light on the head: Photobiomodulation for brain disorders. BBA Clin 6: 113-124.
- Benabid AL (2010) Gene therapy for Parkinson's disease: Do we have the cure? Lancet Neurol 9: 1142-1143.
- El Massri N (2017) Photobiomodulation-induced changes in a monkey model of Parkinson's disease: Changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp Brain Res 235: 1861-1874.
- Reinhart F (2017) The behavioural and neuroprotective outcomes when 670 nm and 810 nm near infrared light are applied together in MPTP-treated mice. Neurosci Res 117: 42-47.
- Moro C (2017) No evidence for toxicity after long-term photobiomodulation in normal non-human primates. Exp Brain Res 235: 3081-3092.
- Darlot F (2016) Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann Neurol 79: 59-75.
- Chen AC (2011) Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One 6: e22453.
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (2017) Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim Biophys Acta 1861: 441-449.
- Fei Q (2017) Near-infrared fluorescent dyes with large Stokes shifts: Light generation in BODIPYs undergoing excited state intramolecular proton transfer. Org Biomol Chem 15: 4072-4076.
- Gulzar A, Xu J, Yang P, He F, Xu L (2017) Upconversion processes: Versatile biological applications and biosafety. Nanoscale 9: 12248-12282.
- Wu B (2017) Near-infrared light-triggered theranostics for tumour-specific enhanced multimodal imaging and photothermal therapy. Int J Nanomed 12: 4467-4478.
- Mebrouk K (2017) Fine and clean photothermally controlled NIR drug delivery from biocompatible nickel-bis(dithiolene)-containing liposomes. ChemMedChem 12: 1753-1758.
- Lajunen T (2016) Light activated liposomes: Functionality and prospects in ocular drug delivery. J Control Release 244: 157-166.
- Lajunen T (2016) Indocyanine green-loaded liposomes for light-triggered drug release. Mol Pharm 13: 2095-2107.
- Li C, Tebo AG, Gautier A (2017) Fluorogenic labeling strategies for biological imaging. Int J Mol Sci 18.
- Wang Y (2017) Affibody-targeted fluorogen activating protein for in vivo tumour imaging. Chem Commun (Camb) 53: 2001-2004.
- He J (2016) A genetically targetable near-infrared photosensitizer. Nat Methods 13: 263-268.
- Piatkevich KD, Subach FV, Verkhusha VV (2013) Engineering of bacterial phytochromes for near-infrared imaging, sensing and light-control in mammals. Chem Soc Rev 42: 3441-3452.
- Bhattacharya S, Auldridge ME, Lehtivuori H, Ihalainen JA, Forest KT (2014) Origins of fluorescence in evolved bacteriophytochromes. J Biol Chem 289: 32144-32152.
- Chernov KG, Redchuk TA, Omelina ES, Verkhusha VV (2017) Near-infrared fluorescent proteins, biosensors and optogenetic tools engineered from phytochromes. Chem Rev 117: 6423-6446.
- Folcher M (2017) Photoactivatable nucleotide cyclases for synthetic photobiology applications. Cambridge: Cambridge University Press. Cambridge University Press Ed Vol Part II, pp: 118-131.
- Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M (2010) Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem 285: 41501-41508.
- Ryu MH (2014) Engineering adenylate cyclases regulated by near-infrared window light. Proc Natl Acad Sci U S A 111: 10167-10172.
- He L (2015) Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. Elife 4.
- Ma G (2017) Optogenetic toolkit for precise control of calcium signaling. Cell Calcium 64: 36-46.
- Michel F, Folcher M (2017) Optogenerapy: When bio-electronic implant enters the modern syringe era. Porto Biomed J 2: 145-149.
- Folcher M (2014) Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant. Nat Commun 5: 5392.
- Xue S (2017) A synthetic-biology-inspired therapeutic strategy for targeting and treating hepatogenous diabetes. Mol Ther 25: 443-455.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences